Production of polycrystalline silicon by chlorination from rice husk and purification of chlorine-containing gases by adsorption method
Автор: Zhanbolot K.Aidaraliev, Imilya A.Rysbaeva, Bekbolot kyzy Baktygul, Mairam K.Chimchikova, Rashid kyzy Burulcha
Журнал: Nanotechnologies in Construction: A Scientific Internet-Journal @nanobuild-en
Рубрика: The results of the specialists’ and scientists’ researches
Статья в выпуске: 6 Vol.15, 2023 года.
Бесплатный доступ
Introduction. In the article we analysed the technology for producing silicon from rice husks. The analysis showed that the production of polycrystalline and amorphous silicon based on rice waste in the form of rice husk solves the simultaneous disposal of rice waste. Rice husk processing produces valuable organic products, and the residual solid waste mainly contains silicon, carbon and other trace metal elements. Therefore, obtaining silicon and silicon-containing materials from rice husk is relevant. Methods and materials. Various methods for obtaining silicon from rice husk are given. Among them, the methods of chlorination and sublimation were chosen, and experimental installations were assembled to conduct the experiment. The object of study was samples obtained from rice husks of Uzgen rice in the Kyrgyz Republic. Results. The composition and structure of rice husks for the production of crystalline silicon were studied. Lime milk was used to purify toxic chlorine-containing gases in the air of the working area and atmospheric air. The condensing system, designed to capture volatile chlorides, has two receivers. In the first receiver at a temperature of 60°C, condensation of iron, aluminum and magnesium chlorides occurs. It has been established that highly volatile silicon (IV) chloride (SiCl4) at a given temperature remains in the gaseous phase and is completely distilled off in the next receiver of the refrigerator. This indicates that the silicon is in the form of SiCl4 (60°C) and condenses only at a lower temperature in the next receiver. The data obtained indicate that when the temperature rises to 200°C, the process of chlorination of metal compounds initiates. The optimal conditions for maximum extraction of metals and silicon tetrachloride from rice husk were identified: temperature 500–550°C and time 120 minutes. Non-volatile chlorides of calcium, sodium, potassium and other elements form a floating mixture at 450°C. During the reaction, metal chlorides harden and settle on the cold walls of the reactor. Therefore, at this temperature there is not enough heat to maintain them in a gaseous state, and they condense to form solid precipitates. Lime milk containing CaO – 130 g/dm2 is a very effective and cheap means for purifying toxic chlorine-containing gases in the air of the working area and atmospheric air. At high temperatures (1050–1100°C), it is possible to activate chemical reactions between the carrier gas (hydrogen) and silicon chloride (SiCl4), which promotes the decomposition of SiCl4 into components, including silicon and hydrogen chloride, and also provides certain conditions for the formation and deposition silicon crystals. Conclusion. A technology for producing polycrystalline silicon by chlorination from rice husks of Uzgen rice of the Kyrgyz Republic has been studied and developed.
Crystalline silicon, rice husk, chlorination, macroelements, purification, sublimation, carrier, chlorination rate, semiconductor, milk of lime, adsorption, kinetics
Короткий адрес: https://sciup.org/142239118
IDR: 142239118 | DOI: 10.15828/2075-8545-2023-15-6-592-601
Текст научной статьи Production of polycrystalline silicon by chlorination from rice husk and purification of chlorine-containing gases by adsorption method
Original article
Silicon and its compounds are a material that has an extremely wide range of useful properties: electronic, electrical, anti-corrosion; due to this, it is increasingly being introduced into technology. To date, silicon has been successfully used in a large number of semiconductor devices, such as thermistors, penzodensors, photore-
RESEARCH RESULTS OF SCIENTISTS AND SPECIALISTS sistors and photocells for recording ultraviolet radiation, etc. [1].
One of the options for the production technology of polycrystalline silicon is presented in the reference [2], where a heat balance analysis was performed. As a result, it was concluded that the main parameter affecting the temperature in the reactor is the wall temperature.
An analysis of existing technological schemes for producing silicon for photoelectric converters (PVCs) was carried out. The results of an experiment to increase the efficiency of silicon-based elements are presented; as a result of research by the authors [3], it was found that increasing the efficiency of solar cells is possible due to the predominant use of silicon single crystals with a complex of new properties.
The article [4] shows the results of a porous silicon study obtained from various types of silicon-containing plant raw materials by the method of magnesium-thermal reduction of silicon dioxide at a temperature of 650оC in an argon atmosphere.
