Разработка вакцин для профилактики коронавирусной инфекции: от SARS и MERS до COVID-19
Автор: Ли Й.-Д., Чи В.-Ю., Су Ц.-Х., Ферралл Л., Хун Ч.-Ф., Ву Ц.-Ч.
Журнал: Juvenis scientia @jscientia
Рубрика: Переводные статьи
Статья в выпуске: 6 т.6, 2020 года.
Бесплатный доступ
Коронавирус тяжелого острого респираторного синдрома (SARS-CoV-2) - это новый вид коронавируса, вызывающий коронавирусную инфекцию 2019 года (COVID-19), которая стала причиной самой серьезной пандемии в текущем столетии. Учитывая высокую летальность и быстрое распространение заболевания, для подавления пандемии необходимо создание эффективной вакцины. С этой целью при тесном сотрудничестве научного сообщества, фармацевтической промышленности и правительственных организаций беспрецедентными темпами осуществляется разработка и тестирование широкого спектра вакцин. В настоящем обзоре выделены наиболее существенные в контексте создания вакцин биологические характеристики коронавирусов, а также кратко изложены ключевые выводы исследований вакцин против коронавируса тяжелого острого респираторного синдрома (SARS-CoV) и коронавируса ближневосточного респираторного синдрома (MERS-CoV) с акцентом на плюсы и минусы каждой стратегии иммунизации. На основе данных о результатах изучения вакцин против этих инфекций обсуждается текущее состояние и потенциальные сложности разработки вакцин для профилактики от COVID-19.
Коронавирусы, sars-cov-2, вакцина, разработка вакцин
Короткий адрес: https://sciup.org/14121386
IDR: 14121386
Текст обзорной статьи Разработка вакцин для профилактики коронавирусной инфекции: от SARS и MERS до COVID-19
Введение. Коронавирусы (CoV) – это группа родственных вирусов, которые могут вызывать у людей респираторные инфекции, протекающие как в легкой форме, так и с летальным исходом. К настоящему времени известно семь видов коронавирусов, поражающих человека [1]. Четыре из них, включая коронавирусы человека 229E (HCoV-229E), OC43 (HCoV-OC43), NL63 (HCoV-NL63) и HKU1 (HCoV-HKU1), вызывают относительно легкие, заканчивающиеся самопроизвольным выздоровлением респираторные инфекции [2]. В то же время три оставшихся коронавируса – коронавирус тяжелого острого респираторного синдрома (SARS-CoV), коронавирус ближневосточного респираторного синдрома (MESR-CoV) и коронавирус тяжелого острого респираторного синдрома 2 (SARS-CoV-2) – являются высокопатогенными и могут привести к тяжелым респираторным заболеваниям и летальному исходу у инфицированных пациентов. Первый коронавирус, вызывающий смертельную инфекцию, SARS-CoV, был обнаружен в 2002 году в провинции Гуандун (Китай).
Во время вспышки 2002-2004 годов вирусом SARS-CoV было заражено 8098 человек, что привело к 774 смертельным исходам (летальность ~ 10%) в 29 странах, прежде чем вспышка заболевания была подавлена [3]. В 2012 году в Саудовской Аравии был обнаружен вирус MERS-CoV. Позднее он вызвал две вспышки заболевания: в Южной Корее в 2015 году и в Саудовской Аравии в 2018 году, а спорадические случаи инфекции регистрируются до сих пор. По состоянию на январь 2020 года в 27 странах мира насчитывается 2519 подтвержденных случаев развития ближневосточного респираторного синдрома и 866 связанных с ним смертельных исходов (летальность ~ 35%) [4]. В декабре 2019 года в г. Ухань (Китай) появился новый тип коронавируса, способный вызывать тяжелое респираторное заболевание. Всемирная организация здравоохранения дала новому вирусу официальное название SARS-CoV-2, а вызываемому им заболеванию – COVID-19 или коронавирус- ная болезнь 2019 года. Клинические проявления COVID-19 варьируют от бессимптомного течения и легких гриппоподобных симптомов до острого респираторного дистресс-синдро-ма и летального исхода. Описаны также хронические осложнения COVID-19 со стороны дыхательной, сердечно-сосудистой и нервной систем [5]. По сравнению с SARS-CoV и MERS-CoV, SARS-CoV-2 демонстрирует высокую кон-тагиозность с приблизительным индексом репродукции 2,2 (т.е. на каждый текущий случай COVID-19 приходится в среднем 2,2 новых случаев заражения) [6]. Кроме того, его способность распространяться бессимптомными носителями значительно снижает эффективность карантинных мероприятий [7]. К октябрю 2020 года вирусом SARS-CoV-2 были инфицированы более 43 миллионов человек, что привело приблизительно к 1,15 миллиона летальных исходов (летальность ~ 3%) в 235 странах, регионах и территориях по всему миру [8]. Вне всякого сомнения, пандемия COVID-19 стала самым серьезным кризисом в сфере общественного здравоохранения, с которым столкнулось современное поколение, и она все еще оказывает ощутимое влияние на мировую экономику и геополитику. Хотя объем наших знаний о патогенных коронавирусах неуклонно увеличивался в течение последних двух десятилетий, до этого года ни одна вакцина для профилактики коронавирусной инфекции человека не была допущена к применению в клинической практике. Учитывая стремительное распространение и высокую смертность от COVID-19, необходимость в эффективной вакцине встала особенно остро. В данном обзоре мы кратко описали наиболее значимые особенности биологии коронавирусов, подытожили стратегии иммунизации против SARS и MERS, а также проанализировали актуальные данные о ходе разработки вакцин против COVID-19. Мы надеемся, что этот обзор будет полезен исследователям, заинтересованным в разработке вакцины против COVID-19.
Биология коронавирусов и ее значение для разработки вакцин. Коронавирусы, на-
Спайковый белок (S)
Белок оболочки (E)
Мембранный белок (M)
Белок нуклеокапсида (N)
Ангиотензинпревращающий фермент 2 (ACE2)
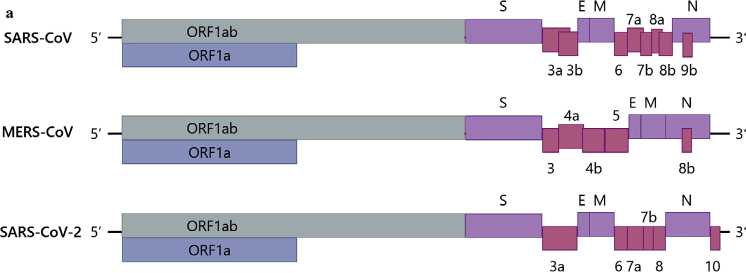
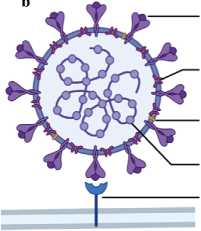
Рисунок 1. Геном и структура вириона коронавирусов (CoV). а Структура генома SARS-CoV, MERS-CoV и SARS-CoV-2 [12-14]. 5'-конец мРНК CoV содержит две перекрывающиеся открытые рамки считывания (ORF): ORF 1a и ORF 1b, охватывающие две трети длины генома. С ORF 1a и ORF 1ab могут быть синтезированы два полипротеина (pp), pp1a и pp1ab, которые далее расщепляются на 16 неструктурных белков (Nsps). 3'-конец мРНК CoV кодирует четыре основных структурных белка в следующем порядке: спайковый белок (S), белок оболочки (E), мембранный белок (M) и белок нуклеокапсида (N). Там же расположены гены родоспецифичных вспомогательных белков. б Структура вириона SARS-CoV-2 [16]. Спайковые белки (S), белки оболочки (E) и мембраны (M) образуют оболочку вириона CoV, а нукле-окапсидные (N) белки образуют капсид, в который упаковывается геномная РНК. Спайковый белок связывается с ангиотензинпревращающим ферментом 2 (ACE2) на клеточной мембране, что позволяет вирусу проникнуть в клетку. (Рисунок создан с помощью BioRender.com.).
звание которых происходит от их характерной короноподобной формы, различимой посредством электронной микроскопии, – это оболочечные РНК-вирусы диаметром примерно 80-160 нм [9, 10]. Геном коронавирусов представляет собой одноцепочечную молекулу (+) РНК размером ~ 30 кб и является самым большим из всех известных геномов РНК-вирусов [9-11]. 5'-конец молекулы РНК CoV содержит две перекрывающиеся открытые рамки считывания (ORFs): ORF 1a и ORF 1b, охватывающие две трети длины генома (рисунок 1а) [9-11]. ORF 1a и ORF 1ab могут быть трансли- рованы в два полипротеина (pp), pp1a и pp1ab, которые далее расщепляются на 16 неструктурных белков (Nsps), участвующих в репликации вирусного генома и синтезе субгеномной мРНК [9-11]. 3'-конец цепочки РНК коронавируса кодирует четыре основных структурных белка в следующем порядке: спайковый белок (S), белок оболочки (E), белок мембраны (M) и белок нуклеокапсида (N) (рисунок 1а) [9-11]. Белки S, E, M формируют оболочку коронавируса, в то время как белок N образует капсид для упаковки геномной РНК (рисунок 1б) [9-11]. 3'-конец нити РНК также кодирует множество вспомогательных белков, которые, как правило, являются родоспецифичными и помогают вирусу избегать клеток иммунной системы организма-хозяина или повышают его вирулентность [9-11]. Например, геном SARS-CoV содержит гены вспомогательных белков ORF 3a, 3b, 6, 7a, 7b, 8a, 8b и 9b, MERS-CoV – ORF 3, 4a, 4b, 5, 8b, а SARS-CoV-2 – ORF 3a, 6, 7a, 7b, 8, 10 (рисунок 1а) [12-14].
Многие вирусные белки являются критически важными для успешного прохождения жизненного цикла коронавируса. Для проникновения в клетки-мишени сначала происходит связывание S-белка с клеточными рецепторами с помощью рецептор-связыва-ющего домена (RBD), а затем комплекс ре-цептор-вирусная частица транспортируется в эндосомы (рисунок 2) [15]. S-белок как SARS-CoV, так и SARS-CoV-2 связывается с ангио-тензинпревращающим ферментом 2 (ACE2), в то время как S-белок MERS-CoV в качестве своего клеточного рецептора использует ди-пептидилпептидазу-4 (DPP4) (рисунок 1б) [16]. В эндосоме S-белок расщепляется на две субъединицы: S1 (с RBD-доменом) и S2 (без RBD), последняя опосредует слияние между вирусной оболочкой и мембраной клетки-хозяина [15]. После проникновения в клетку несколько белков (Nsps), в частности РНК-за-висимая РНК-полимераза (Nsp12) и хеликаза (Nsp13), осуществляют репликацию генома и транскрипцию мРНК коронавируса [17]. С коронавирусной мРНК далее синтезируются различные неструктурные и структурные белки [17]. N-белки связываются с геномной РНК коронавируса, формируя нуклеокапсид, а S, E и M-белки участвуют в образовании оболочки вириона [15]. После сборки вирусные частицы проходят последовательно через эндоплазматический ретикулум (ER) и аппарат Гольджи и выходят из клеток путем экзоцито-за (рисунок 2) [15].
S-белок особенно важен для связывания вируса с клеточными рецепторами и слияния оболочки вируса с мембраной клетки, что позволяет рассматривать его как пер- спективную целевую структуру для конструирования вакцины против коронавирусной инфекции [15]. По данным исследований, антитела, вырабатываемые иммунной системой против вирусного S-белка, длительное время сохраняются в организме и являются иммунодоминантными у пациентов, перенесших SARS [18, 19]. Кроме того, в ряде исследований было показано, что антитела к S-белку могут нейтрализовать SARS-CoV и MERS-CoV и оказывают защитное воздействие на животных и людей [20-22]. Результаты доклинических испытаний также продемонстрировали, что многие вакцины против SARS-CoV и MERS-CoV, сконструированные на основе S-белка, вызывают развитие сильного иммунного ответа и запуск защитных механизмов [23-27]. Это подтверждает предположение, что S-белок коронавируса является идеальной целевой структурой для создания вакцин, эффективно индуцирующих синтез нейтрализующих антител и формирование иммунитета. Помимо S-белка в качестве мишеней для вакцин были также проанализированы и другие структурные белки коронавируса. Так, вакцины на основе N-белка, как правило, не могут индуцировать выработку нейтрализующих антител, вероятно, по той причине, что N-белок не экспонирован на поверхности коронавирусной частицы [16]. Однако, использование N-белка имеет и определенное преимущество, связанное с его большей консервативностью среди всех видов коронавирусов по сравнению с S-белком, и это его свойство может быть полезно для создания универсальной коронавирусной вакцины, индуцирующей Т-клеточный иммунитет [16]. По данным недавнего исследования, вирусная векторная вакцина, экспрессирующая N-белок, обладает способностью индуцировать CD4+ T-клеточный иммунный ответ против SARS-CoV и MERS-CoV, что свидетельствует о возможности создания на основе N-белка вакцины, позволяющий получить клеточный иммунитет против коронавирусов [28]. С другой стороны, присутствие высоких титров антител у имму-
Субгеномная репликация конкретных генов:
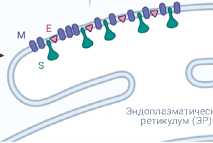
Рисунок 2. Жизненный цикл SARS-CoV-2 [9, 10, 15]. При связывании с мембранным рецептором ACE2 вирион SARS-CoV-2 проникает в клетку хозяина и высвобождает кодирующую нить (+) РНК. С (+) нити РНК синтезируются белки ppla и pplab, которые далее расщепляются на множество неструктурных белков (Nsps), включая РНК-зависимую РНК-полимеразу (Nsp12). РНК-зависимая РНК-полимераза транскрибирует (-) цепь геномной РНК, а потом использует эту (-) РНК как матрицу для синтеза большого количества геномной (+) РНК (полногеномная репликация) и других субгеномных РНК (субгеномная транскрипция). Субгеномные РНК впоследствии транслируются в основные структурные белки (N, S, M, E), которые затем вместе с молекулой геномной (+) РНК формируют зрелый вирион в просвете эндоплазматического ретикулума. На заключительном этапе сформированная вирусная частица покидает клетку путем экзоцитоза. (Репринт схемы «Цикл репликации коронавируса» с сайта BioRender. com (2020). Доступ по ссылке: https://app.biorender.com/biorender-templates ).
низированных животных было показано для вакцин на основе М-белка [29]. Но в то же время результаты доклинических испытаний не продемонстрировали ни образование нейтрализующих антител, ни развитие иммунитета. Что касается вирусного Е-белка, исследований, посвященных его использованию в качестве мишени для создания вакцины, к настоящему времени известно немного, и ни в одном из них не было показано появление нейтрализующих антител или защитного иммунитета [30].
С использованием вакцин против SARS-CoV и MERS-CoV также ассоциировано развитие некоторых иммунопатологических осложнений, что требует их анализа и работы, направленной на дальнейшую оптимизацию данных вакцин. Одним из их неблагоприятных эффектов является антителозависимое усиление инфекции (antibody-dependent enhancement, ADE), которое обычно вызывается вакцинно-индуцированными субоптимальными антителами, облегчающими проникновение вируса в клетку хозяина [11, 31]. В одном из исследований было показано, что вакцина против SARS-CoV на основе полноразмерного S-белка облегчает инфицирование вирусом клеток человека in vitro [32]. Кроме того, два других исследования показали, что введение анти-S-белковой сыворотки также приводит к повышению вирулентности SARS-CoV [33, 34]. Вышеупомянутые результаты порождают сомнения в безопасности вакцин против SARS-CoV и MERS-CoV, сконструированных на основе S-белка. Одной из возможных стратегий предотвращения антителозависимого усиления инфекции является разработка вакцин, содержащих только основные нейтрализующие эпитопы, такие как субъединица S1 или RBD-домен S-белка. Такая стратегия позволяет уменьшить выработку не-нейтра-лизующих антител, вызываемую вакцинами против коронавирусных инфекций, и, следовательно, ослабить эффект антителозависимого усиления инфекции. Другим возможным неблагоприятным эффектом является развитие вакцинно-индуцированных эозинофильных иммунопатологических реакций, представляющих собой нежелательный Th2-поляризованный иммунный ответ, вызванный вакцинацией [11, 35]. По крайней мере в двух исследованиях сообщалось, что вакцина, содержащая инактивированные вирионы SARS-CoV, индуцирует зозинофильный провоспалительный ответ в легких мышей после заражения SARS-CoV [36, 37]. Кроме того, в одном из исследований также отмечалось, что иммунизация вакциной, содержащей SARS-CoV вирусоподобные частицы (virus-like particles, VLP), приводила к эозинофильным иммунопатологическим реакциям в легких после заражения вирусом [37]. Чтобы предотвратить развитие Th2-поляризо-ванного иммунного ответа, в нескольких исследованиях изучался вопрос оптимизации адъюванта. В них было обнаружено, что правильно подобранные адъюванты, такие как агонист Toll-подобных рецепторов и полисахарид дельта-инулин, могут повышать титр нейтрализующих антител в сыворотке крови и уменьшать выраженность эозинофильных иммунопатологических реакций в легких [38, 39]. Эти результаты позволяют наметить перспективную стратегию предотвращения развития Th2-поляризованного иммунного ответа, индуцируемого некоторыми вакцинами против коронавирусов.
