PsbS Dependence in Lipid and Pigment Composition in Rice Plants
Автор: Pashayeva A.
Журнал: Бюллетень науки и практики @bulletennauki
Рубрика: Биологические науки
Статья в выпуске: 9 т.7, 2021 года.
Бесплатный доступ
Plants acclimate to fluctuations in light conditions by adjusting their photosynthetic apparatus. When the light intensity exceeds, an unbalanced excitation of the two photosystems occurs. It results in reduced photosynthetic efficiency. Photosystem II (PSII) is the most susceptible and dynamically regulated part of the light reactions in the thylakoid membrane. Non-photochemical quenching of chlorophyll fluorescence (NPQ) is one of the short-term photoprotective mechanisms, which consist of the number of components. The strongest NPQ component — qE is localized in the PSII antenna and induced in plants by lumen acidification, the activation of the pH sensor PsbS, and the conversion of the violaxanthin to zeaxanthin within the xanthophyll cycle. Here, I present data that characterizes the role of the PsbS protein in organization of PSII structural components in isolated PSII-enriched membranes. The preparations were isolated from wild-type (WT) and PsbS-less (PsbS-KO) mutant rice plant. Based on the obtained results, the PSII-enriched membranes from WT and PsbS-KO differ as in the level of lipids, also in carotenoids. I conclude that the PsbS-dependent changes in membrane fluidity in PsbS-KO mutant plants compensated with increased lipid level in mutant plants.
Carotenoids, lipids, non-photochemical quenching, photosystem II, PsbS, rice, thylakoid membrane
Короткий адрес: https://sciup.org/14119595
IDR: 14119595 | УДК: 598.2. 591.5. 632. 575.12 | DOI: 10.33619/2414-2948/70/05
Текст научной статьи PsbS Dependence in Lipid and Pigment Composition in Rice Plants
Бюллетень науки и практики / Bulletin of Science and Practice
Under natural conditions, land plants constantly undergo different biotic and abiotic stresses. One of the rapidly changing abiotic factors is light intensity. When the light intensity is exceeded, it causes photooxidative damage that destroys the photosynthetic apparatus of plants. When the excited chlorophyll molecules cannot transfer excitation energy to the pigments of the reaction center of photosystem (PS) II and convert it into oxygen, photooxidative damage occurs with excessive formation of reactive oxygen species (ROS) [1, 2]. Effect of the different light intensities on plants has investigated at the level of thylakoid membranes [3], genes [4, 5], proteins [6]. However, less known about the composition of plant metabolites.
Lipids are the main structural component, which is necessary for the functioning of the photosynthetic apparatus, also for different biological processes such as photoprotection, light harvesting, etc. They contribute significantly to plant abiotic stresses response mainly by maintaining the structure of chloroplasts and modifying membrane fluidity through regulating the degree of fatty acids desaturation. Lipid remodeling of photosynthetic membranes has become a vital function in the adaptation of plants to various adverse environmental conditions [7]. The thylakoid membrane of vascular plants mainly contains galactolipids, the most abundant among them are monogalactosyldiacylglycerol (MGDG, ~50% of total lipids), digalactosyldiacylglycerol (DGDG, ~25-30%), sulfoquinovosyl-diacylglycerol (SQDG, ~10-15%), and phosphatidylglycerol (PG, ~10-15%). MGDG, which exists in the membrane in a conical shape, predominantly forms non-bilayer phase, promoting membrane stacking [8], whereas DGDG, SQDG, and PG, cylindrically-shaped lipids, are proficient to assemble into bilayer (lamellar) structures [9]. Galactolipids play a specific role in thylakoid architectural reorganizations in response to high light (HL) stress and they contributing to thylakoid stacking [10]. The lipid composition of the thylakoid membrane influenced by the effect of various stress conditions such as temperature, water or salt stress, nitrogen deficiency, aluminum stress [11, 12]. The observed changes includes in chain length and the number of double bonds in fatty acids, as well as in the ratio between the two major lipids, MGDG and DGDG [13]. The lipid composition of the membrane is essential for the functioning of proteins [14], and it affects the light reactions of the photosynthesis. Thus, the functionality of plastoquinone (PQ) diffusion as an electron transporter can be modified by the thylakoid membrane lipid composition [15].