A literature review on the utilization of rice husks inorganic portions revealed a significant increase in the number of publications on this topic over the past 20 years. This growth is associated with the undisputed leadership of rice as the leading grain crop and an increase in the volume of waste generated during its processing. However, these wastes cannot always be effectively used as complete feed, fertilizer or fuel sources. Thus, researchers are now increasingly looking for new methods for recycling the inorganic portion of rice husks. One of the interesting directions was the study of the silicon carbide producing possibility from these materials. Silicon carbide has a wide range of applications, especially as a raw material for the production of ceramics and new generation refractories [5].
A new and promising source of raw material for the production of silicon and its carbide is rice husk, which contains, along with the organic part, silicon oxide with a mass content of 93%. In [6], a technological scheme for processing rice husks was developed, aimed at obtaining products: amorphous silicon dioxide, cellulose fibrous residue, and the possibility of extracting alkali lignin. The estimated level of profitability of the proposed technology is 30%.
Work [7] presents the results of a study of heat treatment of rice husk samples. During this work, heat treatment was carried out at various temperatures, as well as treatment with hydrochloric acid. The results obtained made it possible to identify the main relationships between the heat treatment process and the characteristics of the samples under study.
To select optimal conditions for preliminary heat treatment, a study was carried out on the firing process at the temperature range from 20 to 500оC of starting materials [8]. It is important to note that a certain amount of active carbon is retained in the material. According to the literature, chlorination in the presence of reducing agents such as coal has long attracted the attention of researchers.
The works [9, 10] determined the optimal conditions for silicon chlorination, the degree of chlorination depending on temperature, time, chlorine supply rate and degree of grinding of the starting material, etc.
There are different opinions regarding the mechanism of the chlorination reaction, especially the interaction with reducing agents. Some researchers suggest that the initially introduced reducing agents react with the oxides, reducing them to a state of lower valence or even to a metallic state. The resulting reaction products then react with chlorine. Other authors believe that chlorine reacts with oxides, displacing oxygen from compounds. In this case, the role of the reducing agent is reduced to binding the released oxygen and removing it from the reaction area.
Rice husk, unlike mineral silicon-containing raw materials, has a stable composition and low content of heavy metals, which is very important for the synthesis of solar silicon. The ability to be used as a raw material to produce silicon that is purer than that obtained from minerals has made rice husk an attractive natural raw material. In addition, rice husks are a source of cellulose and lignin. Cellulose obtained from rice husk can be used as a substrate for nanomodified dressings and medical preparations with reduced toxicity and high prolongation [11].
Silicon dioxide and silica from rice husks are of particular interest as components for producing adsorbents based on them. The chemical stability of these objects, high thermal stability, and the ability to regulate the porosity of the structure provide prospects for creating adsorbents and carriers with a high specific surface area based on them [12].
The problem of obtaining silicon with the complex processing of rice husks indeed remains poorly studied. In this regard, the main goal of our research was to obtain polycrystalline silicon from rice husk, which is a waste product from rice production.
METHODS AND MATERIALS
The iodide method of obtaining pure metals, widely used in technology, is used to isolate the purest silicon. It is based on a reversible reaction [10]:
Si + 2J2 ↔ SiCl4.
The equilibrium of this reaction in the temperature range 750–850оC is almost completely shifted towards the formation of tetraiodide; at temperatures around 1000–1200оC, thermal dissociation of SiJ4 occurs. The production of metal titanium, niobium, tantalum, zirconium, hafnium, rare earth metals, germanium, and silicon is based on the use of chlorine gas [13].
RESEARCH RESULTS OF SCIENTISTS AND SPECIALISTS
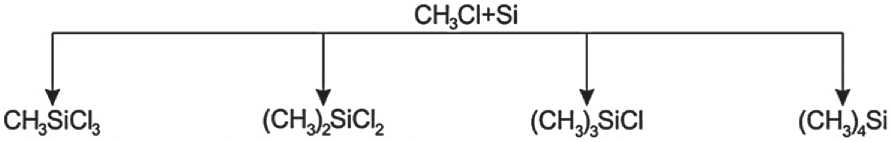
Methyltrichlorsilane Dichlorodimethylsilane Chlorotrimethylsilane Tetramethylsilane(boiling temp. 66 °C) (boiling temp. 70 °C) (boiling temp. 58 °C) (boiling temp. 26 °C)
Fig. 1. Alkylchlorosilane derivatives with various boiling temperatures
According to V.I. Stytsin, the acceleration of the chlorination process in the presence of coal is because of carbon doubles one of the reaction products with chlorine, which, in turn, continuously shifts the equilibrium of the reaction.