Результаты изучения стратегий иммунизации против SARS-CoV и MERS-CoV. Для вакцинации от инфекций, вызываемых вирусами SARS-CoV и MERS-CoV, в свое время были разработаны и испытаны на доклинических моделях различные виды вакцин. Однако лишь немногие из них дошли до клинических испытаний, и ни одна не была одобрена Управлением по контролю за качеством пищевых продуктов и медикаментов США (Food and Drug Administration, FDA). В анализ были включены: вакцины на основе отдельных субъединиц вирусных белков, вакцины на основе вирусоподобных частиц, ДНК-содержащие вакцины, вакцины на основе вирусных векторов, а также цельновирионные инактивированные и живые аттенуированные вакцины. Следующие разделы посвящены описанию принципов разработки различных видов вакцин против SARS-CoV и MERS-CoV (таблица 1), а также содержат актуальные результаты доклинических исследований и клинических испытаний (таблица 2).
Субъединичные (пептидные) вакцины. Субъединичные вакцины состоят из вирусных антигенных пептидных фрагментов, сконструированных на основе рекомбинантных белков. Синтез рекомбинантных белков или их фрагментов не представляет технологической сложности, а продукты этого синтеза относительно безопасны и хорошо переносятся по сравнению с цельновирионными и векторными вакцинами. В свою очередь, недостатком субъединичных вакцин является их низкая иммуногенность. Поэтому для преодоления этого недостатка в состав субъединичных вакцин включают адъюванты и иммуностимулирующие молекулы.
Таблица 1
|
Платформа для разработки вакцины |
Преимущества |
Недостатки |
Примеры вакцин, разрешенных к применению в клинической практике |
|
Цельновирионные инактивированные вакцины |
Более выраженный иммунный ответ; Более высокая безопасность по сравнению с живым аттенуированным вирусом |
Потенциальное изменение эпитопа в процессе инактивации |
Вакцины против брюшного тифа, холеры, вирусного гепатита А, чумы, бешенства, гриппа, полиомиелита (вакцина Солка) |
|
Живые аттенуированные вакцины |
Более выраженный иммунный ответ; Сохранение нативного антигена; Имитация естественной инфекции |
Риск остаточной вирулентности, особенно у людей с ослабленным иммунитетом |
Вакцины против кори, эпидемического паротита, полиомиелита (вакцина Сабина), ротавирусной инфекции, желтой лихорадки, бацилла Кальметта-Герена (БЦЖ), вакцины против краснухи, ветряной оспы |
|
Вакцины на основе вирусных векторов |
Более выраженный иммунный ответ; Сохранение нативного антигена; Имитация естественной инфекции |
Более сложный процесс производства; Риск интеграции в геном; Ослабление иммунного ответа при наличии иммунитета против вируса-вектора |
Вакцина против вируса Эбола |
|
Субъединичные вакцины |
Безопасность и хорошая переносимость |
Более низкая иммуногенность; Необходимость адъюванта или конъюгата для повышения иммуногенности |
Вакцины против коклюша, гриппа, инфекций, вызванных Streptococcus pneumoniae, Haemophilus influenzae типа b |
|
Вакцины, содержащие вирусоподобные частицы |
Безопасность и хорошая переносимость; Имитация нативной конформации вируса |
Более низкая иммуногенность; Более сложный процесс производства |
Вакцины против вирусного гепатита В, папилломавирусной инфекции |
|
ДНК-вакцины |
Безопасность и хорошая переносимость; Стабильность при комнатной температуре; Возможность адаптации к новому патогену; Экспрессия нативного антигена |
Более низкая иммуногенность; Сложный путь введения; Риск интеграции в геном |
Нет данных |
|
РНК-вакцины |
Безопасность и хорошая переносимость; Возможность адаптации к новому патогену; Экспрессия нативного антигена |
Более низкая иммуногенность; Необходимость хранения и транспортировки при низких температурах; Потенциальный риск РНК-индуци-рованного интерферонового ответа |
Нет данных |
Преимущества и недостатки различных платформ для разработки вакцин
В ходе разработки субъединичных вакцин против SARS-CoV исследователи изначально взяли за основу полноразмерный S-белок, а затем сосредоточились на его рецептор-свя-зывающем домене, RBD. Ни одна из субъеди- ничных вакцин против SARS-CoV не дошла до клинических испытаний, хотя на доклинических моделях они демонстрировали выраженную способность индуцировать продукцию антител и оказывать защитные эффекты
Таблица 2
|
Основа |
Вакцина |
Исследовательская группа |
Статус |
Ссылка |
|
Клинические исследования вакцин против SARS |
||||
|
Инактивированный вирус |
Инактивированная вакцина против SARS-CoV (ISCV) |
Sinovac |
Фаза I, завершена |
Lin et al. (2007) [110] Нет регистрационного номера NCT |
|
ДНК-вакцина |
VRC-SRSDNA015-00-VP |
NIAID |
Фаза I, завершена |
Martin et al. (2008) [65] NCT00099463 |
|
Клинические исследования вакцин против MERS |
||||
|
ДНК-вакцина |
GLS-5300 (INO-4700) |
GeneOne Life Science / Inovio Pharmaceuticals / International Vaccine Institute |
Фаза I, завершена |
Modjarrad et al. (2019) [69] NCT02670187 |
|
ДНК-вакцина |
GLS-5300 (INO-4700) |
GeneOne Life Science / Inovio Pharmaceuticals / International Vaccine Institute |
Фаза I/IIa, завершена |
NCT03721718 |
|
Вирусная векторная вакцина |
MVA-MERS-S |
CTC North GmbH & Co. KG |
Фаза I, завершена |
Koch et al. (2020) [102] NCT03615911 |
|
Вирусная векторная вакцина |
MVA-MERS-S_ DF1 |
CTC North GmbH & Co. KG |
Фаза Ib, набор еще не начат |
NCT04119440 |
|
Вирусная векторная вакцина |
ChAdOx1 MERS |
University of Oxford |
Фаза I, идет набор |
Folegatti et al. (2020) [98] NCT03399578 |
|
Вирусная векторная вакцина |
ChAdOx1 MERS |
King Abdullah International Medical Research Center / University of Oxford |
Фаза I, идет набор |
NCT04170829 |
|
Вирусная векторная вакцина |
BVRS-GamVac-Combi |
Gamaleya Research Institute of Epidemiology and Microbiology / Acellena Contract Drug Research and Development |
Фаза I/II, идет набор |
NCT04128059 |
|
Вирусная векторная вакцина |
BVRS-GamVac |
Gamaleya Research Institute of Epidemiology and Microbiology |
Фаза I/II, идет набор |
NCT04130594 |
|
Основа |
Вакцина |
Тип вакцины |
Исследовательская группа |
Статус |
Ссылка |
|
Клинические исследования вакцин против COVID-19 |
|||||
|
Белковая субъединица |
NVX-CoV2373 |
SARS-CoV-2 rS / Matrix-M1 Адъювант |
Novavax |
Фаза III |
Keech et al. (2020) [132] 2020-004123-16 NCT04533399 |
Таблица 2 (продолжение)
|
Основа |
Вакцина |
Тип вакцины |
Исследовательская группа |
Статус |
Ссылка |
|
РНК |
mRNA-1273 |
мРНК, инкапсулированная в липидные наночастицы |
Moderna / NIAID |
Фаза III |
Jackson et al. (2020) [140] Anderson et al. (2020) [141] NCT04470427 |
|
РНК |
BNT162b1 BNT162b2 |
мРНК в липидных наночастицах |
BioNTech / Fosun Pharma / Pfizer |
Фаза III |
Mulligan et al. (2020) [144] Sahin et al. (2020) [145] Walsh et al. (2020) [146] NCT04368728 |
|
Вирусный вектор |
AZD1222 |
ChAdOx1-S |
University of Oxford / AstraZeneca |
Фаза III |
Folegatti et al. (2020) [99] NCT04516746 NCT04540393 ISRCTN89951424 CTRI/2020/08/027170 |
|
Вирусный вектор |
Ad5-nCoV |
Аденовирус 5-го типа |
CanSino Biological Inc. / Beijing Institute of Biotechnology |
Фаза III |
Zhu et al. (2020) [92], Zhu et al. (2020) [93] NCT04526990 NCT04540419 |
|
Вирусный вектор |
Gam-COVID-Vac |
Аденовирусная основа (rAd26-S + rAd5-S) |
Gamaleya Research Institute |
Фаза III |
Logunov et al. (2020) [151] NCT04530396 NCT04564716 |
|
Вирусный вектор |
Ad26.COV2.S |
Аденовирусная основа |
Janssen Pharmaceutical Companies |
Фаза III |
NCT04505722 |
|
Инактивированный вирус |
Адсорбированная (инактивированная) вакцина против COVID-19 |
Инактивированная |
Sinovac |
Фаза III |
NCT04456595 NCT04582344 669/UN6. KEP/EC/2020 |
|
Инактивированный вирус |
Инактивированная вакцина против SARS-CoV-2 (Vero cell) |
Инактивированная |
Wuhan Institute of Biological Products / Sinopharm |
Фаза III |
Xia et al. (2020) [154] ChiCTR2000034780 ChiCTR2000039000 |
|
Инактивированный вирус |
BBIBP-CorV |
Инактивированная |
Beijing Institute of Biological Products / Sinopharm |
Фаза III |
Xia et al. (2020) [156] ChiCTR2000034780 NCT04560881 |
|
Белковая субъединица |
Рекомбинантная вакцина против новой коронавирусной инфекции (CHO cell) |
Рекомбинантный RBD-димер с адъювантом |
Anhui Zhifei Longcom Biopharmaceutical / Institute of Microbiology, Chinese Academy of Sciences |
Фаза II |
NCT04466085 |
|
РНК |
CVnCoV |
мРНК |
Curevac |
Фаза II |
NCT04515147 |
|
Белковая субъединица |
KBP-COVID-19 |
На основе домена RBD S-белка |
Kentucky Bioprocessing, Inc |
Фаза I/II |
NCT04473690 |
|
Белковая субъединица |
Вакцина против SARS-CoV-2 |
S-белок с адъювантом |
Sanofi Pasteur / GSK |
Фаза I/II |
NCT04537208 |
|
РНК |
ARCT-021 |
мРНК |
Arcturus / Duke-NUS |
Фаза I/II |
NCT04480957 |
Таблица 2 (продолжение)
|
Основа |
Вакцина |
Тип вакцины |
Исследовательская группа |
Статус |
Ссылка |
|
ДНК |
INO-4800 |
ДНК-плазмида с электропорацией |
Inovio Pharmaceuticals / International Vaccine Institute |
Фаза I/II |
NCT04447781 NCT04336410 |
|
ДНК |
AG0301-COVID19 |
ДНК-плазмида с адъювантом |
Osaka University / AnGes / Takara Bio |
Фаза I/II |
NCT04463472 NCT04527081 |
|
ДНК |
nCov Vaccine |
ДНК-плазмида |
Cadila Healthcare Limited |
Фаза I/II |
CTRI/2020/07/026352 |
|
ДНК |
GX-19 |
ДНК-вакцина |
Genexine Consortium |
Фаза I/II |
NCT04445389 |
|
Инактивированный вирус |
BBV152A BBV152B BBV152C |
Инактивированная |
Bharat Biotech |
Фаза I/II |
NCT04471519 CTRI/2020/09/027674 |
|
Инактивированный вирус |
Инактивированная вакцина против SARS-CoV-2 |
Инактивированная |
Institute of Medical Biology, Chinese Academy of Medical Sciences |
Фаза I/II |
NCT04470609 |
|
Инактивированный вирус |
QazCovid-in |
Инактивированная |
Research Institute for Biological Safety Problems, Rep of Kazakhstan |
Фаза I/II |
NCT04530357 |
|
Вирусоподобные частицы |
RBD SARS-CoV-2 НВѕАg VLP |
RBD-HBsAg вирусоподобные частицы |
SpyBiotech / Serum Institute of India |
Фаза I/II |
ACTRN12620000 817943 |
|
Белковая субъединица |
SCB-2019 |
S-белок с адъювантом |
Clover Biopharmaceuticals Inc. / GSK / Dynavax |
Фаза I |
NCT04405908 |
|
Белковая субъединица |
COVAX-19 |
S-белок с адъювантом Advax-SM |
Vaxine Pty Ltd / Medytox |
Фаза I |
NCT04453852 |
|
Белковая субъединица |
SARS-CoV-2 Sclamp vaccine |
S-белок, стабилизированный молекулярным зажимом, с адъювантом MF59 |
University of Queensland / CSL / Seqirus |
Фаза I |
ACTRN12620000 674932p ISRCTN51232965 |
|
Белковая субъединица |
MVC-COV1901 |
S-2P белок + CpG 1018 |
Medigen Vaccine Biologics Corporation / NIAID / Dynavax |
Фаза I |
NCT04487210 |
|
Белковая субъединица |
Soberana 01 |
RBD-домен S-белка с адъювантом |
Instituto Finlay de Vacunas, Cuba |
Фаза I |
IFV/COR/04 |
|
Белковая субъединица |
EpiVacCorona |
Адъювантный белковый анти ген |
FBRI SRC VB VECTOR, Rospotrebnadzor, Koltsovo |
Фаза I |
NCT04527575 |
|
Белковая субъединица |
Рекомбинантная вакцина против SARS-CoV-2 |
RBD S-белка (клетки Sf9) |
West China Hospital, Sichuan University |
Фаза I |
ChiCTR2000037518 |
Таблица 2 (продолжение)
|
Основа |
Вакцина |
Тип вакцины |
Исследовательская группа |
Статус |
Ссылка |
|
Белковая субъединица |
IMP (CoVac-1) |
Мультипептид-ная смесь из HLA-DR пептидов SARS-CoV-2 |
University Hospital Tuebingen |
Фаза I |
NCT04546841 |
|
Белковая субъединица |
UB-612 |
S1-RBD-белок |
COVAXX |
Фаза I |
NCT04545749 |
|
РНК |
LNP-nCoVsaRNA |
Самоамплифи-цирующаяся рибонуклеиновая кислота (saRNA), кодирующая S-белок |
Imperial College London |
Фаза I |
ISRCTN17072692 |
|
РНК |
SARS-CoV-2 mRNA vaccine |
мРНК, кодирующая RBD-домен S-белка |
People's Liberation Army (PLA) Academy of Military Sciences / Walvax Biotech |
Фаза I |
ChiCTR2000034112 |
|
Вирусный вектор |
hAd5-S- Fusion + N-ETSD vaccine |
hAd5 Спайк (S) + Нуклеокапсид (N) |
ImmunityBio, Inc. & NantKwest Inc |
Фаза I |
NCT04591717 |
|
Вирусный вектор |
GRAd-COV2 |
Аденовирус обезьян с нарушенной репликацией (GRAd) |
ReiThera / LEUKOCARE / Univercells |
Фаза I |
NCT04528641 |
|
Вирусный вектор |
Ad5-nCoV |
На основе Ad5 |
CanSino Biological Inc. / Institute of Biotechnology, Academy of Military Medical Sciences, PLA of China |
Фаза I |
NCT04552366 |
|
Вирусный вектор |
VXA-CoV2-1 |
дсРНК-адъю-вантная, на основе Ad5 |
Vaxart |
Фаза I |
NCT04563702 |
|
Вирусный вектор |
MVA-SARS-2-S |
Модифицированный вирус осповакцины штамма Анкара + спайковый белок (S) |
Ludwig-Maximilians University of Munich |
Фаза I |
NCT04569383 |
|
Вирусный вектор |
V590 |
VSV + S белок |
Merck Sharp & Dohme / IAVI |
Фаза I |
NCT04569786 |
|
Вирусный вектор |
TMV-083 |
Векторная, на основе вируса кори |
Institute Pasteur / Themis / Univ. of Pittsburg CVR / Merck Sharp & Dohme |
Фаза I |
NCT04497298 |
|
Вирусный вектор |
DelNS1-2019-nCoV-RBD-OPT1 |
Интраназальная, RBD-содержа-щая, на основе вируса гриппа |
Beijing Wantai Biological Pharmacy / Xiamen University |
Фаза I |
ChiCTR2000037782 |
Таблица 2 (окончание)
|
Основа |
Вакцина |
Тип вакцины |
Исследовательская группа |
Статус |
Ссылка |
|
Инактивированный вирус |
Инактивированная вакцина против SARS-CoV-2 |
Инактивированная |
Beijing Minhai Biotechnology |
Фаза I |
ChiCTR2000038804 |
|
Вирусоподобные частицы |
Рекомбинантная вакцина против SARS-CoV-2 на основе вирусоподобных частиц |
Адъювантная, на основе CpG 1018-или AS03-содер-жащих вирусоподобных частиц растительного происхождения |
Medicago Inc |
Фаза I |
NCT04450004 |
Клинические исследования вакцин против SARS, MERS и COVID-19
[23, 24, 32, 40-44]. Исследования показали, что полноразмерный S-белок, внеклеточный домен S-белка и тримеры S-белка (triSpike) обладают иммуногенными свойствами и могут вызывать формирование защитных реакций против инфекции SARS-CoV [23, 24, 32]. Однако работы Kam et al. и Jamue et al. продемонстрировали, что вакцина на основе triSpike в то же время вызывает антителозависимое усиление инфекции SARS-CoV путем связывания с рецептором FcγRII В-лимфоцитов человека in vitro [32, 33]. С другой стороны, вакцины, сконструированные на основе RBD-домена S-белка, способны индуцировать синтез высоких титров нейтрализующих антител, не вызывая явных патогенных эффектов [40-44]. Это, вероятно, связано с тем фактом, что вакцины на основе отдельного RBD-домена вирусного белка не содержат дополнительных не-нейтрализующих эпитопов, как в случае вакцин с полноразмерным S-белком. Согласно одному из исследований, после введения мышам вакцины на основе RBD-домена, у них не только появлялся иммунитет к SARS-CoV и не обнаруживалась вирусная РНК в легких, но также синтезировались S-белок-специфи-ческие антитела, которые затем сохранялись в течение 12 месяцев [42]. Кроме того, было показано, что вакцины на основе RBD-домена белка SARS-CoV также индуцируют RBD-специфичный синтез интерферона гамма, вызывая клеточный иммунный ответ у мышей [44]. Таким образом, RBD-домен S-белка SARS-CoV является основной целевой структурой для разработки вакцин против SARS. Наконец, также были испытаны субъединичные вакцины против SARS-CoV, сконструированные на основе S2-субъединицы, структурных белков N и М [29, 45, 46], но никаких доказательств того, что они могут индуцировать синтез нейтрализующих антител или вызывать защитные реакции против вирусной инфекции, получено не было.