Carotenoids are also an essential component of all photosynthetic organisms, providing photoprotective and antioxidant properties. While the majority of carotenoids bound to specific sites of photosynthetic complexes, approximately 15% of the total carotenoid pool is freely dissipated in the lipid phase in Arabidopsis ( Arabidopsis thaliana ) chloroplasts [16]. Both carotenoids and lipids play a crucial role in PSI and PSII reaction centers. They are necessary for the non-photochemical quenching (NPQ), light-harvesting mechanisms, and protection against reactive oxygen species (ROS).
It is less known about how plants’ metabolite composition responds to fluctuations in environmental conditions. Studies on mutant plants of Arabidopsis that lack PsbS ( npq4-1 ) have shown reduced fitness under natural conditions in the field [17]. In contrast, plants overexpressing
PsbS (oePsbS) demonstrated an increase in triggering of the major, rapidly induced component of NPQ energy quenching (qE) [18, 19]. Because biosynthesis of chloroplast lipids is fine-tuned to thylakoid membrane remodeling during light acclimation [20], I investigated the metabolomics shift associated with lack of PsbS protein using PsbS knockout (PsbS-KO) mutant and wild type (WT) rice plants. While PsbS during NPQ induction promotes a reorganization of the photosynthetic membranes [21, 22], my main objectives were to observe whether the changes in pigment and lipid and composition of PSII enriched membranes depends on PsbS protein of PSII. Also, I investigated if the PsbS-dependent fluctuations in membrane fluidity influence the organization and abundance of the carotenoids and lipids.
Results and Discussion
To analyze differences in the composition of lipids, I have used PSII-enriched membranes (BBY particles), extracted from rice WT and PsbS-KO mutant plant leaves. Total lipids were isolated from BBY particles and further analyzed by gas chromatography. Compared with WT, the amount of main lipids MGDG, DGDG, SQDG and PG increased significantly for the PsbS-KO mutants. While the total amount of lipids for WT was determined as a 252.9 nmol/g, but for PsbS-KO mutants, this number increased to a 354.8 nmol/g. In comparison with WT with an MGDG/DGDG ratio value of 1.6, this ratio increased to 1.8 in PsbS-KO mutant lines. The major increase I observed in the composition of MGDG with the amount of increased by 49% to a 195 nmol/g, whereas the minor increase by 27% to a 23 nmol/g was identified in the amount of PG. Less rise in compare with MGDG demonstrated in the amount of DGDG and SQDG by ~27% and 44%, respectively (Table).
Table
|
Plant |
Lipid classes |
Concentration (nmol/g. fr.wt) |
% of total |
|
MGDG |
131.4±11.3 |
52 |
|
|
DGDG |
83.5±7.2 |
33 |
|
|
WT |
PG |
22.0±1.3 |
8.7 |
|
SQDG |
16.2±1.2 |
6.3 |
|
|
TOTAL |
252.9±23.7 |
100.0 |
|
|
MGDG |
195.4±17.4 |
53.7 |
|
|
DGDG |
109.1±9.8 |
32.2 |
|
|
Psbs-KO |
PG |
27.8±2.5 |
7.7 |
|
SQDG |
22.6±1.9 |
6.4 |
|
|
TOTAL |
354.8±32.3 |
100.0 |
As a next step, to investigate the changes in pigment compositions of PSII-enriched membranes (BBY) of WT and PsbS-KO mutants, we performed the pigment analysis of isolated BBY particles (Figure). The graph below demonstrates that the complex contained neoxanthin (Neo), violaxanthin (Vio), antheraxanthin (Anth), lutein (Lut), chlorophyll (Chl) b, Chl a and β-carotene. No zeaxanthin was detected in both WT and PsbS-KO samples, confirming that the complex was isolated from dark-adapted plant leaves. The pigment analysis of PSII enriched membranes showed that while the amount of Neo, Vio, Lut were higher in PsbS-KO mutant plants, but anth, Chl b and ß-car showed higher results in WT samples. BBY preparations of PsbS-KO mutants associated with the slightly higher number of Neo in comparison with Wt. It has been reported by [23] that Neo and Vio are coordinately bound to LHCII, which is associated with the xanthophyll cycle and the induction of the NPQ [24]. The excited-state quenching in LHCII correlates with Neo distortion [25]. Therefore, I suggest that the higher amount of the Neo and Vio in PsbS-KO mutant may be due to compensation of the absence of PsbS protein. The role of Lut in the charge-separation mechanism for excitation quenching in LHCII also was demonstrated [26]. I found no difference in amount of Chl a between WT and PsbS-KO mutant BBY preparations.