As noted by N.I. Timokhina, coal in a reaction of this type acts together with chlorine and reaction products, as an active principle capable of chlorinating oxides, salts and minerals. These active reagents include carbon tetrachloride, which is Morozov’s extremely active chlorinating agent:
Cl2 + CO = COCl2;
COCl2 + Me2O3 = MeCl3 + CO2;
CO2 + C = CO + CO.
Recently, the chlorine method has been widely used in non-ferrous metallurgy to extract rare and trace elements, including gold and silver. The chlorine method is especially effective for opening refractory ores in which gold and silver are in a finely dispersed condition [14].
In industry, at temperatures from 300 to 850оC, alkylchlorosilanes are obtained by treating a mixture of silicon and copper which is acting as a catalyst (Fig. 1).
The process of trichlorosilane reduction with hydrogen occurs at a temperature of 1000–1100оC [15]:
SiHCl3 + H2 = Si + 3HCl.
The chloride sublimation method was used to isolate pure components from mixtures by separating and condensing volatile chloride compounds (Fig. 2).
The remained amorphous silicon is dried and further used to obtain crystalline silicon. To obtain it, it is nec-
1 —^ 2 —► з —* 4 —- 5
Fig. 2. Chloride sublimation: 1 – reactor (quartz tube), 2 – condenser, 3 – refrigerator, 4 – receiver, 5 – calcium chloride tube essary to use the most effective gas flow method using SiCl4, the vapors of which are transferred by hydrogen or argon (hydrogen was obtained using a Kipp apparatus using the reaction: Zn + HCl = ZnCl2 + H2↑) as shown in Figure 3.
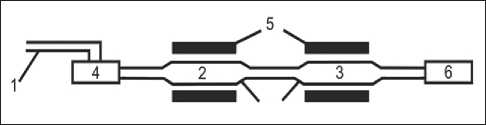
Fig. 3. Hardware and technological diagram of a laboratory installation for deep purification and crystallization of silicon: 1 – gas carrier; 2 – substance to be purified;
3 – purified substance; 4 – transport substance (SiCl4);
5 – electric furnaces; 6 – Migunov aspirator (compressor)
During the extraction of metals and purification of semiconductor elements, a certain amount of toxic chlorine-containing gases is released. Therefore, we investigated the sorption capacity of calcium hydroxide (Ca(OH)2) when washing chlorine-containing gases and in a laboratory installation for cleaning silicon.
The hardware and technological diagram of the laboratory installation (Fig. 4) includes an absorption tube 6, 7, which is a cylindrical glass vessel. This vessel is equipped with a porous gas-permeable partition in the lower part, which creates conditions for the uniform distribution of the chlorine gas mixture entering through the tubes into the bottom of the absorption tube.
The supply of gas from below ensures intensive mixing of the calcium hydroxide, preventing separation of the suspension during the experiment.
Chlorine-containing gases come from a reactor in which semiconductor elements are purified from metals.
For the quantitative analysis of active chlorine during the purification of chlorine-containing gases using calcium hydroxide and its saturated solution Ca(OH)₂, titrimetric, gravimetric methods and express analysis using UG-2 (universal gas analyzer) were used.
RESEARCH RESULTS OF SCIENTISTS AND SPECIALISTS
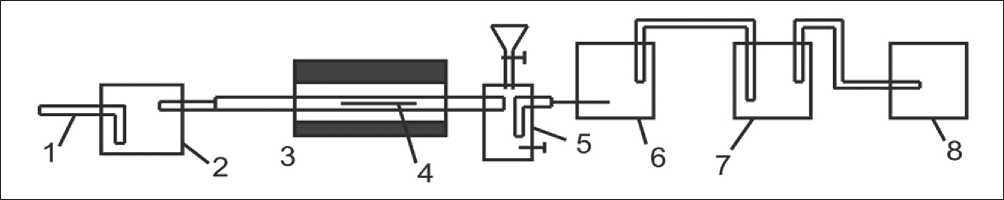
Fig. 4. Hardware and technological diagram of a laboratory installation for determining the sorption capacity of an absorption liquid: 1 – pipe for air intake; 2 – container with carbon tetrachloride; 3 – tube furnace; 4 – reactor with sample; 5 – pipe; 6-7 – absorbers with calcium hydroxide; 8 – Migunov aspirator
The ampoule method can also be used to clean silicon. In this method, 3–4 grams of silicon are placed in ampoules, which are filled with chlorine. Then 1–2 drops of SiCl4 or a small amount of water crystals are introduced into the ampoule. The end of the ampoule with silicon is heated to 1100оC. At this temperature, a reaction occurs between SiCl4 and silicon:
SiCl4 + Si ↔ 2SiCl4.