С учетом предшествующего опыта с SARS-CoV разработка большинства субъединичных вакцин против MERS-CoV также была ориентирована на использование RBD-домена в качестве структурной основы. Вакцины против MERS-CoV на основе RBD, как правило, проявляли высокую иммуногенность и вызывали продукцию эффективных нейтрализующих антител, клеточно-опосредованный иммунный ответ, а также обеспечивали защиту от инфекции MERS-CoV [25, 26]. Исследование, проведенное Tai et al., выявило, что вакцины на основе тримеров RBD могут индуцировать синтез нейтрализующих антител, сохраняющихся в организме в течение 6 месяцев [26]. Другое исследование, также проведенное Tai et al., показало, что рекомбинантные белки с RBD-доменами различных разновидностей MERS-CoV могут индуцировать синтез антител, которые способны перекрестно нейтрализовать MERS-CoV человека и верблюда [25]. Эти результаты свидетельствуют о том, что RBD-домен вируса MERS-CoV является многообещающей целевой структурой для соз- дания вакцины, обладающей способностью вызывать формирование широкого спектра длительно циркулирующих в организме нейтрализующих антител. Было также показано, что, помимо вакцин на основе RBD-домена, индуцировать синтез нейтрализующих антител и обеспечивать защиту от MERS-CoV могут вакцины, которые содержат субъединицу S1, включающую RBD [47, 48]. Примечательно, что N-терминальный домен (NTD) S-белка связывается с сиаловыми кислотами и имеет важное значение для инфицирования MERS-CoV определенных типов клеток. Jiaming et al. показали, что иммунизация вакциной СМ 647 на основе N-концевого домена (NTD) также обеспечивает защиту от MERS-CoV и индуцирует мощный гуморальный и клеточно-опосредованный иммунитет [49]. Однако, поскольку NTD-домен белка SARS-CoV-2 не обладает способностью связываться с сиаловыми кислотами, как белок MERS-CoV, стратегия использования NTD не может быть применена для разработки вакцины против SARS-CoV-2.
Помимо структуры антигена, на эффективность субъединичных вакцин влияет ряд других факторов [16]. В частности, качество и количество синтезируемых белковых субъединиц зависит от конкретной экспрессирующей системы. В своем исследовании Du et al. продемонстрировали, что RBD-содержащий белок SARS-CoV, синтезированный клетками клеточной линии HEK 293 млекопитающих, индуцирует более выраженную выработку нейтрализующих антител, чем RBD-содер-жащие белки, синтезированные клетками насекомых и E. coli. Это, вероятно, связано с приобретением белками более естественной конформации в ходе посттрансляционной модификации, происходящей в клетках млекопитающих [43]. Кроме того, важную роль в повышении иммуногенности субъединичных вакцин играют адъюванты. Zhang et al. изучили широкий спектр соединений (адъювант Фрейнда, алюминий, монофосфорил липид А, монтанид ISA51 и MF59) как вероятных адъювантов для RBD-домен содержащей вакцины против MERS-CoV и обнаружили, что наибольшей способностью потенцировать образование нейтрализующих антител обладает мон-танид MF59 [50]. Полученные этими авторами данные могут стать хорошей отправной точкой для подбора наиболее оптимальных адъювантов субъединичных вакцин против SARS-CoV-2. Более того, на эффективность субъединичной вакцины также влияет способ ее введения, выбор которого зависит от сочетания конкретных антигена и адъюванта. Например, Li et al. показали, что в случае S- и S1-содержащих субъединичных вакцин против SARS-CoV наиболее сильная выработка антител происходит при внутримышечном (в/м), а не подкожном (п/к) введении, в то время как Lan et al. показали, что п/к путь предпочтительнее в/м инъекций в случае RBD-вакцин против MERS-CoV, содержащих адъюванты Фрейнда и CpG [23, 51]. Таким образом, подбор оптимального пути введения должен осуществляться для каждой отдельной вакцины против SARS-CoV-2.
Вакцины на основе вирусоподобных частиц. Вирусоподобные частицы (VLP) – это ансамбли вирусных структурных белков, способных к самосборке, имитирующие конформацию нативных вирионов, но не несущие в себе вирусного генома. В отличие от субъединичных вакцин VLP-вакцины презентируют эпитоп в конформации, более похожей на ту, которую имеет нативный вирус, и это значительно усиливает иммунный ответ. Кроме того, по сравнению с производством цельновирионных вакцин процесс изготовления VLP-вакцин не включает в себя стадии живого вируса и его инактивации, что делает их более безопасными. Большое количество антигенных эпитопов на поверхности вирусоподобных частиц также способствует более мощному гуморальному ответу за счет эффективного перекрестного связывания с рецепторами B-клеток. К настоящему времени в коммерческое производство были выпущены VLP-вакцины против вируса папилломы человека (Cervarix™ и Gardasil®) и вируса гепатита В (Engerix® и Recombivax HB®) [52].
Вплоть до текущего момента имелись сведения лишь о нескольких VLP-вакцинах против SARS-CoV и MERS-CoV. Разрабатывая вакцину против SARS-CoV, Lokugamage et al. показали, что химерные VLP, состоящие из S-белка SARS-CoV и E-, M- и N-белков вируса гепатита мышей, могут индуцировать выработку нейтрализующих антител и снижать титр вируса SARS-CoV в легких мышей после заражения [53]. Согласно результатам, полученным Liu et al., химерные VLP, синтезированные на основе S-белка SARS-CoV и M1-белка вируса гриппа, также вызывают синтез нейтрализующих антител в организме мышей и обеспечивают защиту при их заражении летальной дозой вируса [54]. Однако в другом исследовании анализ тех же, что в работе Lokugamage et al., химерных вирусоподобных частиц показал, что введение данной VLP-вакцины может приводить к развитию иммунопатологических процессов в легких при инфицировании SARS-CoV [37, 53]. Таким образом, при разработке VLP-вакцин против коронавирусной инфекции следует особенно тщательно оценивать возможные нежелательные явления. В отношении VLP-вакцин против MERS-CoV Wang et al. показали, что вирусоподобные частицы, содержащие S-, E- и М-белки MERS-CoV могут вызывать специфический иммунный ответ и запускать формирование Th1-опосредован-ного клеточного иммунитета у макак-резусов [55]. Та же исследовательская группа разработала еще одну химерную VLP-вакцину, состоящую из рецептор-связывающего домена S-белка MERS-CoV и структурного белка VP2 парвовируса собак (CPV) [56]. Они показали, что эта VLP-вакцина индуцирует у мышей образование специфических антител к MERS-CoV и запускает развитие T-клеточного иммунитета [56]. Результаты этих исследований свидетельствуют, что VLP-вакцины обладают потенциальной клинической эффективностью против коронавирусной инфекции.
ДНК-вакцины. ДНК-вакцины содержат гены, кодирующие вирусные антигенные элементы, которые экспрессируются с помощью векторных плазмид и доставляются в клетки посредством электропорации. По сравнению с другими технологическими платформами, используемыми для создания вакцин, технология создания ДНК-вакцин обладает такими преимуществами, как быстрота и гибкость на стадиях разработки и производства, что повышает ее привлекательность в борьбе с эпидемиями, подобными настоящей эпидемии, вызванной SARS-CoV-2. Кроме того, синтез антигенов при введении ДНК-вакцины происходит внутри клеток-мишеней, что позволяет воспроизвести нативную конформацию и осуществить правильную посттрансляционную модификацию вирусных антигенов. Однако существенным недостатком ДНК-вакцин является их ограниченная иммуногенность, обусловленная невозможностью распространения и амплификации in vivo. Поэтому для повышения эффективности ДНК-вакцины необходима ее оптимизация, в частности, добавление адъюванта или вакцинация в режиме прайм-буст. Другой проблемой, касающейся биологической безопасности, является потенциальная интеграция ДНК-вакцин в геном хозяина, что может привести к инициации мутаций и онкогенных процессов [57]. Несмотря на то, что предыдущие исследования охарактеризовали риск внедрения вакцинной плазмиды в хромосому хозяина как весьма низкий, FDA (Управление по контролю за качеством пищевых продуктов и медикаментов) и ВОЗ по-прежнему рекомендуют в рамках оценки безопасности ДНК-вакцин проводить изучение возможности интеграции ДНК в геном [58, 59].
Имеется информация о нескольких канди-датных ДНК-вакцинах против SARS-CoV, включая вакцины на основе S-, M- и N-белков [6064]. Хотя все они могут вызывать выработку определенного уровня антител и формирование клеточного иммунитета, было показано, что только ДНК-вакцина на основе S-белка обеспечивает появление защитного эффекта против инфекции SARS-CoV, вероятно, за счет критически важной роли S-белка на этапе связывания вирусной частицы с рецептором [60].
Yang et al. показали, что все варианты вакцин с ДНК, кодирующей S-белок (полноразмерный, лишенный части цитоплазматического домена, а также лишенный как цитоплазматического, так и трансмембранного доменов), могут индуцировать синтез нейтрализующих антител и развитие Т-клеточных иммунных реакций, а также оказывать защитный эффект против инфекции у мышей [60]. Эти обнадеживающие результаты позволили перейти к следующему этапу, фазе I клинических исследований вакцины, сконструированной на основе гена полноразмерного S-белка SARS-CoV. Исследования подтвердили, что вакцина хорошо переносится пациентами и может индуцировать выработку нейтрализующих антител и развитие Т-клеточного иммунитета у здоровых взрослых [65]. В ходе двух дальнейших исследований с целью повышения эффективности ДНК-вакцины вакцинацию против SARS-CoV проводили в режиме прайм-буст. В работе Zakhartchouk et al. сообщалось, что комбинация ДНК-вакцины и цельновирионной инактивированной вакцины против SARS-CoV может усилить гуморальный иммунный ответ, а также индуцировать развитие более предпочтительного Th1-поляризованного иммунного ответа [66]. Woo et al. продемонстрировали, что использование ДНК-вакцины в качестве праймера и рекомбинантного S-белка, синтезированного E. coli , в качестве бустера позволяет добиться более высоких титров нейтрализирующих антител, нежели иммунизация ДНК-вакциной или субъединичной вакциной по отдельности [67].
Оптимистические результаты, подобные полученным в исследованиях, посвященных SARS-CoV, были также показаны по итогам нескольких работ по разработке вакцины против MERS-CoV. По данным Muthumani et al., ДНК-вакцина, основанная на полноразмерном S-белке MERS-CoV способна индуцировать мощный клеточный иммунитет и выработку антигенспецифических нейтрализующих антител у мышей, макак и верблюдов. При этом у вакцинированных макак при последующем инфицировании MERS-CoV не было отмечено каких-либо клинических или рентгенологических признаков пневмонии [68]. На основе этих обнадеживающих данных ДНК-вакцина против MERS-CoV (GLS-5300 или INO-4700) прошла фазу I клинических исследований [69]. Согласно полученным результатам, GLS-5300 хорошо переносится и не вызывает серьезных побочных эффектов, а также индуцирует развитие стойкого иммунитета у 85% участников, прошедших два этапа иммунизации [69]. Эти данные свидетельствуют о целесообразности дальнейшего исследования GLS-5300. Примечательно, что ДНК-вакцина против SARS-CoV-2, INO-4800, основанная на той же конструкции, что и GLS-5300, в настоящее время находится в фазе I/II клинических исследований (NCT04447781 и NCT04336410) [70]. Помимо этого, в процессе разработки находится еще одна вакцина против MERS-CoV, которая содержит ДНК полноразмерного S-белка в качестве праймера и S1-субъеди-ницу S-белка в качестве бустера. Результаты исследований уже показали высокую нейтрализующую активность вырабатываемых после ее введения антител против нескольких разновидностей MERS-CoV у мышей и макак-резусов [47]. Иммунизация макак-резусов этой вакциной с первичным праймированием ДНК и дальнейшим бустированием белком снижает вероятность развития ассоциированной с MERS-CoV пневмонии по данным рентгенологического исследования, что в очередной раз подтверждает эффективность режима прайм-буст, в том числе в контексте вакцины против MERS-CoV [47]. Целевой структурой для разработки ДНК-вакцины против MERS-CoV может являться не только полноразмерный S-белок, но и его субъединица S1. В исследовании, проведенном Al-Amri et al., сравнивалась иммуногенность вакцин против MERS-CoV на основе гена полноразмерного S-белка (pS) и участка ДНК, кодирующего только субъединицу S1 (pS1), экспрессионный вектор при этом использовался один и тот же [71]. Было обнаружено, что иммунизация вакциной pS1
приводит к развитию иммунного ответа, сбалансированного по соотношению Th1/Th2, и в целом к более высоким уровням выработки всех подклассов IgG по сравнению с иммунизацией pS вакциной. Причиной этого может быть тот факт, что субъединицы S1, лишенные трансмембранного домена, по большей части секретируются во внеклеточное пространство, где более эффективно захватываются антигенпрезентирующими клетками [71]. Авторы данного исследования предполагают, что S1 субъединица может быть более подходящей структурой для конструирования ДНК-вакцины против MERS-CoV, чем полноразмерный S-белок [71].
В целом, результаты изучения ДНК-вакцин против SARS-CoV и MERS-CoV, кодирующих как полноразмерный S-белок, так и субъединицу S1, были обнадеживающими. Данная стратегия разработки ДНК-вакцин, вероятно, может быть использована и в случае с SARS-CoV-2, учитывая его биологическое сходство с SARS-CoV и MERS-CoV.
Вакцины на основе вирусных векторов. Вакцины на основе вирусных векторов — это вакцины, имеющие в составе рекомбинантные вирусные частицы на основе неродственного целевому, модифицированного вируса и ДНК, кодирующую белки-антигены целевого вируса. Они доставляют антиген в клетки, имитируя естественную инфекцию, что способствует per se развитию сильных анти-генспецифических клеточных и гуморальных иммунных реакций, тем самым устраняя потребность в использовании адъювантов. Кроме того, вирусные векторы способны включать в свой геном большие вставки, что обеспечивает гибкость данной платформы с точки зрения выбора антигенов. Однако, помимо преимуществ, данный подход к разработке вакцин имеет несколько недостатков. По сравнению с другими видами вакцин производство вирусных векторных вакцин подразумевает дополнительные трудности, связанные с этапами оптимизации клеточных систем, а также избавления от контаминации, значительно вли- яющей на эффективность вирусных векторов [57]. Другая проблема состоит в том, что геном рекомбинантного вируса с некоторой долей вероятности может интегрироваться в человеческий геном, поэтому перед началом клинических исследований необходимо проводить дополнительную оценку биологической безопасности данного вектора. Наконец, при изучении вирусного вектора, обладающего способностью инфицировать людей в общей популяции, наличие предсуществующего иммунитета к данному вектору может ослаблять вакцинно-индуцированный иммунный ответ. Подобные эффекты были продемонстрированы в работах, посвященных исследованию вакцин, сконструированных на основе аденовирусов и вируса кори [72,73].
Подобно ДНК-вакцинам и субъединичным вакцинам, большинство вирусных векторных вакцин против коронавирусной инфекции нацелены на S-антиген. Для создания вакцин против SARS-CoV и MERS-CoV были протестированы многочисленные вирусные векторы, подробно описанные в ранее опубликованных обзорных статьях [74, 75]. В нижеследующих разделах мы разберем вакцины на основе аденовирусов, модифицированного вируса осповакцины Анкара (MVA) и вируса венесуэльского энцефалита лошадей, которые являются наиболее хорошо изученными вирусными векторными платформами для создания вакцин против коронавирусной инфекции. Мы также кратко опишем другие рекомбинантные вирусные векторы, которые активно тестируются в качестве основы вакцин для профилактики коронавирусной инфекции.
