Pyrimidine
Автор: Farzaliyev V.M., Allahverdiyev M.A., Khalilova A.Z., Aliyeva M.N., Maharramova G.M.
Журнал: Science, Education and Innovations in the Context of Modern Problems @imcra
Статья в выпуске: 3 vol.8, 2025 года.
Бесплатный доступ
Purines and pyrimidines are two of the building blocks of nucleic acids. Only two purines and three pyrimidines occur widely in nucleic acids.
Purines, pyrimidines, nucleic acids, 2, 4, 6-trichloropyrimidine, 2-aminopyrimidines
Короткий адрес: https://sciup.org/16010478
IDR: 16010478 | DOI: 10.56334/sei/8.3.04
Текст научной статьи Pyrimidine

Sci. Educ. Innov. Context Mod. Probl. P-ISSN: 2790-0169 E-ISSN: 2790-0177 Issue 3, Vol. 8, 2025, IMCRA
Pyrimidine is an aromatic heterocyclic organic compound similar to pyridine. [1][2] One of the three diazines (six-membered heterocyclics with two nitrogen atoms in the ring), it has the nitrogens at posi- tions 1 and 3 in the ring.[3] The other diazines are pyrazine (nitrogens 1 and 4) and pyridazine (nitrogens 1 and 2). In nucleic acids, three types of nucleobases are pyrimidine derivatives: cytosine (C), thymine (T), and uracil (U).
As is often the case with parent heterocyclic ring systems, the synthesis of pyrimidine is not that common and is usually performed by removing functional groups from derivatives. Primary syntheses in quantity involving formamide have been reported.[11]
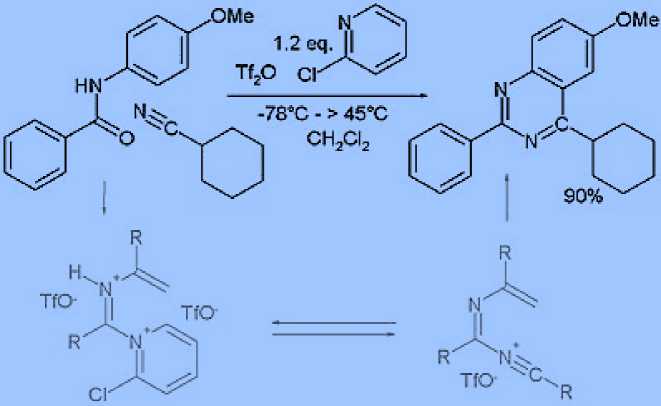
Reaction of the former with amidines to give 2-substituted pyrimidines, with urea to give 2- pyrimidiones, and guanidines to give 2-aminopyrimidines are typical.[12] Pyrimidines can be prepared via the Biginelli reaction. Many other methods rely on condensation of carbonyls with diamines for instance the synthesis of 2-Thio-6-methyluracil from thiourea and ethyl acetoacetate [13] or the synthesis of 4- methylpyrimidine with 4,4-dimethoxy-2-butanone and formamide.[14] A novel method is by reaction of certain amides with carbonitriles under electrophilic activation of the amide with 2-chloro-pyridine and trifluoromethanesulfonic anhydride:[15]

Issue 3, Vol. 8, 2025, IMCRA
Three nucleobases found in nucleic acids, cytosine (C), thymine (T), and uracil(U), are pyrimidine derivatives:
Cytosine (C)
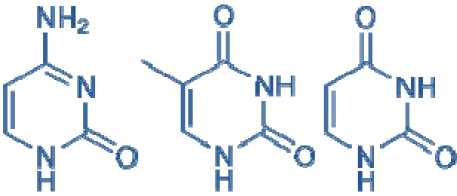
Thymine (T)
Uracil (U)
In DNA and RNA, these bases form hydrogen bonds with their complementarypurines. Thus, in DNA, the purines adenine (A) and guanine (G) pair up with the pyrimidines thymine (T) and cytosine(C), respectively. In RNA, the complement of adenine (A) is uracil (U) instead of thymine (T), so the pairs that form are adenine:uracil and guanine:cytosine. Very rarely, thymine can appear in RNA, or ura- cil in DNA. Other than the three major pyrimidine bases presented, some minor pyrimidine bases can also occur in nucleic acids. These minor pyrimidines are usually methylatedversions of major ones and are postulated to have regulatory functions.[21]
These hydrogen bonding modes are for classical Watson-Crick base pairing. Other hydrogen bond- ing modes ("wobble pairings") are available in both DNA and RNA, although the additional 2'-hydroxyl group of RNA expands the configurations, through which RNA can form hydrogen bonds.
In March 2015, NASA Ames scientists reported that, f or the first time, com-
Sci. Educ. Innov. Context Mod. Probl. P-ISSN: 2790-0169 E-ISSN: 2790-0177 Issue 3, Vol. 8, 2025, IMCRA plex DNA and RNA organic compounds of life, including uracil, cytosine and thymine, have been formed in the laboratory under outer space conditions, using starting chemicals, such as pyrimidine, found in meteorites. Pyrimidine, like polycyclic aromatic hydrocarbons (PAHs), the most carbon-rich chemical found in the Universe, may have been formed in red giants or in interstellar dust and gasclouds, according to the scientists.[22][23][24]