When the temperature decreases to 900оC, SiCl2 dis-proportionates:
2SiCl2 ↔ Si + SiCl4.
When an ampoule with a lower temperature is used, the rate of silicon transport slows down significantly, and the purification process takes several hours [16].
Scanning electron microscopy of all samples was carried out using the following shooting parameters: accelerating voltage is 15 keV, working distance is ≈10 mm.
All samples were secured with double-sided carbon tape. All measurements were carried out in high vacuum mode 10–3 Pa, samples surface Images were obtained.
RESULTS
The composition and structure of rice husk ash from Uzgen rice is shown in Fig. 5 and 6.
The process of low-temperature chlorination is based on a reaction in which oxide forms of metals react with a gas mixture of chlorine and phosgene vapor (COCl2). In the presence of chlorine, metal oxide compounds in the gas mixture undergo an exchange reaction; silicon chloride and chlorides of macroelements (Al, Fe, Ca, Mg, K, Na) are formed in the areas of the raw material under study:
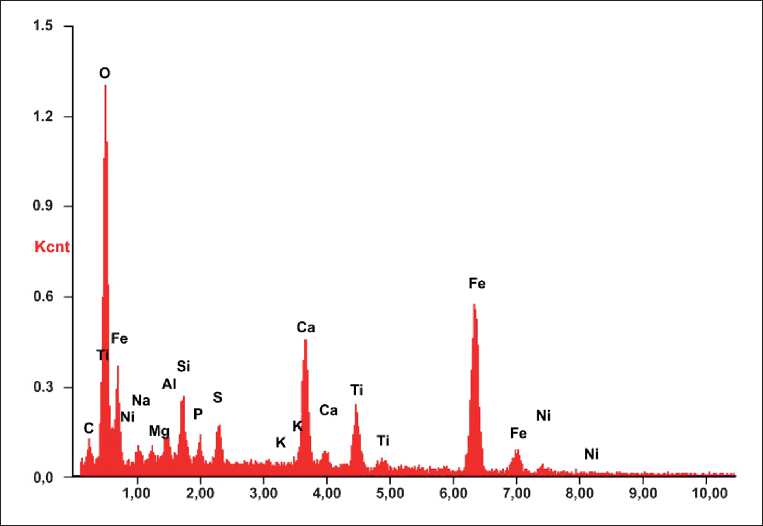
Fig. 5. EDS spectrum of rice husk ash samples
RESEARCH RESULTS OF SCIENTISTS AND SPECIALISTS

Fig. 6. Electronic photograph of the rice husk ash
-
1. 2K2O + COCl2 + 2HCl = 4KCl + CO2 + H2O;
-
2. 2Na2O + COCl2 + 2HCl = 4NaCl + CO2 + H2O;
-
3. 2MgO + COCl2 + 2HCl = 2MgCl2 + CO2 + H2O;
-
4. 2CaO + COCl2 + 2HCl = 2CaCl2 + CO2 + H2O;
-
5. Fe2O3 + 2COCl2 + 2HCl = 2FeCl2 + 2CO2 + H2O;
-
6. Al2O3 + 2COCl2 + 2HCl = 2AlCl3 + CO2 + H2O;
-
7. SiO2 + COCl2 + 2HCl = SiCl4 + CO2 + H2O.
These reactions demonstrate the process of chlorination of metal oxide forms in the presence of COCl2 and HCl. In this case, volatile chlorides (MgCl2, FeCl2, AlCl3) are formed, which condense in the cold part of the reactor. Non-volatile chlorides of calcium (CaCl2), sodium (NaCl) and potassium (KCl) have a higher melting point. This means they form a melting mixture at 490оC and the remainder of the process can be processed and valuable products recovered.
The residue after chlorination, containing illiquid chlorides and other reaction products, is leached with water. During leaching, calcium chlorides and alkali metal chlorides go into solution. Liquid silicon tetrachloride (SiCl4) and its vapor are cooled in a refrigerator and collected in a receiver.
Thorough purification of silicon by the diffusiontransport method was carried out by heating it in SiCl4 vapor and the minimum operating temperature was 850– 900оC. At higher temperatures, a significant portion of the processed silicon could interact with its tetrachloride and be transferred as a result of the reaction to the cold walls of the tube.