Вирусные векторные вакцины против SARS-CoV. Векторные вакцины на основе аденовирусов широко распространены и успешно протестированы в клинических исследованиях как средство профилактики большого количества заболеваний. Эффективность вакцин на основе аденовирусов изучалась несколькими группами исследователей также и в контексте профилактики инфекции, вызванной SARS-CoV. Возможность создания аденовирусной векторной вакцины против SARS была впервые продемонстрирована в двух исследованиях Gao et al. и Liu et al. [76, 77]. Они показали, что аденовирусный вектор, экспрессирующий субъединицу S1, может индуцировать синтез нейтрализующих антител у обезьян и крыс, но ни в одном из исследований не был продемонстрирован защитный эффект in vivo после инфицирования SARS-CoV [76, 77]. Позже See et al. сравнили эффективность аденовирусной вакцины, экспрессирующей S-белок, и цельновирионной инактивированной вакцины против SARS-CoV [78]. Они обнаружили, что обе вакцины индуцируют развитие защитных реакций у мышей в ответ на инфицирование SARS-CoV, но гуморальный иммунный ответ при использовании векторной вакцины был выражен слабее по сравнению с эффектом инактивированной вакцины [78]. Кроме того, Kobinger et al. также протестировали прайм-буст иммунизацию на хорьках, которым вводили экспрессирующие S-белок векторы на основе аденовируса человека 5-го типа и аденовирусов шимпанзе [79]. Полученные ими данные показали, что такая иммунизация иммунизация приводит к существенному снижению вирусной нагрузки и риска развития пневмонии у хорьков после инфицирования SARS-CoV [79]. Итоговые результаты стали надежным подспорьем для дальнейшей разработки вакцин против MERS и COVID-19 на основе аденовирусов.
Еще одной хорошо зарекомендовавшей себя платформой для создания вакцин в условиях возникающих инфекций является модифицированный вирус осповакцины Анкара (MVA) [80]. Bisht et al. было показано, что интраназальная или внутримышечная иммунизация высокоаттенуированным МVА, содержащим ген полноразмерного S-белка, индуцирует как образование нейтрализующих антител, так и развитие защитного иммунитета у мышей, что подтверждается снижением количества вирусных частиц в легких мышей после инфицирования SARS-CoV [81]. Другое исследование, проведенное Chen et al., пока- зало, что рекомбинантный MVA, экспрессирующий S-белок SARS-CoV, инициирует продукцию нейтрализующих антител у мышей, хорьков и обезьян, но в ходе этой работы не проводились эксперименты для проверки защитного эффекта вакцины на основе MVA [82]. Однако, еще два исследования, проведенные Weingartl et al. и Czub et al. показали, что вакцина на основе MVA, экспрессирующая S-белок SARS-CoV, не оказывает защитного действия после иммунизации хорьков и даже индуцирует воспалительные реакции и очаговые некрозы в печени [83, 84]. Это демонстрирует важность учета потенциальных побочных эффектов при разработке S-белковой вакцины против SARS-CoV на основе MVA.
При анализе вакцины против SARS-CoV, сконструированной на основе вируса венесуэльского энцефалита лошадей (VEE), исследовательской группой Deming et al. было выявлено, что репликон-несущие вирусоподобные частицы VEE (VRP), экспрессирующие S-белок, обеспечивают кратковременную и долгосрочную защиту от гомологичных вариантов вируса у молодых и стареющих мышей [85]. Чтобы еще больше повысить эффективность VEE вакцины при инфицировании гетерологичным вариантом SARS-CoV, Sheahan et al. повысили иммуногенность S-белковой VRP вакцины путем замены гликопротеина аттенуированного VEE его аналогом дикого типа. Результаты подтвердили, что улучшенная S-белковая VRP вакцина дает защитный эффект при инфицировании старых мышей гетерологичными вариантами SARS-CoV [86].
Помимо MVA и VEE, еще несколько вирусных векторов рассматриваются в качестве основы для вакцин против SARS-CoV. В своих работах Buchholz et al. и Bukreyev et al. использовали аттенуированный вирус парагриппа в качестве вектора для экспрессии S-белка SARS-CoV, показав, что вакцина на основе вируса парагриппа может индуцировать синтез нейтрализующих антител и возникновение защитного эффекта у хомяков и обезьян при инфицировании SARS-CoV [30, 87].
Кроме того, Kapadia et al. в качестве вектора для вакцины против SARS-CoV протестировали аттенуированный вирус везикулярного стоматита (VSV) [88]. Их данные демонстрируют, что иммунизация рекомбинантным, экспрессирующим S-белок VSV может вызывать продукцию SARS-нейтрализующих антител у мышей и оказывает защитный эффект против инфекции, вызванной SARS-CoV [88].
Вирусные векторные вакцины против MERS-CoV. Несколько вакцин против MERS-CoV в своей основе имеют аденовирусный вектор. Было показано, что аденовирусы человека типов 5 (Ad5) и 41 (Ad41), экспрессирующие S- или S1-белок MERS-CoV, индуцируют выработку нейтрализующих антител у мышей [89, 90]. Однако в этих работах отсутствует оценка степени развития защитного эффекта после вакцинации [89, 90]. В другом исследовании вакцина Ad5-MERS-S тестировалась в комплексе с S-белковыми наночастицами [91]. Гетерологичная иммунизация в режиме прайм-буст посредством введения Ad5/MERS и последующего введения наночастиц спай-кового белка привела не только к возникновению защитного эффекта против MERS-CoV у hDPP4-трансдуцированных мышей, но и вызвала более сбалансированный с точки зрения соотношения Th1/Th2 иммунный ответ, нежели прайм-буст иммунизация только Аd5 или наночастицами [91]. Кроме того, вектор Ad5 уже используется при разработке вакцины против SARS-CoV-2, и результаты I и II фаз клинических исследований выглядят обнадеживающе [92, 93].
Для решения проблемы снижения эффективности иммунизации из-за наличия у пациентов иммунитета к человеческим аденовирусам в качестве вирусного вектора был использован аденовирус шимпанзе. Так, было показано, что S-белковая вакцина против MERS-CoV на основе вектора аденовируса шимпанзе (ChAdOxl) индуцирует выработку высоких концентраций нейтрализующих антител и развитие клеточно-опосредованного иммунитета у мышей, а также защищает hDPP4-трансдуцированных мышей при заражении летальной дозой MERS-CoV [94, 95]. Помимо прочего, было показано, что вакцина ChAdOxl-MERS также снижает вирусную нагрузку у одногорбых верблюдов и обеспечивает развитие защитного иммунитета у макак-резусов [96, 97]. Ввиду перспективности результатов доклинических исследований вакцина ChAdOxl-MERS вступила в фазу I клинических исследований, показавших, что ChAdOxl-MERS безопасна и хорошо переносится во всех протестированных дозах, и ее однократное введение способно индуцировать как гуморальные, так и клеточные иммунные реакции против MERS-CoV [98]. Та же исследовательская группа использовала платформу ChAdOxl для разработки вакцины против SARS-CoV-2, и их продукт AZD1222 (или ChAdOxl-nCoV-19) в настоящее время является наиболее перспективным среди вакцин против COVID-19 [99].
Сообщалось, что вакцина на основе модифицированного вируса осповакцины Анкара (MVA), экспрессирующего полноразмерный S-белок MERS-CoV, индуцирует не только выработку вируснейтрализующих антител и специфичный для MERS-CoV CD8+ Т-клеточ-ный ответ, но и обеспечивает защитный эффект против MERS-CoV у DPP4-трансду-цированных мышей [100]. Кроме того, у одногорбых верблюдов, иммунизированных MVA вакциной на основе S-белка MERS-CoV, происходит синтез нейтрализующих антител, и при этом наблюдается сниженное выделение вирусных частиц после заражения MERS-CoV [101]. Поскольку верблюд является основным природным резервуаром MERS-CoV, эта вакцина позволяет эффективно контролировать передачу вируса от верблюда человеку [101]. Наконец, I фаза клинических исследований показала, что S-белковая вакцина MVA-MERS имеет благоприятный профиль безопасности, а гомологичная прайм-буст иммунизация вакциной MVA-MERS-S индуцирует гуморальный и клеточно-опосредованный иммунный ответ против MERS-CoV, что позволяет перейти к тестированию вакцины MVA-MERS-S на большей группе людей [102].
Еще одним объектом исследований стали вакцины против MERS-CoV, сконструированные на основе вируса венесуэльского энцефалита лошадей (VEE). Agnihothram et al. продемонстрировали, что репликон-несущие вирусоподобные векторные частицы VEE, синтезирующие S-белок MERS-CoV, могут индуцировать синтез нейтрализующих антител у молодых и старых мышей [103]. Еще одно исследование, проведенное Zhao et al. установило, что вакцина на основе VRP, синтезирующих N-белок MERS, может индуцировать опосредованный клетками CD4+ иммунный ответ и обеспечивать формирование защитного иммунитета против MERS-CoV у hDPP4-трансду-цированных мышей [28]. Поскольку N-белок коронавирусов более консервативен, чем S-белок, такой подход обладает потенциалом для разработки универсальной вакцины для профилактики коронавирусной инфекции [28].
Для разработки вакцины против MERS-CoV были задействованы и другие платформы. В частности, было показано, что S-белковые вакцины против MERS-CoV на основе вирусов кори и бешенства индуцируют образование нейтрализующих антител и оказывают защитный эффект против MERS-CoV у hDDP4-трансдуцированных мышей [104, 105]. Векторы на основе вирусов псевдочумы птиц и везикулярного стоматита также использовались в качестве основы для вакцин, экспрессирующих S-белок MERS [106, 107]. Однако в статьях были описаны только данные о нейтрализации вируса in vitro , но не о формировании защитного иммунитета in vivo после введения этих вакцин [106, 107].
Таким образом, было показано, что вакцины против SARS-CoV и MERS-CoV, основанные на вирусных векторах, включая аденовирусы, модифицированный вирус осповакцины Анкара, вирус венесуэльского энцефалита лошадей, вирус парагриппа, вирус везикулярного стоматита, вирус кори и вирус бешенства, вызывают формирование защитного иммуните- та против вирусной инфекции. Некоторые из этих вирусных векторов уже рассматриваются как перспективные платформы для разработки вакцины против SARS-CoV-2.
Цельновирионные инактивированные вакцины. Цельновирионные инактивированные вакцины состоят из химически или радиационно инактивированных вирусных частиц. Они содержат полный состав иммуногенных компонентов исходного вируса, но по сравнению с аттенуированными вирусами при условии правильной инактивации для них не свойственен риск реактивации. Хотя они безопаснее вакцин, содержащих живые аттенуированные вирионы, структура иммуногенных эпитопов инактивированных вирусов может быть деформирована в процессе инактивации, что, в свою очередь, ослабляет обеспечиваемый ими защитный эффект. Более того, есть данные, что введение цельновирионных инактивированных вакцин как против SARS-CoV, так и против MERS-CoV приводит к развитию эозинофильных патологических процессов в легких [36, 37]. Данный недостаток цельновирионных инактивированных вакцин снижает их привлекательность для исследователей, занимающихся разработкой вакцины для профилактики коронавирусной инфекции.
На ранних этапах разработки вакцин против SARS-CoV ведущим подходом оставалась иммунизация посредством введения инактивированного вируса. Исследования показали, что инактивированный двойным воздействием ультрафиолетового излучения и формальдегида SARS-CoV обладает способностью вызывать образование нейтрализующих антител. К тому же в ходе фазы I клинических исследований вакцины, содержащей частицы SARS-CoV, инактивированные β-пропиолакто-ном, были показаны ее безопасность, хорошая переносимость и способность вызывать продукцию SARS-CoV-специфических нейтрализующих антител [108-110]. Тем не менее, более поздние исследования показали, что введение мышам дважды инактивированной вакцины против SARS-CoV, как содержащей алюминий в качестве адъюванта, так и без него, не обеспечивает формирование необходимого иммунитета и индуцирует эозинофильную воспалительную реакцию в легких после инфицирования [36]. Тот же побочный эффект был обнаружен и у инактивированной гамма-излучением вакцины против MERS-CoV, включающей в состав такие адъюванты, как алюминий или MF59, несмотря на ее способность индуцировать выработку нейтрализующих антител [111]. Данные результаты значительно ослабили энтузиазм ученых в отношении инактивированных вакцин против коронавирусной инфекции. Тем не менее, два недавних исследования выявили, что введение инактивированного ультрафиолетом вируса SARS-CoV в сочетании с агонистами Toll-подобных рецепторов в качестве адъюванта и формальдегид-инактивированного вируса MERS-CoV с адъювантом из смеси алюминия и неметилированных CpG позволяет уменьшить вероятность или полностью предотвратить развитие патологии легких с Тһ2-поляризованным иммунным ответом после заражения вирусом [38, 112]. Эти результаты продемонстрировали, что при условии подбора удачной комбинации адъювант/ способ инактивации использование такой платформы как инактивированный вирус дает возможность создания конкурентоспособной вакцины против коронавирусной инфекции.
Живые аттенуированные вакцины. Живые аттенуированные вакцины содержат живые вирусы, ослабленные путем удаления или мутационной модификации патогенной части вирусного генома. Подобно инактивированным вакцинам они обладают почти всеми иммуногенными свойствами нативного вируса. Кроме того, они сохраняют нативную конформацию вирусных антигенов и презентируют антигены иммунной системе таким же образом, как это происходит при естественном течении инфекции. Поэтому живые аттенуированные вакцины являются наиболее иммуногенным типом вакцин и долгое время успешно используются в борьбе с различными инфекционными заболеваниями [113]. Однако иммунизация живыми аттенуированными вакцинами по сравнению с другими типами вакцин сопряжена с более высоким риском, который заключается в возможной реверсии вируса в вирулентное состояние, а также в опасности развития персистирующей инфекции у пациентов с ослабленным иммунитетом. Таким образом, биологическая безопасность живых аттенуированных вакцин должна быть тщательно исследована и оценена перед переходом к применению в медицинской практике.
Несмотря на то, что в отношении нескольких живых аттенуированных вакцин против SARS-CoV и MERS-CoV была показана эффективность на животных моделях, ни одна из них еще не прошла клинических исследований [114-117]. Оболочечный белок Е, помимо своей структурной функции, играет важную роль в активации инфламмасом, и считается, что он участвует в развитии усиленной воспалительной реакции в легких [118]. Соответственно, делеция в гене оболочечного белка может привести к снижению вирулентности коронавируса [119]. По данным Lamirande et al., мутантные варианты SARS-CoV, лишенные гена оболочечного белка, могут обеспечивать защиту хомяков от инфекции, вызванной SARS-CoV [114]. Кроме того, еще одной возможной мишенью для создания вакцины против коронавирусной инфекции является неструктурный белок 16 (nsp16). Nsp16 кодирует ген 2'-O-рибоза метилтрансферазы, фермента, необходимого для 5'-кэпирова-ния вирусной РНК [120]. Это метилирование помогает коронавирусу избежать узнавания клетками иммунной системы, что обычно приводит к синтезу интерферона I типа и последующей активации механизмов врожденного иммунитета. Следовательно, делеция по гену nsp16 приводит к снижению вирулентности вируса [120]. По данным исследований, вакцины, содержащие мутантные по гену nsp16 разновидности как SARS-CoV, так и
MERS-CoV, способны обеспечивать защиту от коронавирусной инфекции [115, 116]. Другой подходящей целевой структурой для создания живой аттенуированной вакцины против коронавирусной инфекции является неструктурный белок 14 (nsp14), который кодирует экзорибонуклеазу (ExoN), участвующую в редактировании цепочки РНК во время репликации [121]. Потеря гена ExoN приводит к значительному снижению точности репликации и, соответственно, к ослаблению патогенных свойств коронавируса [121]. Graham et al. показали, что делеция в гене ExoN снижает вирулентность SARS-CoV при заражении молодых, старых и иммунодефицитных мышей, а вакцина на основе ExoN(-) SARS-CoV помогает сформировать иммунитет против коронавирусной инфекции у этих животных [117]. Таким образом, все вышеперечисленные целевые структуры являются потенциальными мишенями для разработки живой аттенуированной вакцины против SARS-CoV-2.
Актуальная информация о разработке вакцин против SARS-CoV-2. По сравнению с SARS и MERS, региональные вспышки которых, как правило, спонтанно угасали сами собой, общемировой масштаб пандемии COVID-19 определил беспрецедентные темпы разработки вакцин. Эта острая потребность в вакцине привела к появлению множества различных подходов к разработке вакцин. Во-первых, в погоне за созданием вакцины против COVID-19 на первое место выходят такие нестандартные подходы как разработка вакцин на основе нуклеиновых кислот или вирусных векторов. Такие вакцины обладают большей предпочтительностью в силу того, что для их разработки необходима только генетическая последовательность вируса, но не сам вирус [122]. Таким образом, для этих подходов характерна высокая адаптивность методик к особенностям нового возбудителя, в то время как их профили безопасности уже были хорошо изучены в ходе недавних вспышек гриппа, геморрагической лихорадки Эбола и лихорадки Зика [57]. Во-вторых, процесс клинических исследований при разработке вакцины против COVID-19 был ускорен за счет проведения параллельных исследований, а не последовательного проведения отдельных этапов. Например, многие вакцины-кандидаты против COVID-19 вошли в фазу клинических исследований еще до получения доклинических данных на животных моделях, а в ходе клинических исследований некоторых вакцин фазы I/II или II/III были совмещены для экономии времени [123]. Что немаловажно, чтобы удовлетворить мировую потребность в большом количестве доз вакцин против COVID-19, компании-разработчики вакцин, в частности ведущие, нарастили свои производственные мощности до объема в примерно 1 миллиард доз в год [124-126]. Правительства Соединенных Штатов Америки и ряда других стран также вносят важный вклад в обеспечение населения вакцинами путем финансирования расширения производств потенциально эффективных вакцин [127-129].