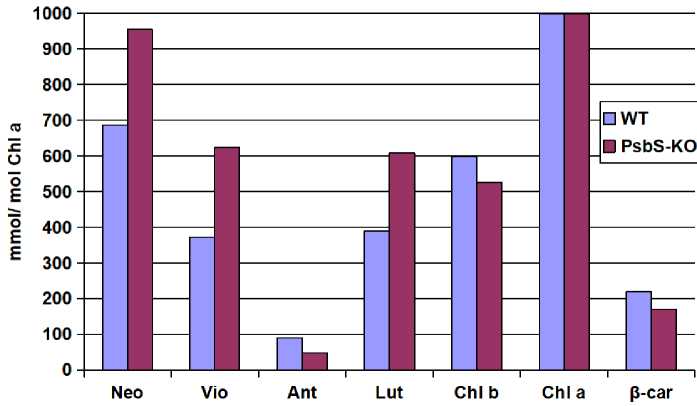
Figure. Composition of photosynthetic pigments in PSII-enriched membranes (BBY) of WT and PsbS-KO mutant lines
I found significant differences in lipid and pigment composition between the BBY preparations of WT and PsbS-KO rice leaves. It was earlier reported that under the influence of various stress factors, membrane restructuring is possible, where long-chain lipids are replaced by short-chain ones, and their saturation also increases [27]. Plants can rapidly modify previously accumulated lipids to compensate for the needs of the cell (for example, for fatty acids) under stressful conditions (changes in illumination, temperature, composition of the medium). The plants lacking PsbS induce a metabolic and transcriptomic shift that activates protective response mechanisms to increase resistance against biotic stress [12]. The synthesis of lipids is also apparently associated with the response cells to oxidative stress. So as the PsbS-KO mutant plants produce more ROS, I suggest that it results in enhanced synthesis of lipids and carotenoids. Also, the modulation of the membrane fluidity is directly associated with the composition of lipid-phase located carotenoids [16]. Following the previously published results [28, 29], I consider that the PsbS-dependent decrease in membrane fluidity in the bulk in thylakoid membranes of PsbS-KO mutant plants appears due to the lack of the PsbS protein and it compensated with the increased amount of lipids. Neoxanthin causes a largescale conformational change in the LHCII proteins [30], such unquenched LHCII intermediate with higher neoxanthin amount helps to understand the molecular mechanism of quenching. In this context, data obtained by me may indicate that LHCII in PsbS-KO plants might be under a quenching state.
Material and Method
Plants and growth conditions
One-month-old seedlings of wild-type (WT) and PsbS-knockout mutant (PsbS-KO) rice ( Oryza sativa L .) plants were grown in soil in a greenhouse under 30/26 °C (day/night), with a light photoperiod of 16 h of light/8 h of dark.
Isolation of PSII enriched complexes (BBY)
PSII complexes (BBY particles) isolated from WT and PsbS-KO mutant rice leaves as described by [31, 32] and then were stored at -80°C in a re-suspension medium containing 400 mM sucrose, 15 mM NaCl, 5 mM MgCl2, and 40 mM Mes (pH 6.5).
