Развитие ферментативной теории восстановления аромата
Автор: Дубова Г.Е., Безусов А.Т.
Журнал: Вестник Воронежского государственного университета инженерных технологий @vestnik-vsuet
Рубрика: Пищевая биотехнология
Статья в выпуске: 2 (60), 2014 года.
Бесплатный доступ
Новыми аспектами в образовании свежих ароматов могут служить условия предварительной обработки плодов и последующие условия протекания ферментативных реакций. Синтез ароматических компонентов свежей травы, арбузов, зеленых листьев происходит с участием растительных липоксигеназ. Основой этого исследования является факт, что молекулы соединения-предшественника могут выдерживать режимы переработки, тогда как ферменты и ароматические соединения зачастую разрушаются. Растительные гомогенаты - потенциальные источники ферментов, которые продуцируют натуральные ароматические вещества. Образование свежего аромата происходит в результате реакции между полиненасыщенными жирными кислотами клеточных мембран и липоксигеназами и гидропероксид лиазами растительного сырья и является наиболее ощутимым. Предварительная подготовка образцов положительно влияет на энергию связывания в комплексе фермент-субстрат. Изменение йодного числа в обработанных гомогенатах, по сравнению со свежими, показывает изомеризацию предшественников аромата. Определено минимальное количество внесенных гомогенатов (до 20 г) и время протекания реакции восстановления аромата (5-7 минут). Предварительное охлаждение гомогенатов активизирует ферменты, усиливает окисляемость ПНЖК и приводит к восстановлению свежего аромата растительного сырья. В условиях инактивации ферментов ароматы не синтезируются. В случае межфазной активации между субстратом и ферментами получен стойкий аромат в пищевой глазури, пене.
Аромат, гомогенат, предшественник, жирные кислоты, ферменты, субстрат
Короткий адрес: https://sciup.org/14040222
IDR: 14040222 | УДК: 66.091-026.785
Текст научной статьи Развитие ферментативной теории восстановления аромата
At the same time, the most sought-after products are those that are natural and have no additives. The development of flavor industry in the last decade was focused on the analysis of natural compounds and finding new aromatic components from which one can restore the natural flavors [1].
The most commonly used flavors in food industry are obtained by chemical synthesis or isolated by chemical processes. These flavors are used repeatedly in various products, however, their repeated use may result in an overabundance of them in an organism. For example, the fruit flavoring substance usually includes isobutyl acetate and ethyl formate and it is used in beverages, yogurts, ice creams, fruit fillings, and tea.
The issue of preservation of aromatic substances is mostly solved in the production of canned fruit and berry juices. Aromatic substances are condensed from a fruit mass by distillation, and then concentrated 100-200 times by absorption, extraction and distillation. The resulting concentrate of aromatic substances is stored separately from the juices.The use of enzymes is another way to restore the fresh flavor of food in processed products. The aroma of dried vegetables found in products can be restored by adding to the processed products the liquid extract of fresh raw materials [2].
To continue the line of research, Schwimmer demonstrated that blanched, dried or canned beans, peas, broccoli, carrots, tomatoes, and cabbage change their flavor under the influence of enzymes isolated from fresh raw vegetables, genetically related to the main product or from mustard. Thus, he formulated the theory of possible aroma recovery based on enzymatic processes. According to this theory, aroma recovery is dependent on the presence of the precursors and on the availability of enzymes that then specifically form natural aromas from these precursors.
One of the major achievements in understanding the mechanisms of flavor formation is this knowledge of the reactions of flavor precursors. Extensive work has been done since to identify flavor precursors of meat, cheese, fish, beer, champagne, bananas, apples, coffee and other products. Knowledge of the flavor precursors and pathways leading to the formation of taste and flavor in fruits and vegetables has been slowly accumulating over the years [3]. The theory of flavor recovery has not been further developed due to the energy barrier between enzymes and a substrate precursor in natural flavor-forming reactions [2].
The authors conducted a literature review of sources published in the last 60 years on the issue of fresh flavor recovery and the lost aroma during thermal processing or dehydration.
Preparation of suspension samples with the active complex of enzymes . Ukrainian grown fresh ripe tomatoes, sweet pepper, cucumbers, and watermelons were purchased from local farmers with the shelf life being a few hours. The vegetables were kept refrigerated for 60 hours at the temperature of about 5 °C. Then vegetables were finely shredded for 7 minutes in a Fillips blender; cellular fluid was collected by squeezing, the hard part was convectively frozen at the temperature of below -18 °C. During the technological use, the hard residue was thawed in a microwave oven in a thawing mode.
Iodine number. The chosen method is based on the effect of iodide acid upon essential fatty acids. The ten-gram sample was placed in a retort with a ground-in stopper and then it was dissolved in 15 ml of 95% alcohol on the water bath at the temperature of 50-60 °C. The retort was cooled to the room temperature; 20 ml of 0,2n. iodine alcoholic solution and 200 ml of distilled water (at the 30 °C temperature) were added. The retort was closed, vigorously agitated, mixed well and left alone for 5 minute. The iodine excess was titrated by 0,1 n hyposulphite solution.