В данном разделе мы обсудим актуальные результаты доклинических и клинических исследований вакцин против COVID-19 (по состоянию на 26 октября 2020 года). Мы опишем репрезентативную выборку вакцин против COVID-19, составленную из представителей каждой крупной технологической платформы, по которым имеются опубликованные клинические данные (таблица 2).
Субъединичные (пептидные) вакцины. К настоящему времени в клинические исследования вошли 13 субъединичных вакцин против SARS-CoV-2 [130]. Среди них ведущей является вакцина NVX-CoV2373 компании Novavax, которая вступила в IIб фазу клинических исследований в Южной Африке (NCT04533399) и III фазу клинических исследований в Великобритании (2020-004123-16). NVX-CoV2373 содержит префузионно стабилизированный полноразмерный спайковый белок вируса в комплексе с запатентованным компанией адъювантом на основе сапонина [131, 132]. По результатам доклинических исследований вакцина успешно индуциро- вала продукцию нейтрализующих антител и предотвращала репликацию вируса в дыхательных путях у макак, зараженных вирусом [131]. Вакцина также индуцировала синтез связывающих и нейтрализующих антител у всех участников I фазы исследования [132]. В ходе фазы I исследователи также наблюдали снижение эффективной дозы вакцины при добавлении адъюванта. Ими было обнаружено, что обе схемы вакцинации с дозами в 5 и 25 мкг в присутствии адъюванта индуцировали значительно более высокие титры нейтрализующих антител по сравнению с группой плацебо и группой, вакцинированной дозой в 25 мкг без адъюванта. Другая вакцина, вошедшая в фазу II исследований, – это рекомбинантная вакцина против коронавирусной инфекции компании Anhui Zhifei Longcom (NCT04466085). Вместо полноразмерного S-белка вакцина Anhui Zhifei Longcom содержит только RBD-домен S-белка SARS-CoV-2. Однако никаких дополнительных данных по этой вакцине до сих пор представлено не было. Большинство других субъединичных вакцин-кандидатов против SARS-CoV-2 также содержат либо полноразмерный S-белок, либо RBD-домен S-белка в качестве своего вакцинного антигена. В недавнем исследовании также была описана обобщенная стратегия повышения иммуногенности субъединичных вакцин против COVID-19 [133]. Исследователи изучили дисульфид-связанную димерную форму RBD-домена MERS, которая обладает значительно большей иммуногенностью и способностью вызывать более выраженный иммунный ответ, чем его обычный мономерный аналог. Применив ту же стратегию к SARS-CoV-2, они продемонстрировали десятистократное увеличение титров нейтрализующих антител [133]. Существует вероятность, что подобные иммуногенные структуры могут быть универсально использованы во всех субъединичных вакцинах против коронавирусной инфекции в будущем.
ДНК-вакцины. В настоящее время в стадии клинических исследований находятся четыре
ДНК-вакцины против SARS-CoV-2 [130]. Среди их разработчиков ведущей является компания Inovio, опубликовавшая результаты по ДНК-вакцинам против MERS-CoV и SARS-CoV-2. ДНК-вакцина Inovio против SARS-CoV-2 (INO-4800) содержит ген полноразмерного S-белка и вводится внутрикожно с помощью ручного устройства CELLECTRA для электропорации клеток кожи [70, 134]. Имея предшествующий опыт проведения I/IIa фаз клинических исследований вакцины против MERS (INO-4700), компания использует ту же технологическую платформу для разработки вакцины против SARS-CoV-2 (INO-4800) [69, 70]. Было продемонстрировано, что вакцина индуцирует продукцию нейтрализующих антител и вызывает Th1-поляризованные иммунные реакции на животных моделях, в том числе у мышей, морских свинок и макак-резусов [70, 135]. Вакцина в настоящее время находится в двух исследованиях I/II фазы (NCT04447781 и NCT04336410). Согласно данным промежуточного анализа двух исследований фазы I, вакцина вызывала развитие гуморальных и Т-клеточных иммунных реакций у 94% участников после введения двух доз, при этом степень тяжести нежелательных явлений не превышала легкую [136].
РНК-вакцины. Несмотря на то, что за последние два десятилетия не было проведено ни одного исследования РНК-вакцин против SARS-CoV или MERS-CoV, с момента начала эпидемии COVID-19 в фазу клинических исследований вошли шесть новых РНК-вакцин против SARS-CoV-2 [130]. РНК-вакцины состоят из вирусных антиген-кодирующих мРНК, которые могут быть транслированы клетками человека в антигенные белки для стимуляции иммунной системы. Для повышения эффективности РНК-вакцины обычно доставляются в клетку в комплексе с дополнительными агентами, такими как протамин или наночастицы на основе липидов и полимеров [137]. Подобно ДНК-вакцинам, РНК-вакцины могут быть легко адаптированы к новым патогенам и позволяют воспроизводить нативные конформацию и модификации вирусных белков. Однако по сравнению с ДНК-вакцинами РНК-вакцины имеют некоторые дополнительные преимущества. В отличие от ДНК, РНК вируса не взаимодействует с геномом клетки-хозяина, что, следовательно, устраняет риск геномной интеграции. Кроме того, РНК-вакцины могут быть введены несколькими путями, включая традиционную внутривенную инъекцию, в то время как для введения ДНК-вакцин необходимы специальные техники и устройства, такие как электропорация или генная пушка. Тем не менее, РНК-вакцины имеют и некоторые недостатки. Экзогенная РНК может активировать интерферон-опосредованный противовирусный иммунный ответ и, соответственно, привести к остановке трансляции и деградации мРНК, что значительно снижает эффективность РНК-вакцин [138]. Кроме того, действие интерферона связано с процессами воспаления и развития аутоиммунных реакций [139]. Несмотря на то, что до текущего момента не было зарегистрировано случаев серьезных аутоиммунных заболеваний, спровоцированных РНК-вакцинами, исследователям необходимо внимательно оценивать риски появления побочных эффектов.
Moderna и BioNTech/Pfizer являются двумя ведущими разработчиками РНК-вакцин против SARS-CoV-2. Вакцина мРНК-1273 Moderna кодирует тример спайкового белка вируса, в котором аминокислоты в позициях 986 и 987 заменены пролином для стабилизации префузионной конформации белка [140]. Нуклеотиды в мРНК также модифицированы таким образом, чтобы одновременно усилить трансляцию и увеличить период полужизни, а также чтобы избежать активации интерфе-рон-ассоциированных генов при проникновении мРНК в клетку [140]. Предварительные данные по фазе I клинических исследований показали, что: (1) у всех 45 пациентов пациентов после двухэтапной иммунизации были обнаружены нейтрализующие антитела; (2) титры антител в сыворотке иммунизированных пациентов после вакцинации двумя доза- ми были выше, чем в сыворотке реконвалесцентов; (3) у иммунизированных пациентов наблюдался Th1-поляризованный иммунный ответ [140]. Было зарегистрировано несколько случаев развития нежелательных явлений системного характера после введения второй дозы, однако ни одного случая нежелательных явлений 4-й степени тяжести зафиксировано не было [140]. Исследователи пришли к выводу, что доза 100 мкг обеспечивает удовлетворительный иммунный ответ, и поэтому эта доза будет тестироваться в клинических исследованиях III фазы (NCT04470427) [140]. Кроме того, они расширили исследования фазы I, включив в них 40 участников в возрасте старше 55 лет [141]. Результаты показали, что доза мРНК-1273 100 мкг индуцировала более высокие титры агглютинирующих и нейтрализующих антител, чем доза 25 мкг, а связанные с мРНК-1273 нежелательные явления у этих участников старшего возраста были легкими или умеренными [141]. 16 ноября 2020 года Moderna опубликовала первые результаты промежуточного анализа III фазы клинических исследований (NCT04470427) [142]. По итогам анализа данных 95 человек, у которых проявились симптомы COVID-19 после добровольного участия в исследовании, только 5 входили в группу вакцинированных мРНК-1273, а остальные 90 получили плацебо. Эффективность вакцины, таким образом, составила 94,5% [142]. Кроме того, все 11 добровольцев, у которых развились тяжелые симптомы COVID-19, относились к группе плацебо, то есть ни один из них не был вакцинирован мРНК-1273 [142]. Проведенная параллельно оценка профиля безопасности вакцины также не выявила каких-либо серьезных проблем с биологической безопасностью [142]. Суммируя все вышесказанное, результаты клинических исследований показали, что вакцина мРНК-1273 безопасна и эффективна для предотвращения клинически выраженного заболевания, вызванного SARS-CoV-2.
Вакцина BioNTech и Pfizer имеет 4 варианта: BNT162b1, BNT162b2, BNT162a1 и
BNT162c2. Варианты BNT162b1 и BNT162b2 являются нуклеозид-модифицированными мРНК (modRNA) вакцинами [143]. BNT162b1 кодирует тример RBD-домена спайково-го белка, в то время как BNT162b2 кодирует полноразмерный спайковый белок [143]. В отличие от них BNT162a1 - это вакцина на основе мРНК, содержащей уридин (uRNA), а BNT162c2 – вакцина на основе самоамплифи-цирующейся мРНК (saRNA) [143]. К текущему моменту BioNTech и Pfizer опубликовали два отчета по I/II фазе клинических исследований BNT162b1, которые были проведены в Германии (NCT04380701) и США (NCT04368728), соответственно [144, 145]. Оба исследования показали, что двухэтапная схема введения BNT162b1 обеспечивала синтез RBD-до-мен-связывающих и нейтрализующих антител в более высоких, чем в реконвалесцентной сыворотке, титрах [144, 145]. В отношении клеточно-опосредованного иммунитета было показано, что у большинства участников развивался Th1-поляризованный ответ, о чем свидетельствовало выявление в сыворотке иммунизированных повышенных концентраций интерферона гамма, интерлейкина-2 и интерлейкина-12, но не интерлейкина-4 [144, 145]. Хотя немецкое и американское исследования тестировали разные дозы вакцины, их результаты хорошо согласуются друг с другом, демонстрируя, что вакцинирование дозой 3050 мкг на 1-й и 22-й день индуцирует развитие благоприятного иммунного ответа без серьезных побочных эффектов [144, 145]. Вслед за этими двумя работами было опубликовано еще одно исследование, сравнивающее ответ на вакцинацию BNT162b1 и BNT162b2 [146]. В нем описывалось, что BNT162b1 и BNT162b2 индуцируют продукцию сопоставимых уровней нейтрализующих антител у молодых людей и людей старшего возраста [146]. Однако для BNT162b2 характерна меньшая частота системных реакций у лиц старшего возраста [146]. Поэтому разработчики выбрали именно BNT162b2, а не BNT162b1 для проведения III фазы клинических исследований
(NCT04368728). 18 ноября 2020 года Pfizer и BioNTech предоставили результаты анализа эффективности по итогам III фазы клинических исследований (NCT04368728) после достижения первичных конечных точек, отражающих эффективность вакцины [147]. Их оценка показала, что эффективность BNT162b2 против COVID-19 составляет 95% [147]. Этот коэффициент основан на анализе 170 подтвержденных случаев COVID-19, из которых 162 случая наблюдались в группе плацебо, и только 8 - в группе иммунизированных BNT162b2 [147]. Кроме того, из 10 случаев COVID-19 тяжелого течения, наблюдавшихся в этом исследовании, 9 развились у участников группы плацебо и только 1 был зарегистрирован в группе BNT162b2 [147]. Следует отметить, что у пожилых людей наблюдаемая эффективность составила более 94%, что означает способность вакцины защитить от COVID-19 наиболее уязвимую часть населения [147]. В ходе исследований, включивших 43000 участников, серьезных проблем, связанных с безопасностью вакцины, выявлено не было [147]. Эти данные указывают на то, что BNT162b2 является еще одной хорошо переносимой и эффективной вакциной против COVID-19.
AZD1222 – это вирусная векторная вакцина (ChAdOx1) на основе аденовируса шимпанзе, экспрессирующая спайковый белок SARS-CoV-2 [99]. Платформа ChAdOx1 была использована для разработки вакцины против MERS-CoV, и результаты ее доклинических и клинических исследований I фазы выглядят многообещающе [94-98]. Команда исследователей, занимающихся разработкой вакцины AZD1222, в июле 2020 года опубликовала свой промежуточный отчет по результатам фазы I/II клинических исследований и продемонстрировала, что AZD1222 может вызывать продукцию антител к S-белку и Т-клеточный иммунный ответ, а также индуцировать синтез нейтрализующих антител у всех участников после вакцинации в режиме прайм-буст [99]. Серьезных побочных эффектов при этом не наблюдалось [99]. Основываясь на этих обнадеживающих данных, разработчики инициировали II/III фазу исследований AZD1222 в Великобритании (2020-00122832) и III фазу в Бразилии (ISRCTN89951424), США (NCT04516746), России (NCT04540393) и Индии (CTRI/2020/08/027170). В сентябре
Цельновирионные инактивированные вакцины . В настоящее время в процессе клинических исследований находится 7 цельновирионных инактивированных вакцин против COVID-19 [130]. По данным предшествующего опыта разработки вакцин против SARS-CoV и MERS-CoV, после введения инактивированного вируса в числе побочных эффектов могут быть эозинофильные иммунопатологические процессы в легких, что было показано в доклинических исследованиях [36, 37]. Несмотря на то, что пока не было зарегистрировано никаких серьезных побочных эффектов применения инактивированных вакцин против COVID-19, важно, чтобы исследовательское сообщество помнило об уже имеющихся прецедентах и тщательно оценивало наличие возможных нежелательных явлений. Что касается изучения инактивированных вакцин против COVID-19, на данный момент три команды исследователей опубликовали данные своих клинических исследований. Компания SinoVac Inc. разработала вакцину CoronaVac (также известную как PiCoVacc), которая представляет собой цельновирионную вакцину на основе полученной от пациента разновидности SARS-CoV-2. Вакцина синтезирована клетками клеточной линии Vero и инактивирована β-пропиолактоном [153]. По данным доклинических исследований, PiCoVacc индуцирует широкий спектр нейтрализующих антител против 10 репрезентативных вариантов
SARS-CoV-2 у мышей, крыс и обезьян [153]. Иммунизация макак тремя дозами PiCoVacc обеспечивает развитие у них защитного иммунитета от SARS CoV-2, не вызывая эффекта антителозависимого усиления инфекции [153]. Вслед за доклиническими исследованиями вакцины CoronaVac были проведены два исследования I/II фазы (NCT04383574 и NCT04352608, результаты еще не опубликованы). В настоящее время начинается III фаза клинических исследований в Бразилии (NCT04456595), Индонезии (669/UN6.KEP/ EC/2020) и Турции (NCT04582344). Кроме того, Sinopharm Inc. и Институт биологических продуктов г. Ухань разработали другую инактивированную вакцину против COVID-19 (без зарегистрированного названия). Для создания этой вакцины вариант WIV04 был выделен у пациента с COVID-19 из г. Ухань, а затем разработчики осуществили размножение вируса в клетках линии Vero и его двукратную инактивацию β-пропиолактоном [154]. Исследователи протестировали три различные дозы и три различных схемы иммунизации в ходе фаз I и II клинических исследований. Промежуточный отчет по результатам фазы I/ II показал, что у всех пациентов, вакцинированных различными дозами препарата, наблюдался синтез нейтрализующих антител при низкой частоте побочных реакций [154]. В настоящее время стартовали клинические исследования III фазы в Объединенных Арабских Эмиратах (ChiCTR2000034780) и Кувейте (ChiCTR2000039000). Помимо прочего, Sinopharm Inc. в сотрудничестве с Институтом биологических продуктов г. Пекин разработали еще одну инактивированную вакцину против COVID-19 – BBIBP-CorV [155, 156]. Процесс производства BBIBP-CorV очень схож с описанным выше в отношении другой вакцины Sinopharm Inc., за исключением того, что в случае BBIBP-CorV использован вариант коронавируса HB02, а не WIV04 [155]. Разработчики протестировали BBIBP-CorV на доклинических моделях и показали, что двухэтапная иммунизация BBIBP-CorV оказы- вает защитный эффект при инфицировании макак-резусов SARS-CoV-2 [155]. После этого было проведено исследование I/II фазы, в ходе которого было продемонстрировано, что данная вакцина безопасна и хорошо переносится участниками двух возрастных групп во всех тестируемых дозах [156]. Полученные данные также свидетельствуют о том, что вакцина демонстрирует хорошую иммуногенность и обеспечивает развитие гуморального иммунного ответа против SARS-CoV-2 у всех участников исследования через 42 дня после иммунизации [156]. В настоящее время эта вакцина проходит III фазу клинических исследований в Объединенных Арабских Эмиратах (ChiCTR2000034780) и Аргентине (NCT04560881).