Lipid analysis
Total lipids extracted from PSII enriched membranes of two-week-old WT and PsbS-KO mutant plant leaves. Individual lipids were purified by one-dimensional thin layer chromatography as described by [12].
Pigment analysis
Pigments were extracted from PSII-enriched membranes (BBY) preparations of wild type (WT) and PsbS-KO mutant plants by gently agitating in ice-cold 100% acetone for 1 h. Samples centrifuged and the extracts filtered through a 0.2-μm syringe filter. The pigment composition of thylakoid membranes was determined by reversed-phase HPLC (HP 1100 series, Hewlett-Packard, Waldbronn, Germany) as described by [33].
Список литературы PsbS Dependence in Lipid and Pigment Composition in Rice Plants
- Aliyeva, D. R., Aydinli, L. M., Pashayeva, A. N., Zulfugarov, I. S., & Huseynova, I. M. (2020). Photosynthetic machinery and antioxidant status of wheat genotypes under drought stress followed by rewatering. Photosynthetica, 58(5), 1217 1225. https://doi.org/10.32615/ps.2020.074
- Zulfugarov, I. S., Tovuu, A., Kim, J. H., & Lee, C. H. (2011). Detection of Reactive Oxygen Species in Higher Plants. Journal of Plant Biology, 54(6), 351 357. https://doi.org/10.1007/s12374 011 9177 4
- Eberhard, S., Finazzi, G. & Wollman, F.A. (2008) The dynamics of photosynthesis. Annual Review of Genetics 42, 463 515. https://doi.org/10.1146/annurev.genet.42.110807.091452
- Kimura, M., Yamamoto, Y. Y., Seki, M., Sakurai, T., Sato, M., Abe, T., Yoshida, S., Manabe, K., Shinozaki, K. & Matsui, M. (2003) Iden tification of Arabidopsis genes regulated by high light stress using cDNA microarray. Photochemistry and Photobiology 77, 226 233. https://doi.org/10.1562/0031 8655(2003)0770226IOAGRB2.0.CO2
- Rutitzky, M., Ghiglione, H. O., Curá, J. A., Casal, J. J., & Yanovsky, M. J. (2009). Comparative genomic analysis of light regulated transcripts in the Solanaceae. BMC genomics, 10(1), 1 14. https://doi.org/10.1186/1471 2164 10 60
- Pashayeva, A., Wu, G., Huseynova, I., Lee, C. H., & Zulfugarov, I. S. (2021). Role of Thylakoid Protein Phosphorylation in Energy Dependent Quenching of Chlorophyll Fluorescence in Rice Plants. International journal of molecular sciences, 22(15), 7978. https://doi.org/10.3390/ijms22157978
- Liu, X., Ma, D., Zhang, Z., Wang, S., Du, S., Deng, X., & Yin, L. (2019). Plant lipid remodeling in response to abiotic stresses. Environmental and Experimental Botany, 165(June), 174 184. https://doi.org/10.1016/j.envexpbot.2019.06.005
- Seiwert, D., Witt, H., Ritz, S., Janshoff, A., & Paulsen, H. (2018). The Nonbilayer Lipid MGDG and the Major Light Harvesting Complex (LHCII) Promote Membrane Stacking in Supported Lipid Bilayers. Biochemistry, 57(15), 2278 2288. https://doi.org/10.1021/acs.biochem.8b00118
- Dlouhý, O., Kurasová, I., Karlický, V., Javornik, U., Šket, P., Petrova, N. Z., Krumova, S. B., Plavec, J., Ughy, B., Špunda, V., & Garab, G. (2020). Modulation of non bilayer lipid phases and the structure and functions of thylakoid membranes: effects on the water soluble enzyme violaxanthin de epoxidase. Scientific Reports, 10(1), 1 14. https://doi.org/10.1038/s41598 02068854 x