During such purification, there is a significant reduction in the aluminum content in aluminothermic silicon, as well as zinc in silicon obtained by reduction with zinc:
2Zn + SiCl4 ↔ 2ZnCl2 + Si.
The most effective is the gas method using SiCl4, the vapors of which are carried by hydrogen or argon. In this case, the carrier gas (Ar, H2) must be slowly pumped through the SiCl4 to avoid SiCl4 vapors slipping through the silicon being purified.
However, this method has a drawback: during the disproportionation reaction of SiCl2, SiCl4 is again formed according to the following reaction:
2SiCl2 ↔ Si + SiCl4.
This may reduce the effectiveness of the method, since SiCl4 will be returned to the purification process. The amount of non-chlorinated part of the original sample is determined. This residue was analyzed for the following elements: calcium, sodium, potassium, iron, aluminum, magnesium and silicon. The composition of the residue was then analyzed and the overall recovery of these components as a result of the chlorination process was calculated. The results of the experiments are shown in Table 1–3.
Table 1
Effect of temperature on the degree of chlorination of metal oxides and silicon
|
Elements compounds |
Processing temperature, оС |
Sublimation, % |
Residues, % |
|
Fe 2 O 3 |
350 |
99.9 |
0.1 |
|
SiO2 |
360 |
99.8 |
0.2 |
|
Al 2 O 3 |
380 |
97.9 |
2.1 |
|
Na2O |
450 |
– |
100 |
|
K 2 O |
450 |
0.1 |
99.9 |
|
CaO |
450 |
- |
100 |
|
MgO |
520 |
82.5 |
17.5 |
Table 2
Effect of time on the degree of chlorination of metal oxides and silicon
|
Elements compounds |
Chlorination time, min |
Sublimation, % |
Residues, % |
|
Fe 2 O 3 |
120 |
0.1 |
99.9 |
|
SiO2 |
– |
100 |
|
|
Al 2 O 3 |
0.2 |
99.8 |
|
|
Na2O |
– |
100 |
|
|
K 2 O |
– |
100 |
|
|
CaO |
– |
100 |
|
|
MgO |
0.5 |
99.5 |
RESEARCH RESULTS OF SCIENTISTS AND SPECIALISTS
Table 3
The influence of chlorine supply rate on the degree of chlorination of silicon oxide and the production of silicon (IV) chloride
|
Experiment No. |
Chlorine consumption g/hour |
Sublimation, % |
Residue, % |
||
|
1 |
12 |
SiCl4 |
96.4 |
SiO2 |
3.6 |
|
2 |
24 |
SiCl4 |
99.8 |
SiO2 |
0.2 |
|
3 |
36 |
SiCl4 |
100 |
SiO2 |
– |
In the course of experimental studies, we found that during the recovery of rice husk ash, it is divided into silicon tetrachloride and metal chlorides (Al, Fe, Ca, Mg, K, Na). It has also been found that the reduction reaction is highly dependent on several parameters such as coal content, temperature, time and chlorine feed rate, and these factors have a significant impact on the recovery rate of the feedstock [13, 17].
The resulting highly volatile silicon chlorides SiCl4 in the liquid state were condensed in a receiver with a temperature of 60оC. Volatile metal chlorides (Al, Fe, Mg) sublimed and condensed in the wide part of the condenser, and non-volatile calcium, sodium, and potassium chlorides formed a melting mixture at a temperature of 450оC in the reactor. At the end of the reaction, non-volatile chlorides were removed from the reaction zone by aqueous treatment and obtained in the form of a melt.
The results of metals (Fe, Al, Mg, Ca, Na, K) and silicon chlorination in a chlorine gas mixture of carbon tetrachloride and air depending on temperature and time are shown in Figures 7 and 8 [13, 17].
DISCUSSION
Iron chlorination begins with the formation of volatile chlorides at a temperature of 350оC. At these temperature, aluminum and magnesium do not sublimate. Aluminum is chlorinated and sublimated at a temperature of 380оC, magnesium at a temperature of 520оC, and silicon at a temperature of 360оC. The degree of chlorination of iron, aluminum, magnesium and silicon increases greatly with increasing temperature, at a temperature of 550оC and amounts to 99.9%, 97.9%, 82.5% and 99.9%, respectively. To reduce silicon (IV) chloride at a temperature of 950–1000оC, it is necessary to carry out the following reactions:
-
1. 2Zn + SiCl4 → 2ZnCl2 + Si;
-
2. 2Mg + SiCl4 → 2MgCl2 + Si.