Другие платформы для создания вакцин. Существует также несколько вакцин-кандидатов против COVID-19, для создания которых были использованы технологии, отличные от упомянутых выше. Вакцины на основе вирусоподобных частиц, показавшие свою эффективность против SARS-CoV и MERS-CoV в доклинических моделях, также тестируются как вакцины-кандидаты против COVID-19. В настоящее время одна из них находится в I фазе клинических исследований (NCT04450004), а еще 14 вакцин-кандидатов находятся в доклинической разработке [130]. Однако ни одна из команд исследователей, разрабатывающих вакцины на основе вирусоподобных частиц, пока не опубликовала результаты исследований. В данный момент известно о трех доклинических исследованиях живых аттенуированных вакцин, которые, как это было показано для SARS-CoV и MERS-CoV, обеспечивают формирование защитных реакций у инфицированных мышей [130]. Повышенный риск развития побочных эффектов делает живую аттенуированную вакцину менее привлекательной платформой, учитывая значимость временного фактора в случае разработки вакцины против COVID-19. Тем не менее, при условии успешной разработки, вакцинация живой аттенуированной вакциной может обеспечить формирование наиболее сильного иммунитета благодаря высокой степени сходства вакцинного вируса с возбудителем заболевания.
Помимо обращения к перечисленным общепринятым технологическим платформам, ученые также занимаются разработкой вакцины против COVID-19 с использованием нестандартных подходов. Компания Aivita Biomedical Inc. разработала вакцину AV-COVID-19, которая содержит аутологичные дендритные клетки, несущие антигены SARS-CoV-2 [157]. Для получения AV-COVID-19 собственные моноциты периферической крови пациента подвергаются in vitro дифференцировке в дендритные клетки и инкубируются с антигенами SARS-CoV-2, после чего возвращаются в кровоток этого же пациента [157]. Компания запустила клиническое исследование фазы I/II для оценки профиля безопасности и эффективности вакцины при иммунизации взрослых (NCT04386252). Другой разработкой является вакцина bacTRL-Spike от Symvivo Corporation, которая представляет собой живую бифидобактериальную вакцину, сконструированную для доставки в организм человека синтетической ДНК-плазмиды, кодирующей S-белок SARS-CoV-2. Вакцина вошла в I фазу клинических исследований для оценки ее безопасности (NCT04334980). Кроме того, группа ученых из Нанкинского университета обнаружила, что растительная микроРНК MIR2911 может взаимодействовать с SARS-CoV-2, избирательно связываясь с мРНК и блокируя процесс трансляции [158]. Их данные показали, что MIR2911 ингибирует репликацию SARS-CoV-2 и сокращает период до получения отрицательных тестов у инфицированных пациентов [158]. После получения таких результатов была начата фаза I клинических исследований (ChiCTR2000031432) в Китае для оценки безопасности и переносимости MIR2911.
Наконец, некоторые группы исследователей тестируют уже существующие лицензированные вакцины и пытаются переориенти- ровать их для борьбы с COVID-19. Так, было показано, что противотуберкулезная вакцина БЦЖ может активировать врожденный иммунитет и индуцировать неспецифический иммунный ответ против вирусных патогенов, включая респираторно-синцитиальный вирус (RSV), вирус гриппа А и вирус простого герпеса 2-го типа (HSV2) [159-162]. Интерес вызывает исследование, в котором был проведен сравнительный анализ последствий пандемии COVID-19 в отдельных странах, по результатам которого авторы выявили их взаимосвязь с особенностями подходов к противотуберкулезной вакцинации [163]. Они обнаружили, что страны, не проводящие всеобщей вакцинации от туберкулеза, пострадали от коронавирусной инфекции сильнее по сравнению со странами, которые в течение многих лет придерживались политики всеобщей противотуберкулезной вакцинации [163]. На основе выводов, сделанных в данном исследовании, было инициировано по меньшей мере 13 клинических исследований III фазы, направленных на изучение возможности снижения заболеваемости и смертности среди медицинских работников после вакцинации БЦЖ (NCT04328441, NCT04327206, NCT04350931, NCT04348370, NCT04362124, NCT04369794, NCT04373291, NCT04379336, NCT04384549, NCT04439045, NCT04387409, NCT04417335, NCT04414267).
Другие аспекты разработки вакцины против SARS-CoV-2. Учитывая быструю передачу и бессимптомное распространение вируса, очевидно, что для возвращения людей к нормальной жизни необходима эффективная вакцина и общемировой охват иммунизацией. Однако даже при условии появления эффективной вакцины против SARS-CoV-2 срок сохранения иммунитета после вакцинации по большому счету остается неизвестным. Предыдущие исследования SARS показали, что SARS-специфичные IgG и нейтрализующие антитела у пациентов, которые перенесли инфекцию, вызванную SARS-CoV, сохранялись только в течение приблизительно двух лет
[164, 165]. Таким образом, формирование постоянного иммунитета в случае вакцин против COVID-19 маловероятно, и в будущем может потребоваться практика регулярной вакцинации. К тому же на настоящий момент не определен минимальный титр нейтрализующих антител, способный обеспечить защитный эффект против SARS-CoV-2. Считается, что чем больше нейтрализующих антител индуцирует вакцинация, тем сильнее будет ее защитный эффект. Это согласуется с наблюдениями, что в большинстве случаев повторного заражения SARS-CoV-2 во время первого заболевания наблюдались только умеренные симптомы, либо заболевание вовсе протекало бессимптомно, и этого было недостаточно для индукции выработки большого количества нейтрализующих антител [166, 167]. Поэтому очень важно, чтобы дальнейшие исследования вакцин против COVID-19 велись с учетом оценки корреляции между количеством нейтрализующих антител и степенью выраженности защитного эффекта после вакцинации. Наконец, что не менее важно, в геноме SARS-CoV-2 были обнаружены различные мутации, наиболее распространенной из которых является мутация D614G [168]. D614G - это миссенс-мутация в гене S-белка, которая увеличивает инфекционные свойства SARS-CoV-2 за счет уменьшения шеддинга (отщепления) S1 субъединицы и повышенного включения S-белка в вирион [169, 170]. При этом в качестве благоприятного обстоятельства следует отметить, что мутация D614G не препятствует связыванию нейтрализующих антител с вирионами SARS-CoV-2 и, таким образом, не снижает эффективность вакцинации [170]. Однако не исключено, что такие «ускользающие» от иммунной системы мутации появятся в будущем и значительно затруднят разработку вакцин для профилактики COVID-19.
Заключительные комментарии. С момента открытия коронавирусов человека в 1960х годах появилось несколько новых видов коронавирусов, которые со временем стали серьезной угрозой для мирового здравоохра- нения. Несмотря на то, что с момента первой вспышки коронавирусной инфекции прошло уже почти два десятилетия, научное и медицинское сообщество оказалось недостаточно подготовленным к эффективной борьбе с этими патогенами. Один из уроков, который мы вынесли, заключается в том, что механизмы финансирования и регулирования фармацевтического рынка на текущий момент не способны стимулировать предварительную разработку вакцин, до того, как произойдет вспышка смертельного заболевания. Чтобы восполнить эти пробелы, в настоящее время научные институты и фармацевтические компании по всему миру разрабатывают огромное количество вакцин-кандидатов и проводят клинические исследования в сжатые сроки. Благоприятным обстоятельством можно считать то, что результаты исследований биологических особенностей и клинической значимости SARS-CoV и MERS-CoV, а также накопленный опыт в разработке вакцин против других инфекций дали прочную опору для создания множества перспективных вакцин-кандидатов. Кроме того, в кратчайшие сроки были исследованы эффекты большого количества лекарственных препаратов, действие которых нацелено на молекулы SARS-CoV-2, необходимые для жизненного цикла вируса, а также на регулирование иммунного ответа против SARS-CoV-2. Ведущими препаратами из этого списка являются ремдесивир и дексаметазон, клинические исследования которых продемонстрировали ускорение выздоровления и снижение летальности при COVID-19 [171, 172]. Эти терапевтические средства, наряду с вакцинацией против SARS-CoV-2, могут быть использованы в составе комплексных мер для смягчения последствий пандемии COVID-19. В заключение мы выражаем надежду, что страны всего мира, независимо от политических идеологий, смогут объединиться и сотрудничать с целью быстрой и успешной разработки вакцин против COVID-19 в ближайшем будущем.
Аббревиатуры: COVID-19: коронавирусная инфекция 2019 года; CoV: коронавирус; SARS-CoV: коронавирус тяжелого острого респираторного синдрома; MERS-CoV: коронавирус ближневосточного респираторного синдрома; SARS-CoV-2: коронавирус тяжелого острого респираторного синдрома 2; pp: полипротеин; Nsps: неструктурные белки; S: шип (спайк); E: оболочка; M: мембрана; N: нуклеокапсид; RBD: рецептор-связыва-ющий домен; ACE2: ангиотензинпревращающий фермент 2; DPP4: дипептидилпептидаза-4; ЭР: эндоплазматический ретикулум; ADE: антите- лозависимое усиление инфекции; NTD: N-концевой домен; в/м: внутримышечная инъекция; п/к: подкожная инъекция; VLP: вирусоподобные частицы; CPV: парвовирус собак; VEE: вирус венесуэльского энцефалита лошадей; VRP: репликон-несущие вирусоподобные частицы; Ad#: аденовирус человека типа #; ChAdOxl: вектор аденовируса шимпанзе; ExoN: экзорибуноклеаза; modRNA: модифицированная РНК; uRNA: РНК, содержащая уридин; saRNA: самоамплифицирующаяся РНК; RSV: респираторно-синцитиальный вирус человека; HSV: вирус простого герпеса.
to 31 July 2003 . Geneva: World-Health-Organization; 2003 .
2020b .
2020 ;586(7830):589-593. DOI: 10.1038/s41586-020-2639-4
Перевод поступил в редакцию: 29.12.2020
Список литературы Разработка вакцин для профилактики коронавирусной инфекции: от SARS и MERS до COVID-19
- Centers‑for‑Disease‑Control‑and‑Prevention. Human Coronavirus Types. 2020. https://www.cdc.gov/coronavirus/types.html.
- van der Hoek L. Human coronaviruses: what do they cause?. Antivir Ther. 2007;12(4 Pt B):651-658.
- World‑Health‑Organization. Summary of probable SARS cases with onset of illness from 1 November 2002 to 31 July 2003. Geneva: World‑Health‑Organization; 2003.
- World‑Health‑Organization. MERS situation update, January 2020. Geneva: World‑Health‑Organization; 2020a.
- Saag MS, Gandhi RT, Hoy JF, et al. Antiretroviral Drugs for Treatment and Prevention of HIV Infection in Adults: 2020 Recommendations of the International Antiviral Society-USA Panel. JAMA. 2020;324(16):1651-1669. DOI: 10.1001/jama.2020.17025
- Li Q, Guan X, Wu P, et al. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumonia. N Engl J Med. 2020;382(13):1199-1207. DOI: 10.1056/NEJMoa2001316
- Gandhi M, Yokoe DS, Havlir DV. Asymptomatic Transmission, the Achilles' Heel of Current Strategies toControl Covid-19. N Engl J Med. 2020;382(22):2158-2160. DOI: 10.1056/NEJMe2009758
- World‑Health‑Organization. Coronavirus-disease (COVID‑19) pandemic. Geneva: World‑Health‑Organization; 2020b.
- Masters PS. The molecular biology of coronaviruses. Adv Virus Res. 2006;66:193-292. DOI: 10.1016/S0065-3527(06)66005-3
- Stadler K, Masignani V, Eickmann M, et al. SARS-beginning to understand a new virus. Nat Rev Microbiol. 2003;1(3):209-218. DOI: 10.1038/nrmicro775
- Enjuanes L, Zuñiga S, Castaño-Rodriguez C, et al. Molecular Basis of Coronavirus Virulence and Vaccine Development. Adv Virus Res. 2016;96:245-286. DOI: 10.1016/bs.aivir.2016.08.003
- NCBI‑Reference‑Sequence. SARS coronavirus Tor2, complete genome. 2020.
- NCBI‑Reference‑Sequence. Middle East respiratory syndrome‑related coronavirus isolate HCoV‑EMC/2012, complete genome. 2020.
- NCBI‑Reference‑Sequence. Severe acute respiratory syndrome coronavirus 2 isolate Wuhan‑Hu‑1, complete genome. 2020.
- Du L, He Y, Zhou Y, et al. The spike protein of SARS-CoV-a target for vaccine and therapeutic development. Nat Rev Microbiol. 2009;7(3):226-236. DOI: 10.1038/nrmicro2090
- Wang N, Shang J, Jiang S, Du L. Subunit Vaccines Against Emerging Pathogenic Human Coronaviruses. Front Microbiol. 2020;11:298. DOI: 10.3389/fmicb.2020.00298
- Snijder EJ, Decroly E, Ziebuhr J. The Nonstructural Proteins Directing Coronavirus RNA Synthesis and Processing. Adv Virus Res. 2016;96:59-126. DOI: 10.1016/bs.aivir.2016.08.008
- Cao Z, Liu L, Du L, et al. Potent and persistent antibody responses against the receptor-binding domain of SARS-CoV spike protein in recovered patients. Virol J. 2010;7:299. DOI: 10.1186/1743-422X-7-299
- Zhong X, Yang H, Guo ZF, et al. B-cell responses in patients who have recovered from severe acute respiratory syndrome target a dominant site in the S2 domain of the surface spike glycoprotein. J Virol. 2005;79(6):3401-3408. DOI: 10.1128/JVI.79.6.3401-3408.2005
- Qiu M, Shi Y, Guo Z, et al. Antibody responses to individual proteins of SARS coronavirus and their neutralization activities. Microbes Infect. 2005;7(5-6):882-889. DOI: 10.1016/j.micinf.2005.02.006
- Tang XC, Agnihothram SS, Jiao Y, et al. Identification of human neutralizing antibodies against MERS-CoV and their role in virus adaptive evolution. Proc Natl Acad Sci U S A. 2014;111(19):E2018-E2026. DOI: 10.1073/pnas.1402074111
- Li Y, Wan Y, Liu P, et al. A humanized neutralizing antibody against MERS-CoV targeting the receptor-binding domain of the spike protein. Cell Res. 2015;25(11):1237-1249. DOI: 10.1038/cr.2015.113
- Li J, Ulitzky L, Silberstein E, et al. Immunogenicity and protection efficacy of monomeric and trimeric recombinant SARS coronavirus spike protein subunit vaccine candidates. Viral Immunol. 2013;26(2):126-132. DOI: 10.1089/vim.2012.0076
- He Y, Li J, Heck S, et al. Antigenic and immunogenic characterization of recombinant baculovirus-expressed severe acute respiratory syndrome coronavirus spike protein: implication for vaccine design. J Virol. 2006;80(12):5757-5767. DOI: 10.1128/JVI.00083-06
- Tai W, Wang Y, Fett CA, et al. Recombinant Receptor-Binding Domains of Multiple Middle East Respiratory Syndrome Coronaviruses (MERS-CoVs) Induce Cross-Neutralizing Antibodies against Divergent Human and Camel MERS-CoVs and Antibody Escape Mutants. J Virol. 2016;91(1):e01651-16. DOI: 10.1128/JVI.01651-16
- Tai W, Zhao G, Sun S, et al. A recombinant receptor-binding domain of MERS-CoV in trimeric form protects human dipeptidyl peptidase 4 (hDPP4) transgenic mice from MERS-CoV infection. Virology. 2016;499:375-382. DOI: 10.1016/j.virol.2016.10.005
- Wang Y, Tai W, Yang J, et al. Receptor-binding domain of MERS-CoV with optimal immunogen dosage and immunization interval protects human transgenic mice from MERS-CoV infection. Hum Vaccin Immunother. 2017;13(7):1615-1624. DOI: 10.1080/21645515.2017.1296994
- Zhao J, Zhao J, Mangalam AK, et al. Airway Memory CD4(+) T Cells Mediate Protective Immunity against Emerging Respiratory Coronaviruses. Immunity. 2016;44(6):1379-1391. DOI: 10.1016/j.immuni.2016.05.006
- He Y, Zhou Y, Siddiqui P, Niu J, Jiang S. Identification of immunodominant epitopes on the membrane protein of the severe acute respiratory syndrome-associated coronavirus. J Clin Microbiol. 2005;43(8):3718-3726. DOI: 10.1128/JCM.43.8.3718-3726.2005
- Buchholz UJ, Bukreyev A, Yang L, et al. Contributions of the structural proteins of severe acute respiratory syndrome coronavirus to protective immunity. Proc Natl Acad Sci USA. 2004;101(26):9804-9809. DOI: 10.1073/pnas.0403492101
- Huisman W, Martina BE, Rimmelzwaan GF, et al. Vaccine-induced enhancement of viral infections. Vaccine. 2009;27(4):505-512. DOI: 10.1016/j.vaccine.2008.10.087
- Kam YW, Kien F, Roberts A, et al. Antibodies against trimeric S glycoprotein protect hamsters against SARSCoV challenge despite their capacity to mediate FcgammaRII-dependent entry into B cells in vitro. Vaccine. 2007;25(4):729-740. DOI: 10.1016/j.vaccine.2006.08.011
- Jaume M, Yip MS, Cheung CY, et al. Anti-severe acute respiratory syndrome coronavirus spike antibodies trigger infection of human immune cells via a pH- and cysteine protease-independent FcγR pathway. J Virol. 2011;85(20):10582-10597. DOI: 10.1128/JVI.00671-11
- Wang SF, Tseng SP, Yen CH, et al. Antibody-dependent SARS coronavirus infection is mediated by antibodies against spike proteins. Biochem Biophys Res Commun. 2014;451(2):208-214. DOI: 10.1016/j.bbrc.2014.07.090
- Rosenthal KS, Zimmerman DH. Vaccines: all things considered. Clin Vaccine Immunol. 2006;13(8):821-829. DOI: 10.1128/CVI.00152-06
- Bolles M, Deming D, Long K, et al. A double-inactivated severe acute respiratory syndrome coronavirus vaccine provides incomplete protection in mice and induces increased eosinophilic proinflammatory pulmonary response upon challenge. J Virol. 2011;85(23):12201-12215. DOI: 10.1128/JVI.06048-11
- Tseng CT, Sbrana E, Iwata-Yoshikawa N, et al. Immunization with SARS coronavirus vaccines leads to pulmonary immunopathology on challenge with the SARS virus. PLoS One. 2012;7(4):e35421. DOI: 10.1371/journal.pone.0035421
- Iwata-Yoshikawa N, Uda A, Suzuki T, et al. Effects of Toll-like receptor stimulation on eosinophilic infiltration in lungs of BALB/c mice immunized with UV-inactivated severe acute respiratory syndrome-related coronavirus vaccine. J Virol. 2014;88(15):8597-8614. DOI: 10.1128/JVI.00983-14
- Honda-Okubo Y, Barnard D, Ong CH, et al. Severe acute respiratory syndrome-associated coronavirus vaccines formulated with delta inulin adjuvants provide enhanced protection while ameliorating lung eosinophilic immunopathology. J Virol. 2015;89(6):2995-3007. DOI: 10.1128/JVI.02980-14
- He Y, Zhou Y, Liu S, et al. Receptor-binding domain of SARS-CoV spike protein induces highly potent neutralizing antibodies: implication for developing subunit vaccine. Biochem Biophys Res Commun. 2004;324(2):773-781. DOI: 10.1016/j.bbrc.2004.09.106
- Du L, Zhao G, Li L, et al. Antigenicity and immunogenicity of SARS-CoV S protein receptor-binding domain stably expressed in CHO cells. Biochem Biophys Res Commun. 2009;384(4):486-490. DOI: 10.1016/j.bbrc.2009.05.003
- Du L, Zhao G, He Y, et al. Receptor-binding domain of SARS-CoV spike protein induces long-term protective immunity in an animal model. Vaccine. 2007;25(15):2832-2838. DOI: 10.1016/j.vaccine.2006.10.031
- Du L, Zhao G, Chan CC, et al. Recombinant receptor-binding domain of SARS-CoV spike protein expressed in mammalian, insect and E. coli cells elicits potent neutralizing antibody and protective immunity. Virology. 2009;393(1):144-150. DOI: 10.1016/j.virol.2009.07.018
- Du L, Zhao G, Chan CC, et al. A 219-mer CHO-expressing receptor-binding domain of SARS-CoV S protein induces potent immune responses and protective immunity. Viral Immunol. 2010;23(2):211-219. DOI: 10.1089/vim.2009.0090
- Guo Y, Sun S, Wang K, et al. Elicitation of immunity in mice after immunization with the S2 subunit of the severe acute respiratory syndrome coronavirus. DNA Cell Biol. 2005;24(8):510-515. DOI: 10.1089/dna.2005.24.510
- Liu SJ, Leng CH, Lien SP, et al. Immunological characterizations of the nucleocapsid protein based SARS vaccine candidates. Vaccine. 2006;24(16):3100-3108. DOI: 10.1016/j.vaccine.2006.01.058.