- Yu, L., Fan, J., Zhou, C., & Xu, C. (2021). Chloroplast lipid biosynthesis is fine tuned to thylakoid membrane remodeling during light acclimation. Plant Physiology, 185(1), 94 107. https://doi.org/10.1093/plphys/kiaa013
- Nami, F., Tian, L., Huber, M., Croce, R., & Pandit, A. (2021). Lipid and protein dynamics of stacked and cation depletion induced unstacked thylakoid membranes. BBA Advances, June, 100015. https://doi.org/10.1016/j.bbadva.2021.100015
- Tovuu, A., Zulfugarov, I. S., Wu, G., Kang, I. S., Kim, C., Moon, B. Y., An, G., & Lee, C. H. (2016). Rice mutants deficient in ω 3 fatty acid desaturase (FAD8) fail to acclimate to cold temperatures. Plant Physiology and Biochemistry, 109, 525 535. https://doi.org/10.1016/j.plaphy.2016.11.001
- Yang, C., Boggasch, S., Haase, W., & Paulsen, H. (2006). Thermal stability of trimeric light harvesting chlorophyll a/b complex (LHCIIb) in liposomes of thylakoid lipids. Biochimica et Biophysica Acta Bioenergetics, 1757(12), 1642 1648. https://doi.org/10.1016/j.bbabio.2006.08.010
- Van Eerden, F. J., Melo, M. N., Frederix, P. W. J. M., & Marrink, S. J. (2017). Prediction of Thylakoid Lipid Binding Sites on Photosystem II. Biophysical Journal, 113(12), 2669 2681. https://doi.org/10.1016/j.bpj.2017.09.039
- Sattari Vayghan, H., Tavalaei, S., Grillon, A., Meyer, L., Ballabani, G., Glauser, G., & Longoni, P. (2020). Growth Temperature Influence on Lipids and Photosynthesis in Lepidium sativum. Frontiers in Plant Science, 11. https://doi.org/10.3389/fpls.2020.00745
- Bykowski, M., Mazur, R., Wójtowicz, J., Suski, S., Garstka, M., Mostowska, A., & Kowalewska, Ł. (2021). Too rigid to fold: Carotenoid dependent decrease in thylakoid fluidity hampers the formation of chloroplast grana. Plant Physiology, 185(1), 210 227. https://doi.org/10.1093/plphys/kiaa009
- Külheim, C., Ågren, J., & Jansson, S. (2002). Rapid regulation of light harvesting and plant fitness in the field. Science, 297(5578), 91 93. https://doi.org/10.1126/science.1072359
- Li, X. P., Müller Moulé, P., Gilmore, A. M., & Niyogi, K. K. (2002). PsbS dependent enhancement of feedback de excitation protects photosystem II from photoinhibition. Proceedings of the National Academy of Sciences, 99(23), 15222 15227. https://doi.org/10.1073/pnas.232447699
- Johansson Jänkänpää, H., Frenkel, M., Zulfugarov, I., Reichelt, M., Krieger Liszkay, A., Mishra, Y., Gershenzon, J., Moen, J., Lee, C. H., & Jansson, S. (2013). Non Photochemical Quenching Capacity in Arabidopsis thaliana Affects Herbivore Behaviour. PLoS ONE, 8(1). https://doi.org/10.1371/journal.pone.0053232
- Unal, D., García Caparrós, P., Kumar, V., & Dietz, K. J. (2020). Chloroplast associated molecular patterns as concept for fine tuned operational retrograde signalling. Philosophical Transactions of the Royal Society B, 375(1801), 20190443. https://doi.org/10.1098/rstb.2019.0443
- Zulfugarov, I.S., Tovuu, A., Dogsom, B., Lee, C.Y. and Lee, C.H. (2010). PsbS specific zeaxanthin independent changes in fluorescence emission spectrum as a signature of energy dependent non photochemical quenching in higher plants. Photochemical & Photobiological Sciences, 9(5), pp.697 703. https://doi.org/10.1039/B9PP00132H