In these reactions, metals are removed in the form of volatile chloride and deposited on the cold walls of the reactor, while amorphous silicon remains in the elevated temperature zone of the reactor. The remain-
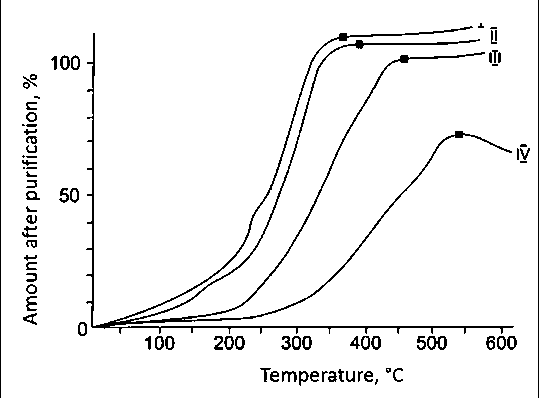
Fig. 7. Chlorination of metals and silicon depending on the temperature
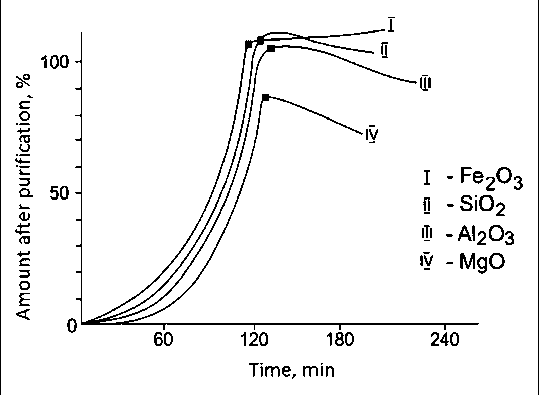
Fig. 8. Chlorination of metals and silicon depending on time
RESEARCH RESULTS OF SCIENTISTS AND SPECIALISTS ing silicon after reduction is leached with hot distilled water, and metal chlorides pass into the solution during leaching.
Amorphous silicon is placed in the first compartment of the installation. We slowly pass the carrier gas (H2) through liquid SiCl4 so that there is no leakage of SiCl4 vapor through the silicon being purified. We heat the electric furnace to up 1050–1100оC.
SiHCl3 ↔ SiCl2 + HCl;
SiCL2 + H2 ↔ SiH2Cl2;
SiH2Cl2 ↔ Si + 2HCl;
2SiCl2 ↔ Si + SiCl4;
SiCl4 + H2 ↔ SiHCl3 + HCl.
The overall equation for the main reactions is:
SiCl4 + H2 = SiHCl3 + HCl;
SiHCl3 + H2 = Si + 3HCl.
Exothermic reactions are characterized by the movement of a substance flow from a zone with a higher temperature (T2) to a zone with a lower temperature (T1), where T2 > T1. In the case of endothermic reactions, the reverse movement is observed: the flow of substance goes from a zone with a lower temperature (T1) to a zone with a higher temperature (T2), where T2 > T1.
When carrying out the reactions, by-products of trichlorosilane, polysilane, and chlorides are formed. Let’s consider methods for purifying silicon chlorides with silica gel and a 30% solution of potassium hydroxide.
In addition to the gas flow method, the silicon purification process is also performed using the diffusiontransport method, which involves heating silicon in SiCl4 vapor. In this method, the cleaning temperature is maintained in the range of 830–900оC.
Given the higher temperature, a significant proportion of silicon interacts with silicon tetrachloride and is transferred through a transport reaction to the cold part of the wall of the tubular reactor.
To purify chlorine-containing gases, calcium hydroxide with a CaO content of 130 g/dm3 was used as an adsorbent in one series of experiments; in another series, lime water was used (saturated solution of Ca(OH)2 of 1.4 g/dm3). In each experiment of one series, the initial chemical capacity of the absorption liquid remained constant. The initial CaCl₂ content in the absorption liquid varied from 0 to 335 g/dm³. This range of CaCl2 concentration changes corresponds to the values that have been previously established by numerous studies for the change in CaCl2 concentration in circulating lime milk in a laboratory gas treatment.
The formation of CaCl2 occurs through chemisorption of gases HCl and Cl2:
Cl2 + H2O = HCl + HClO; 2HCl + Ca(OH)2 = CaCl2 + 2H2O.
The experimental results are presented in Figure 9 in the form of the dependence of the concentration of calcium hydroxide (curves 1, 2) and lime water (curve 3) on the initial content of CaCl2 in them, which characterizes their sorption capacity for chlorine. Curve 1 corresponds to the appearance of traces of Cl2 breakthrough, curve 2 to the complete absorption of bubbled Cl2.