- Wang L, Shi W, Joyce MG, et al. Evaluation of candidate vaccine approaches for MERS-CoV. Nat Commun. 2015;6:7712. DOI: 10.1038/ncomms8712
- Eyer P, Lierheimer E, Schneller M. Reactions of nitrosochloramphenicol in blood. Biochem Pharmacol. 1984;33(14):2299-2308. DOI: 10.1016/0006-2952(84)90670-1
- Jiaming L, Yanfeng Y, Yao D, et al. The recombinant N-terminal domain of spike proteins is a potential vaccine against Middle East respiratory syndrome coronavirus (MERS-CoV) infection. Vaccine. 2017;35(1):10-18. DOI: 10.1016/j.vaccine.2016.11.064
- Zhang N, Channappanavar R, Ma C, et al. Identification of an ideal adjuvant for receptor-binding domainbased subunit vaccines against Middle East respiratory syndrome coronavirus. Cell Mol Immunol. 2016;13(2):180-190. DOI: 10.1038/cmi.2015.03
- Lan J, Deng Y, Chen H, et al. Tailoring subunit vaccine immunity with adjuvant combinations and delivery routes using the Middle East respiratory coronavirus (MERS-CoV) receptor-binding domain as an antigen. PLoS One. 2014;9(11):e112602. DOI: 10.1371/journal.pone.0112602
- Qian C, Liu X, Xu Q, et al. Recent Progress on the Versatility of Virus-Like Particles. Vaccines. 2020; 8(1):139. DOI: 10.3390/vaccines8010139
- Lokugamage KG, Yoshikawa-Iwata N, Ito N, et al. Chimeric coronavirus-like particles carrying severe acute respiratory syndrome coronavirus (SCoV) S protein protect mice against challenge with SCoV. Vaccine.2008;26(6):797-808. DOI: 10.1016/j.vaccine.2007.11.092
- Liu YV, Massare MJ, Barnard DL, et al. Chimeric severe acute respiratory syndrome coronavirus (SARS-CoV) S glycoprotein and influenza matrix 1 efficiently form virus-like particles (VLPs) that protect mice against challenge with SARS-CoV. Vaccine. 2011;29(38):6606-6613. DOI: 10.1016/j.vaccine.2011.06.111
- Wang C, Zheng X, Gai W, et al. MERS-CoV virus-like particles produced in insect cells induce specific humoural and cellular imminity in rhesus macaques. Oncotarget. 2017;8(8):12686-12694. DOI: 10.18632/oncotarget.8475
- Wang C, Zheng X, Gai W, et al. Novel chimeric virus-like particles vaccine displaying MERS-CoV receptorbinding domain induce specific humoral and cellular immune response in mice. Antiviral Res. 2017;140:55-61. DOI: 10.1016/j.antiviral.2016.12.019
- Rauch S, Jasny E, Schmidt KE, Petsch B. New Vaccine Technologies to Combat Outbreak Situations. Front Immunol. 2018;9:1963. DOI: 10.3389/fimmu.2018.01963
- Wang Z, Troilo PJ, Wang X, et al. Detection of integration of plasmid DNA into host genomic DNA following intramuscular injection and electroporation. Gene Ther. 2004;11(8):711-721. DOI: 10.1038/sj.gt.3302213
- Schalk JA, Mooi FR, Berbers GA, et al. Preclinical and clinical safety studies on DNA vaccines. Hum Vaccin. 2006;2(2):45-53. DOI: 10.4161/hv.2.2.2620
- Yang ZY, Kong WP, Huang Y, et al. A DNA vaccine induces SARS coronavirus neutralization and protective immunity in mice. Nature. 2004;428(6982):561-564. DOI: 10.1038/nature02463
- Kim TW, Lee JH, Hung CF, et al. Generation and characterization of DNA vaccines targeting the nucleocapsid protein of severe acute respiratory syndrome coronavirus. J Virol. 2004;78(9):4638-4645. DOI: 10.1128/jvi.78.9.4638-4645.2004
- Zhao P, Cao J, Zhao LJ, et al. Immune responses against SARS-coronavirus nucleocapsid protein induced by DNA vaccine. Virology. 2005;331(1):128-135. DOI: 10.1016/j.virol.2004.10.016
- Okada M, Okuno Y, Hashimoto S, et al. Development of vaccines and passive immunotherapy against SARS corona virus using SCID-PBL/hu mouse models. Vaccine. 2007;25(16):3038-3040. DOI: 10.1016/j.vaccine.2007.01.032
- Wang Z, Yuan Z, Matsumoto M, et al. Immune responses with DNA vaccines encoded different gene fragments of severe acute respiratory syndrome coronavirus in BALB/c mice. Biochem Biophys Res Commun.2005;327(1):130-135. DOI: 10.1016/j.bbrc.2004.11.147
- Martin JE, Louder MK, Holman LA, et al. A SARS DNA vaccine induces neutralizing antibody and cellular immune responses in healthy adults in a Phase I clinical trial. Vaccine. 2008;26(50):6338-6343. DOI: 10.1016/j.vaccine.2008.09.026
- Zakhartchouk AN, Liu Q, Petric M, Babiuk LA. Augmentation of immune responses to SARS coronavirus by a combination of DNA and whole killed virus vaccines. Vaccine. 2005;23(35):4385-4391. DOI: 10.1016/j.vaccine.2005.04.011
- Woo PC, Lau SK, Tsoi HW, et al. SARS coronavirus spike polypeptide DNA vaccine priming with recombinant spike polypeptide from Escherichia coli as booster induces high titer of neutralizing antibody against SARS coronavirus. Vaccine. 2005;23(42):4959-4968. DOI: 10.1016/j.vaccine.2005.05.023
- Muthumani K, Falzarano D, Reuschel EL, et al. A synthetic consensus anti-spike protein DNA vaccine induces protective immunity against Middle East respiratory syndrome coronavirus in nonhuman primates. Sci Transl Med. 2015;7(301):301ra132. DOI: 10.1126/scitranslmed.aac7462
- Modjarrad K, Roberts CC, Mills KT, et al. Safety and immunogenicity of an anti-Middle East respiratory syndrome coronavirus DNA vaccine: a phase 1, open-label, single-arm, dose-escalation trial. Lancet Infect Dis. 2019;19(9):1013-1022. DOI: 10.1016/S1473-3099(19)30266-X
- Smith TRF, Patel A, Ramos S, et al. Immunogenicity of a DNA vaccine candidate for COVID-19. Nat Commun. 2020;11(1):2601. DOI: 10.1038/s41467-020-16505-0
- Al-Amri SS, Abbas AT, Siddiq LA, et al. Immunogenicity of Candidate MERS-CoV DNA Vaccines Based on the Spike Protein. Sci Rep. 2017;7:44875. DOI: 10.1038/srep44875
- Fausther-Bovendo H, Kobinger GP. Pre-existing immunity against Ad vectors: humoral, cellular, and innate response, what's important?. Hum Vaccin Immunother. 2014;10(10):2875-2884. DOI: 10.4161/hv.29594
- Knuchel MC, Marty RR, Morin TN, et al. Relevance of a pre‑existing measles immunity prior immunization with a recombi‑ nant measles virus vector. Hum Vacc Immunother. 2013;9(3):599-606. DOI: 10.4161/hv.23241
- Enjuanes L, Dediego ML, Alvarez E, et al. Vaccines to prevent severe acute respiratory syndrome coronavirusinduced disease. Virus Res. 2008;133(1):45-62. DOI: 10.1016/j.virusres.2007.01.021
- Schindewolf C, Menachery VD. Middle East Respiratory Syndrome Vaccine Candidates: Cautious Optimism. Viruses. 2019;11(1):74. DOI: 10.3390/v11010074
- Gao W, Tamin A, Soloff A, et al. Effects of a SARS-associated coronavirus vaccine in monkeys. Lancet. 2003;362(9399):1895-1896. DOI: 10.1016/S0140-6736(03)14962-8
- Liu RY, Wu LZ, Huang BJ, et al. Adenoviral expression of a truncated S1 subunit of SARS-CoV spike protein results in specific humoral immune responses against SARS-CoV in rats. Virus Res. 2005;112(1-2):24-31. DOI: 10.1016/j.virusres.2005.02.009
- See RH, Petric M, Lawrence DJ, et al. Severe acute respiratory syndrome vaccine efficacy in ferrets: whole killed virus and adenovirus-vectored vaccines. J Gen Virol. 2008;89(Pt 9):2136-2146. DOI: 10.1099/vir.0.2008/001891-0
- Kobinger GP, Figueredo JM, Rowe T, et al. Adenovirus-based vaccine prevents pneumonia in ferrets challenged with the SARS coronavirus and stimulates robust immune responses in macaques. Vaccine. 2007;25(28):5220-5231. DOI: 10.1016/j.vaccine.2007.04.065
- Volz A, Sutter G. Modified Vaccinia Virus Ankara: History, Value in Basic Research, and Current Perspectives for Vaccine Development. Adv Virus Res. 2017;97:187-243. DOI: 10.1016/bs.aivir.2016.07.001
- Bisht H, Roberts A, Vogel L, et al. Severe acute respiratory syndrome coronavirus spike protein expressed by attenuated vaccinia virus protectively immunizes mice. Proc Natl Acad Sci U S A. 2004;101(17):6641-6646. DOI: 10.1073/pnas.0401939101
- Chen Z, Zhang L, Qin C, et al. Recombinant modified vaccinia virus Ankara expressing the spike glycoprotein of severe acute respiratory syndrome coronavirus induces protective neutralizing antibodies primarily targeting the receptor binding region. J Virol. 2005;79(5):2678-2688. DOI: 10.1128/JVI.79.5.2678-2688.2005
- Czub M, Weingartl H, Czub S, et al. Evaluation of modified vaccinia virus Ankara based recombinant SARS vaccine in ferrets. Vaccine. 2005;23(17-18):2273-2279. DOI: 10.1016/j.vaccine.2005.01.033
- Weingartl H, Czub M, Czub S, et al. Immunization with modified vaccinia virus Ankara-based recombinant vaccine against severe acute respiratory syndrome is associated with enhanced hepatitis in ferrets. J Virol. 2004;78(22):12672-12676. DOI: 10.1128/JVI.78.22.12672-12676.2004
- Deming D, Sheahan T, Heise M, et al. Vaccine efficacy in senescent mice challenged with recombinant SARS-CoV bearing epidemic and zoonotic spike variants. PLoS Med. 2006;3(12):e525. DOI: 10.1371/journal.pmed.0030525
- Sheahan T, Whitmore A, Long K, et al. Successful vaccination strategies that protect aged mice from lethal challenge from influenza virus and heterologous severe acute respiratory syndrome coronavirus. J Virol. 2011;85(1):217-230. DOI: 10.1128/JVI.01805-10
- Bukreyev A, Lamirande EW, Buchholz UJ, et al. Mucosal immunisation of African green monkeys (Cercopithecus aethiops) with an attenuated parainfluenza virus expressing the SARS coronavirus spike protein for the prevention of SARS. Lancet. 2004;363(9427):2122-2127. DOI: 10.1016/S0140-6736(04)16501-X
- Kapadia SU, Rose JK, Lamirande E, et al. Long-term protection from SARS coronavirus infection conferred by a single immunization with an attenuated VSV-based vaccine. Virology. 2005;340(2):174-182. DOI: 10.1016/j.virol.2005.06.016
- Kim E, Okada K, Kenniston T, et al. Immunogenicity of an adenoviral-based Middle East Respiratory Syndrome coronavirus vaccine in BALB/c mice. Vaccine. 2014;32(45):5975-5982. DOI: 10.1016/j.vaccine.2014.08.058
- Guo X, Deng Y, Chen H, et al. Systemic and mucosal immunity in mice elicited by a single immunization with human adenovirus type 5 or 41 vector-based vaccines carrying the spike protein of Middle East respiratory syndrome coronavirus. Immunology. 2015;145(4):476-484. DOI: 10.1111/imm.12462
- Jung SY, Kang KW, Lee EY, et al. Heterologous prime-boost vaccination with adenoviral vector and protein nanoparticles induces both Th1 and Th2 responses against Middle East respiratory syndrome coronavirus. Vaccine. 2018;36(24):3468-3476. DOI: 10.1016/j.vaccine.2018.04.082
- Zhu FC, Li YH, Guan XH, et al. Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: a dose-escalation, open-label, non-randomised, first-in-human trial. Lancet. 2020;395(10240):1845-1854. DOI: 10.1016/S0140-6736(20)31208-3
- Zhu FC, Guan XH, Li YH, et al. Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 2020;396(10249):479-488. DOI: 10.1016/S0140-6736(20)31605-6
- Alharbi NK, Padron-Regalado E, Thompson CP, et al. ChAdOx1 and MVA based vaccine candidates against MERS-CoV elicit neutralising antibodies and cellular immune responses in mice. Vaccine. 2017;35(30):3780-3788. DOI: 10.1016/j.vaccine.2017.05.032
- Munster VJ, Wells D, Lambe T, et al. Protective efficacy of a novel simian adenovirus vaccine against lethal MERS-CoV challenge in a transgenic human DPP4 mouse model. NPJ Vaccines. 2017;2:28. DOI: 10.1038/s41541-017-0029-1
- Alharbi NK, Qasim I, Almasoud A, et al. Humoral Immunogenicity and Efficacy of a Single Dose of ChAdOx1 MERS Vaccine Candidate in Dromedary Camels. Sci Rep. 2019;9(1):16292. DOI: 10.1038/s41598-019-52730-4
- van Doremalen N, Haddock E, Feldmann F, et al. A single dose of ChAdOx1 MERS provides protective immunity in rhesus macaques. Sci Adv. 2020;6(24):eaba8399. DOI: 10.1126/sciadv.aba8399
- Folegatti PM, Bittaye M, Flaxman A, et al. Safety and immunogenicity of a candidate Middle East respiratory syndrome coronavirus viral-vectored vaccine: a dose-escalation, open-label, non-randomised, uncontrolled, phase 1 trial. Lancet Infect Dis. 2020;20(7):816-826. DOI: 10.1016/S1473-3099(20)30160-2
- Folegatti PM, Ewer KJ, Aley PK, et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet.2020;396(10249):467-478. DOI: 10.1016/S0140-6736(20)31604-4
- Volz A, Kupke A, Song F, et al. Protective Efficacy of Recombinant Modified Vaccinia Virus Ankara Delivering Middle East Respiratory Syndrome Coronavirus Spike Glycoprotein. J Virol. 2015;89(16):8651-8656.DOI: 10.1128/JVI.00614-15
- Haagmans BL, van den Brand JM, Raj VS, et al. An orthopoxvirus-based vaccine reduces virus excretion after MERS-CoV infection in dromedary camels. Science. 2016;351(6268):77-81. DOI: 10.1126/science.aad1283
- Koch T, Dahlke C, Fathi A, et al. Safety and immunogenicity of a modified vaccinia virus Ankara vector vaccine candidate for Middle East respiratory syndrome: an open-label, phase 1 trial. Lancet Infect Dis.2020;20(7):827-838. DOI: 10.1016/S1473-3099(20)30248-6
- Agnihothram S, Gopal R, Yount BL Jr, et al. Evaluation of serologic and antigenic relationships between middle eastern respiratory syndrome coronavirus and other coronaviruses to develop vaccine platforms for the rapid response to emerging coronaviruses. J Infect Dis. 2014;209(7):995-1006. DOI: 10.1093/infdis/jit609
- Malczyk AH, Kupke A, Prüfer S, et al. A Highly Immunogenic and Protective Middle East Respiratory Syndrome Coronavirus Vaccine Based on a Recombinant Measles Virus Vaccine Platform. J Virol. 2015;89(22):11654-11667. DOI: 10.1128/JVI.01815-15
- Wirblich C, Coleman CM, Kurup D, et al. One-Health: a Safe, Efficient, Dual-Use Vaccine for Humans and Animals against Middle East Respiratory Syndrome Coronavirus and Rabies Virus. J Virol. 2017;91(2):e02040-16. DOI: 10.1128/JVI.02040-16
- Liu RQ, Ge JY, Wang JL, et al. Newcastle disease virus-based MERS-CoV candidate vaccine elicits high-level and lasting neutralizing antibodies in Bactrian camels. J Integr Agric. 2017;16(10):2264-2273. DOI: 10.1016/S2095-3119(17)61660-5
- Liu R, Wang J, Shao Y, et al. A recombinant VSV-vectored MERS-CoV vaccine induces neutralizing antibody and T cell responses in rhesus monkeys after single dose immunization. Antiviral Res. 2018;150:30-38. DOI: 10.1016/j.antiviral.2017.12.007
- Takasuka N, Fujii H, Takahashi Y, et al. A subcutaneously injected UV-inactivated SARS coronavirus vaccine elicits systemic humoral immunity in mice. Int Immunol. 2004;16(10):1423-1430. DOI: 10.1093/intimm/dxh143
- Qu D, Zheng B, Yao X, et al. Intranasal immunization with inactivated SARS-CoV (SARS-associated coronavirus) induced local and serum antibodies in mice. Vaccine. 2005;23(7):924-931. DOI: 10.1016/j.vaccine.2004.07.031
- Lin JT, Zhang JS, Su N, et al. Safety and immunogenicity from a phase I trial of inactivated severe acute respiratory syndrome coronavirus vaccine. Antivir Ther. 2007;12(7):1107-1113.