- Tibiletti, T., Auroy, P., Peltier, G., & Caffarri, S. (2016). Chlamydomonas reinhardtii PsbS protein is functional and accumulates rapidly and transiently under high light. Plant Physiology, 171(4), 2717 2730. https://doi.org/10.1104/pp.16.00572
- Tu, W., Wu, L., Zhang, C., Sun, R., Wang, L., Yang, W., Yang, C., & Liu, C. (2020). Neoxanthin affects the stability of the C2S2M2 type photosystem II supercomplexes and the kinetics of state transition in Arabidopsis. Plant Journal, 104(6), 1724 1735. https://doi.org/10.1111/tpj.15033
- Wang, K., Tu, W., Liu, C., Rao, Y., Gao, Z., & Yang, C. (2017). 9 cis Neoxanthin in light harvesting complexes of photosystem II regulates the binding of violaxanthin and xanthophyll cycle. Plant physiology, 174(1), 86 96. https://doi.org/10.1104/pp.17.00029
- Haferkamp, S., Haase, W., Pascal, A. A., Van Amerongen, H., & Kirchhoff, H. (2010). Efficient light harvesting by photosystem II requires an optimized protein packing density in grana thylakoids. Journal of Biological Chemistry, 285(22), 17020 17028. https://doi.org/10.1074/jbc.M109.077750
- Cupellini, L., Calvani, D., Jacquemin, D., & Mennucci, B. (2020). Charge transfer from the carotenoid can quench chlorophyll excitation in antenna complexes of plants. Nature Communications, 11(1). https://doi.org/10.1038/s41467 020 14488 6
- Rodin, R. V. (2021). Obrazovanie lipidov v kletke mikrovodorosli Chlorella vulgaris. Alleja Nauki, 2(53), 20 24. (in Russian).
- Goral, T. K., Johnson, M. P., Duffy, C. D., Brain, A. P., Ruban, A. V. & Mullineaux, C. W., (2012). Light‐harvesting antenna composition controls the macrostructure and dynamics of thylakoid membranes in Arabidopsis. The Plant Journal, 69(2), 289 301. https://doi.org/10.1111/j.1365 313X.2011.04790.x
- Tovuu, A., Zulfugarov, I. S., & Lee, C. H. (2013). Correlations between the temperature dependence of chlorophyll fluorescence and the fluidity of thylakoid membranes. Physiologia plantarum, 147(4), 409 416. https://doi.org/10.1111/j.1399 3054.2012.01700.x
- Li, F., Liu, C., Streckaite, S., Yang, C., Xu, P., Llansola Portoles, M. J., Ilioaia, C., Pascal, A. A., Croce, R. & Robert, B. (2021). A new, unquenched intermediate of LHCII. Journal of Biological Chemistry, 296. https://doi.org/10.1016/j.jbc.2021.100322
- Ford, R. C., & Evans, M. C. W. (1983). Isolation of a photosystem 2 preparation from higher plants with highly enriched oxygen evolution activity. FEBS letters, 160(1 2), 159 164. https://doi.org/10.1016/0014 5793(83)80957 0
- Zulfugarov, I. S., Tovuu, A., Eu, Y. J., Dogsom, B., Poudyal, R. S., Nath, K., Hall, M., Banerjee, M., Yoon, U. C., Moon, Y. H., An, G., Jansson, S., & Lee, C. H. (2014). Production of superoxide from Photosystem II in a rice (Oryza sativa L.) mutant lacking PsbS. BMC Plant Biology, 14(1), 1 15. https://doi.org/10.1186/s12870 014 0242 2
- Zulfugarov, I. S., Ham, O. K., Mishra, S. R., Kim, J. Y., Nath, K., Koo, H. Y., Kim, H. S., Moon, Y. H., An, G., & Lee, C. H. (2007). Dependence of reaction center type energy dependent quenching on photosystem II antenna size. Biochimica et Biophysica Acta Bioenergetics, 1767(6), 773 780. https://doi.org/10.1016/j.bbabio.2007.02.021