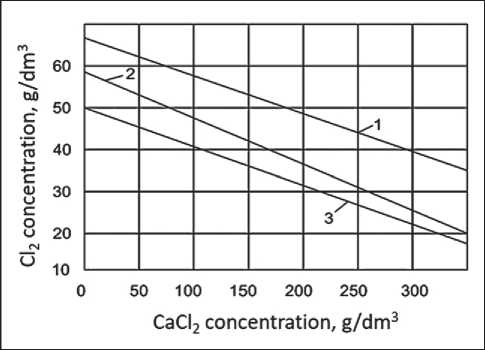
Fig. 9. Dependence of the sorption capacity of calcium hydroxide on the initial content of calcium chloride in them: 1 – traces of chlorine leakage through the volume of calcium hydroxide; 2 – complete (100%) capture of chlorine by calcium hydroxide; 3 – traces of chlorine breakthrough through the volume of calcium hydroxide
Above curve 1 there is an area in which gas purification to sanitary standards is not ensured, below curve 2 there is an area of complete purification (100%) of the chlorine gas mixture. Based on the results of a series of experiments with calcium hydroxide, curve 3, similar to curve 1, was obtained.
Analysis of these data allows us to conclude that with an increase in the initial content of CaCl₂, the amount of Cl₂ that can absorb calcium hydroxide significantly decreases, while maintaining the ability to purify the chlorine-gas mixture to sanitary standards (curve 2). For example, the amount of Cl2 decreases from 57 g/dm³ (in the absence of CaCl2) to 22 g/dm³ (with 335 g/dm³ CaCl2). There is also a noticeable decrease in the Cl2 content to traces of breakthrough (curve 1) at the outlet of the reactor – from 65 g/dm³ (in the absence of CaCl2) to 28 g/dm³ (at 335 g/dm³ CaCl2). For lime water, the corresponding values are 2.8 g/dm³ and 0.8 g/dm³, respectively.
The general technological scheme for obtaining crystalline silicon from rice husk is shown in Figure 10.
The chemical composition of rice husks before and after chemical treatments shown in Table 4.
RESEARCH RESULTS OF SCIENTISTS AND SPECIALISTS
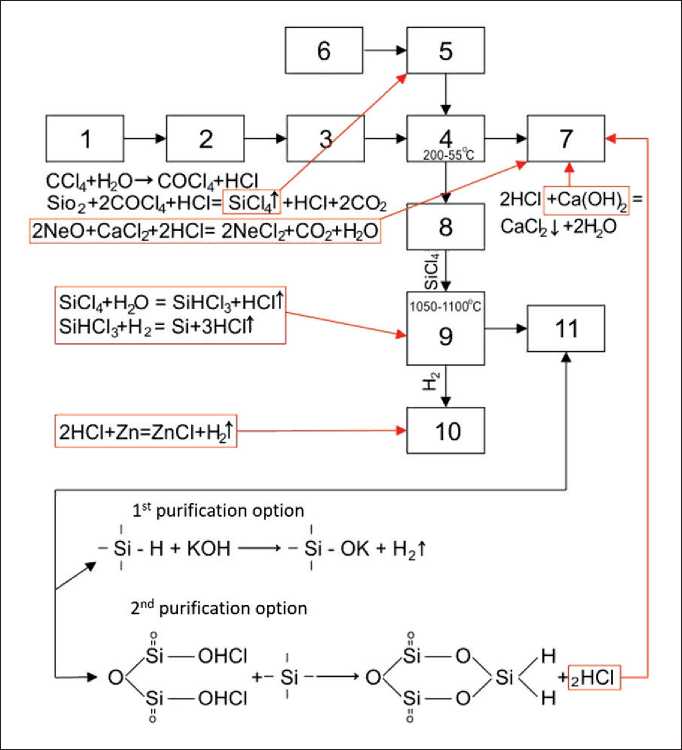
Fig. 10. General technological scheme for obtaining crystalline silicon from rice husks: 1 – mechanical cleaning and washing of rice husks; 2 – drying rice husk; 3 – roasting rice husks; 4 – tubular furnace for chlorination of rice husk ash; 5 – container for carbon tetrachloride; 6 – mini compressor for blowing carbon tetrachloride (SiCl4); 7 – adsorption column for purification of chlorine-containing gases with Ca(OH)2 solution (plumbing unit);
9 – electric furnace for deep purification of silicon and production of crystalline silicon; 10 – instrumentation apparatus for hydrogen production; 11 – column for purification of by-products (silica gel, KOH, HCl)
Table 4
Chemical composition of rice husk
|
Metall compounds |
SiO2 |
K2O |
Na2O |
CaO |
MgO |
Fe2O3 |
Al2O3 |
|
Rice husk before treatment |
30 |
0.18 |
0.12 |
0.32 |
0.2 |
0.5 |
0.5 |
|
Metals |
Si |
K |
Na |
Ca |
Mg |
Fe |
Al |
|
Rice husk after treatment |
99.65 |
0.02 |
0.04 |
0.12 |
0.11 |
0.04 |
0.02 |
CONCLUSION
-
1. The composition and structure of rice husk ash was studied to obtain polycrystalline silicon. It has been established that the supply rate of the chlorine gas mixture (phosgene and hydrogen chloride) for the chlorination of metal oxides is 2.5 cm2/min at various temperature ranges (200, 300, 400, 550oC).