- Agrawal AS, Tao X, Algaissi A, et al. Immunization with inactivated Middle East Respiratory Syndrome coronavirus vaccine leads to lung immunopathology on challenge with live virus. Hum Vaccin Immunother. 2016;12(9):2351-2356. DOI: 10.1080/21645515.2016.1177688
- Deng Y, Lan J, Bao L, et al. Enhanced protection in mice induced by immunization with inactivated whole viruses compare to spike protein of middle east respiratory syndrome coronavirus. Emerg Microbes Infect. 2018;7(1):60. DOI: 10.1038/s41426-018-0056-7
- Minor PD. Live attenuated vaccines: Historical successes and current challenges. Virology. 2015;479-480:379-392. DOI: 10.1016/j.virol.2015.03.032
- Lamirande EW, DeDiego ML, Roberts A, et al. A live attenuated severe acute respiratory syndrome coronavirus is immunogenic and efficacious in golden Syrian hamsters. J Virol. 2008;82(15):7721-7724. DOI: 10.1128/JVI.00304-08
- Menachery VD, Gralinski LE, Mitchell HD, et al. Combination Attenuation Offers Strategy for Live Attenuated Coronavirus Vaccines. J Virol. 2018;92(17):e00710-18. DOI: 10.1128/JVI.00710-18
- Menachery VD, Gralinski LE, Mitchell HD, et al. Middle East Respiratory Syndrome Coronavirus Nonstructural Protein 16 Is Necessary for Interferon Resistance and Viral Pathogenesis. mSphere. 2017;2(6):e00346-17.DOI: 10.1128/mSphere.00346-17
- Graham RL, Becker MM, Eckerle LD, et al. A live, impaired-fidelity coronavirus vaccine protects in an aged, immunocompromised mouse model of lethal disease. Nat Med. 2012;18(12):1820-1826. DOI: 10.1038/nm.2972
- Schoeman D, Fielding BC. Coronavirus envelope protein: current knowledge. Virol J. 2019;16(1):69. DOI: 10.1186/s12985-019-1182-0
- DeDiego ML, Nieto-Torres JL, Jimenez-Guardeño JM, et al. Coronavirus virulence genes with main focus on SARS-CoV envelope gene. Virus Res. 2014;194:124-137. DOI: 10.1016/j.virusres.2014.07.024
- Menachery VD, Debbink K, Baric RS. Coronavirus non-structural protein 16: evasion, attenuation, and possible treatments. Virus Res. 2014;194:191-199. DOI: 10.1016/j.virusres.2014.09.009
- Robson F, Khan KS, Le TK, et al. Coronavirus RNA Proofreading: Molecular Basis and Therapeutic Targeting. Mol Cell. 2020;79(5):710-727. DOI: 10.1016/j.molcel.2020.07.027
- van Riel D, de Wit E. Next-generation vaccine platforms for COVID-19. Nat Mater. 2020;19(8):810-812. DOI: 10.1038/s41563-020-0746-0
- Lurie N, Saville M, Hatchett R, Halton J. Developing Covid-19 Vaccines at Pandemic Speed. N Engl J Med. 2020;382(21):1969-1973. DOI: 10.1056/NEJMp2005630
- Johnson-&-Johnson. Johnson & Johnson announces a lead vaccine candidate for COVID-19; landmark new partnership with U.S. Department of Health & Human Services; and commitment to supply one billion vaccines worldwide for emergency pandemic use. 2020.
- GSK. GSK announces intention to produce 1 billion doses of pandemic vaccine adjuvant in 2021 to support multiple COVID-19 vaccine collaborations. 2020.
- Chemical-&-Engineering-News. Moderna picks Lonza to make 1 billion doses of its coronavirus vaccine. 2020.
- CNN-Health. US taxpayers are funding six Covid vaccines. Here's how they work. 2020.
- REUTERS. EU to use $2.7 billion fund to buy promising COVID-19 vaccines. 2020.
- AP-News. China aims to make 1 billion COVID-19 vaccine doses a year. 2020.
- World-Health-Organization. Draft landscape of COVID-19 candidate vaccines. Geneva: World-Health-Organization; 2020c.
- Novavax. NVX-CoV2373 COVID-19 Vaccine candidate phase 1/2, part 1, clinical trial results. 2020.
- Keech C, Albert G, Cho I, et al. Phase 1-2 Trial of a SARS-CoV-2 Recombinant Spike Protein Nanoparticle Vaccine. N Engl J Med. 2020;383(24):2320-2332. DOI: 10.1056/NEJMoa2026920
- Dai L, Zheng T, Xu K, et al. A Universal Design of Betacoronavirus Vaccines against COVID-19, MERS, and SARS. Cell. 2020;182(3):722-733.e11. DOI: 10.1016/j.cell.2020.06.035
- Diehl MC, Lee JC, Daniels SE, et al. Tolerability of intramuscular and intradermal delivery by CELLECTRA(®) adaptive constant current electroporation device in healthy volunteers. Hum Vaccin Immunother. 2013;9(10):2246-2252. DOI: 10.4161/hv.24702
- Kared H, Redd AD, Bloch EM, et al. CD8+ T cell responses in convalescent COVID-19 individuals target epitopes from the entire SARS-CoV-2 proteome and show kinetics of early differentiation. Preprint. bioRxiv. 2020;2020.10.08.330688. DOI: 10.1101/2020.10.08.330688
- INOVIO-Pharmaceuticals. INOVIO announces positive interim phase 1 data for INO-4800 vaccine for COVID-19. 2020. URL: http://ir.inovio.com/news-releases/news-releases-details/2020/INOVIO-Announces-Positive-Interim-Phase-1-Data-For-INO-4800-Vaccine-for-COVID-19/default.aspx
- Kauffman KJ, Webber MJ, Anderson DG. Materials for non-viral intracellular delivery of messenger RNA therapeutics. J Control Release. 2016;240:227-234. DOI: 10.1016/j.jconrel.2015.12.032
- Sahin U, Karikó K, Türeci Ö. mRNA-based therapeutics-developing a new class of drugs. Nat Rev Drug Discov. 2014;13(10):759-780. DOI: 10.1038/nrd4278
- Pardi N, Hogan MJ, Porter FW, Weissman D. mRNA vaccines − a new era in vaccinology. Nat Rev Drug Discov. 2018;17(4):261-279. DOI: 10.1038/nrd.2017.243
- Jackson LA, Anderson EJ, Rouphael NG, et al. An mRNA vaccine against SARS-CoV-2—preliminary report. N Engl J Med. 2020. DOI: 10.1056/NEJMoa2022483
- Anderson EJ, Rouphael NG, Widge AT, et al. Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N Engl J Med. 2020. DOI: 10.1056/NEJMoa2028436
- Moderna’s COVID-19 Vaccine Candidate Meets its Primary Efficacy Endpoint in the First Interim Analysis of the Phase 3 COVE Study. Moderna, 2020. URL: https://investors.modernatx.com/news-releases/newsrelease- details/modernas-covid-19-vaccine-candidate-meets-its-primary-efficacy.
- Genetic-Engineering-&-Biotechnology-News. BioNTech, Pfizer, and Fosun Pharma—BNT162. Genetic Engineering & Biotechnology News. 2020.
- Mulligan MJ, Lyke KE, Kitchin N, et al. Phase I/II study of COVID-19 RNA vaccine BNT162b1 in adults. Nature. 2020;586(7830):589-593. DOI: 10.1038/s41586-020-2639-4
- Sahin U, Muik A, Derhovanessian E, et al. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature. 2020;586(7830):594-599. DOI: 10.1038/s41586-020-2814-7
- Walsh EE, Frenck RW Jr, Falsey AR, et al. Safety and Immunogenicity of Two RNA-Based Covid-19 Vaccine Candidates. N Engl J Med. 2020;383(25):2439-2450. DOI: 10.1056/NEJMoa2027906
- Pfizer and BioNTech Conclude Phase 3 Study of COVID-19 Vaccine Candidate, Meeting All Primary Efficacy Endpoints. Pfizer, 2020. URL: https://www.pfizer.com/news/press-release/press-release-detail/pfizer-andbiontech-conclude-phase-3-study-covid-19-vaccine.
- The-New-York-Times. AstraZeneca Pauses Vaccine Trial for Safety Review. 2020.
- Astrazeneca. COVID-19 vaccine AZD1222 clinical trials resumed in the UK. 2020.
- AZD1222 vaccine met primary efficacy endpoint in preventing COVID-19. Astrazeneca, 2020. URL: https://www.astrazeneca.com/media-centre/press-releases/2020/azd1222hlr.html.
- Logunov DY, Dolzhikova IV, Zubkova OV, et al. Safety and immunogenicity of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine in two formulations: two open, non-randomised phase ½ studies from Russia. Lancet. 2020;396(10255):887-897. DOI: 10.1016/S0140-6736(20)31866-3
- Second Interim Analysis of Clinical Trial Data Showed a 91.4% Efficacy for the Sputnik V Vaccine on Day 28 After the First Dose; Vaccine Efficacy is Over 95% 42 Days After the First Dose. Sputnik V, 2020. URL: https://sputnikvaccine.com/newsroom/pressreleases/second-interim-analysis-of-clinical-trial-data-showed-a-91-4-efficacy-for-the-sputnik-v-vaccine-on-d/.
- Gao Q, Bao L, Mao H, et al. Development of an inactivated vaccine candidate for SARS-CoV-2. Science. 2020;369(6499):77-81. DOI: 10.1126/science.abc1932
- Xia S, Duan K, Zhang Y, et al. Effect of an Inactivated Vaccine Against SARS-CoV-2 on Safety and Immunogenicity Outcomes: Interim Analysis of 2 Randomized Clinical Trials. JAMA. 2020;324(10):951-960. DOI: 10.1001/jama.2020.15543
- Wang H, Zhang Y, Huang B, et al. Development of an Inactivated Vaccine Candidate, BBIBP-CorV, with Potent Protection against SARS-CoV-2. Cell. 2020;182(3):713-721.e9. DOI: 10.1016/j.cell.2020.06.008
- Xia S, Zhang Y, Wang Y, et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: a randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect Dis. 2020. DOI: 10.1016/S1473-3099(20)30831-8
- AIVITA-Biomedical. SARS-COV-2 VACCINE. 2020.
- Zhou LK, Zhou Z, Jiang XM, et al. Absorbed plant MIR2911 in honeysuckle decoction inhibits SARS-CoV-2 replication and accelerates the negative conversion of infected patients. Cell Discov. 2020;6:54. DOI: 10.1038/s41421-020-00197-3
- Stensballe LG, Nante E, Jensen IP, et al. Acute lower respiratory tract infections and respiratory syncytial virus in infants in Guinea-Bissau: a beneficial effect of BCG vaccination for girls community based casecontrol study. Vaccine. 2005;23(10):1251-1257. DOI: 10.1016/j.vaccine.2004.09.006
- Spencer JC, Ganguly R, Waldman RH. Nonspecific protection of mice against influenza virus infection by local or systemic immunization with Bacille Calmette-Guérin. J Infect Dis. 1977;136(2):171-175. DOI: 10.1093/infdis/136.2.171
- Starr SE, Visintine AM, Tomeh MO, Nahmias AJ. Effects of immunostimulants on resistance of newborn mice to herpes simplex type 2 infection. Proc Soc Exp Biol Med. 1976;152(1):57-60. DOI: 10.3181/00379727-152-39327
- O'Neill LAJ, Netea MG. BCG-induced trained immunity: can it offer protection against COVID-19?. Nat Rev Immunol. 2020;20(6):335-337. DOI: 10.1038/s41577-020-0337-y
- Pilarowski G, Lebel P, Sunshine S, et al. Performance characteristics of a rapid SARS-CoV-2 antigen detection assay at a public plaza testing site in San Francisco. Preprint. medRxiv. 2020;2020.11.02.20223891. DOI: 10.1101/2020.11.02.20223891
- Cao WC, Liu W, Zhang PH, et al. Disappearance of antibodies to SARS-associated coronavirus after recovery. N Engl J Med. 2007;357(11):1162-1163. DOI: 10.1056/NEJMc070348
- Wu LP, Wang NC, Chang YH, et al. Duration of antibody responses after severe acute respiratory syndrome. Emerg Infect Dis. 2007;13(10):1562-1564. DOI: 10.3201/eid1310.070576
- Iwasaki A. What reinfections mean for COVID-19. Lancet Infect Dis. 2020. DOI: 10.1016/S1473-3099(20)30783-0
- Craviso GL, Musacchio JM. High-affinity binding of the antitussive dextromethorphan to guinea-pig brain. Eur J Pharmacol. 1980;65(4):451-453. DOI: 10.1016/0014-2999(80)90354-4
- Callaway E. The coronavirus is mutating − does it matter?. Nature. 2020;585(7824):174-177. DOI: 10.1038/d41586-020-02544-6
- Korber B, Fischer WM, Gnanakaran S, et al. Tracking Changes in SARS-CoV-2 Spike: Evidence that D614G Increases Infectivity of the COVID-19 Virus. Cell. 2020;182(4):812-827.e19. DOI: 10.1016/j.cell.2020.06.043
- Zhang L, Richards A, Khalil A, et al. SARS-CoV-2 RNA reverse-transcribed and integrated into the human genome. Preprint. bioRxiv. 2020;2020.12.12.422516. DOI: 10.1101/2020.12.12.422516
- Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of covid-19—final report. N Engl J Med. 2020. DOI: 10.1056/NEJMoa2007764
- Group RC, Horby P, Lim WS, et al. Dexamethasone in hospitalized patients with covid-19—preliminary report. N Engl J Med. 2020. DOI: 10.1056/NEJMoa2021436.