-
2. It was discovered that during this process toxic chlorine-containing gases with a characteristic fishy odor are released in the air of the working area and atmospheric air, exceeding the maximum permissible concentration (MPC). If the salt content in lime milk is more than 355 g/dm3, it is necessary to replace the solution for purifying technical gases. Therefore, lime milk with a CaO content of 130 g/dm3 is an effective
-
3. A technological scheme for producing polycrystalline silicon from rice husks using the chlorination method has been developed.
RESEARCH RESULTS OF SCIENTISTS AND SPECIALISTS and economical means for purifying toxic chlorinecontaining gases in the working area and atmospheric air.
Список литературы Production of polycrystalline silicon by chlorination from rice husk and purification of chlorine-containing gases by adsorption method
- Noshelsky A.Ya. Production of semiconductor materials. Metallurgy; 1989. 210 p.
- Ermolaev A.A. Silicon in agriculture. M: 1992; 253 p.
- Vlasov A.S., Zakharov A.I., Sarkisyan O.A., Lukosheva N.A. Preparation of silicon carbide from rice husk processing products. Fireproof materials. 1991; 10 (15-17).
- Sharikov V.I., Spotnitsky S.A. and others. Technology of hydrolysis production. M: Timber industry. 1973; P. 403.
- Ismanov E.M., Tashpolotov I.T., Omurbekova G.K., Aidaraliev Zh.K., Sadykov E.S. Technology for producing silicon using organic and inorganic raw materials. Science and new technologies. Bishkek: 2001; 1: 22-24.
- Ugai Y.A. Introduction to semiconductor chemistry. M: Higher School; 1965. 333 p.
- Gorichev I.G., Zaitsev B.E., Kipryanov N.A., Gromov D.N. Guide to inorganic synthesis. M.; “Chemistry”. 1997. 319 p.
- Morozov I.S. The use of chlorine in metallurgy or rare non-ferrous metals. M: The science; 1966. P. 252.
- Handbook ed. L.A. Oshina. Industrial organochlorine products. M: The science; 1976. 646 p.
- Senotova S. A. Mathematical model of the process of producing polycrystalline silicon. Bulletin of ISTU. 2013; 10 (81): 239-241.
- Bekbolot kyzy B., Murzubraimov B.M. Problems of utilization of rice waste and prospects for their use. News of the National Academy of Sciences of the Kyrgyz Republic. 2010; 3: 128-131.
- Bekbolot kyzy B. Preparation of silicon dioxide nanoparticles from rice husks. Bekbolot kyzy B. Bulletin of KGUSTA. 2014; 1(43):142-145.
- Ismanov E.M., Omurbekova G.K., Baydolotov R.R., Tashpolotov I.T. Study of optimal conditions for chlorination of silicon in rice husks. Science and new technologies. 2003; 3:39-40.
- Popov V.M. Organic chemistry. M: Education; 1976. P. 163.
- Syman A.D., Yablonsky A.P., Kashko I.A., Girel K.V., Bondarenko A.V. Structural properties of porous silicon particles formed by magnesium-thermal reduction of silicon dioxide made from silicon-containing plants. Reports of BSUIR. 2016; 1 (95): 19-25.
- Usubakunov M.U., Chukulova U.E., Bleshinsky S.V. Complex processing of antimony and pyrite concentrates containing noble metals by chlorination with carbon tetrachloride. Science and new technologies. 2000; 2:102-104.
- Ismanov E.M., Omurbekova G.K., Baydolotov R.R., Tashpolotov I.T. Kinetics of low-temperature chlorination of metal oxides and silicon in rice husks. Science and new technologies. 2013; 3: 20-22.