It is difficult to overestimate the role of enzymes in flavor formation: just in a matter of minutes lipids, carbohydrates, proteins and amino acids found in fruits and vegetables are converted to volatile compounds. As a result of homogenization, plant enzymes lead to the formation or enhancement of fruit and vegetable flavors. The destruction of plant tissues by using enzyme solu- tions, as well as the hydrolysis of the glycosidic linkages of precursors, provides a significant increase in the yield of flavoring substances (Oey, 2010). The most promising area of application of fermentolise technology is the production of natural flavoring from fruit processing waste (peels, pulp, seeds). Industries commonly use microorganisms (yeast, fungi, bacteria) for their fermentative production. The potential to obtain flavor through biotransformation of precursors is intensively studied [4]. Plant homogenates, or homogeneous micronized suspensions of plant materials, represent potential sources of natural aromatic substances. Enzyme activity in such a suspension can be instrumental in flavoring processes that avoid laborious isolation and purification of enzymes from plant raw materials and save on energy costs associated with dialysis or lyophilization. The availability of these organic components is the main advantage of this method, while their shortterm effect, limited to a few hours, is the main drawback. In some patented flavoring processes of this kind, strawberry leaves, soybeans, and mushrooms are used as homogenates. However, during the concentration or distillation of these "key" aromatic components obtained through such process, e.g. cis-3-hexenal or 1-octen-3-ol, their yields will be low, averaging 10%.
The short-term effect of flavoring is not necessarily a negative factor in the case of preparing small quantities of food, such as in a restaurant or a cafe. Furthermore, there is no need to isolate flavors from homogenates in commercial quantities. Sometimes it is sufficient to restore or to add the “key” flavor ingredient: hexanal for wheat bread and apple juice, hexenal for chopped strawberries or bananas, nonadienal for cucumbers, and decadienal for potato chips. In these cases, the effect is obtained from the use of natural flavor precursors and enzymes from homogenates of plant materials. The renowned flavor specialist Blumenthal enhanced the flavor and smell of the product by mixing pepper and strawberry homogenates [5]. The author of this article finds value in creating edible films or glazes, where enzymes found in homogenates will serve as the source of flavor formation. The impact of strengthening the aromatic profile of products will be seen in lower quantities of salt used during the preparation.
The prospects for the use of enzyme theory have been described in patents on including enzymes in processed foods immediately before eating for strengthening taste and aroma. Lipid degradation and availability of polyunsaturated fatty acids for enzymatic oxidation reactions are important factors for the formation of certain flavor components. First, lipids are hard to reach due to their location in the cytoplasmic membrane. Segregation of lipids is correlated with its destruction by means of heating, freezing, or other physical methods. It is plausible to assume that freezing yields better outcomes, since it results not only in destruction of cytoplasmic membrane of the cell, but also in special isomerization of lipids. Furthermore, decreasing the temperature causes a decrease in membrane fluidity, offset by desaturation of membrane lipid in fatty acids caused by desaturases. Such processes in isolated fruits are yet to be studied.
Most of the enzymes are still active after the harvesting of fruits and vegetables and this continuing enzymatic activity upon storage affects their quality [7]. The 10 ° C drop in the environmental temperature for isolated ripe fruits is sufficient for activation of the membrane PUFA desaturation and over time one can observe a change in physical properties of the cytoplasmic membrane, such as an increase in the degree of unsaturation of the fatty acid residues in the cellular membranes [8]. Wang et al. (1996) demonstrated that the successful introduction of the yeast Δ-9 desaturase in transgenic tomato plants leads to an increase in levels of palmitoleic acid, 9, 12 - hexadienoic acid, and linoleic acid being accompanied by a decrease in palmitic acid and stearic acid. Change in the profile of fatty acids is due to a change in certain aromatic compounds derived from fatty acids, namely the cis-3-hexenol, 1-hexanol, hexanal, and cis-3-hexenal. The findings show the change in iodine number in test samples of the fresh homogenates after a short storage and freezing (Figure 1).
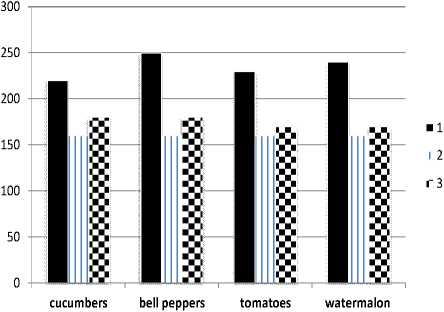
Figure 1. Changes in iodine number of homogenates after freezing (1), fresh (2), after a short storage (3)
The average 75 g increase of iodine number in frozen homogenates as compared to fresh ones is the evidence for the arrangement of double bonds in PUFA and enhanced activity of desaturases. When the cellular systems are damaged by freezing, most of the enzymes exhibit significant activity upon thawing. The reason for this increase in the rate of enzyme-catalyzed reactions is the rupture of the membranes, organelles, particularly sensitive lysosom, and failure in insulation of enzymes, enzyme substrates, and enzyme activators. Some studies report that lipoxygenase exhibit significant activity after freezing and thawing [9]. Lipoxygenase and hydroperoxide lyase are so closely associated in lysosomes that any extract from homogenates upon thawing contained a critical concentration of both enzymes. In part this increase in the activity of enzymes can be explained by the change in properties of the components of the cytoplasmic membrane. Preliminary preparation by cooling results in adequacy of the enzyme and substrate which positively influences binding energy.
Desaturases prepares the substrate to be influenced by the aroma-forming enzymes and as the result fresh flavor is formed de novo. Careful thawing plays an important role in these processes, for instance in microwave oven with low power setting in order not to inactivate enzymes. Lipase, lipoxygenase, and hydroperoxide lyase are then released from lysosomes and mitochondria, while being less accessible in fresh raw materials even after a fairly thin homogenization. The treatment of fresh homogenates in the microwave field at low power (up to 70 W) causes the change in binding energy influencing the enzyme-substrate complex. The specific heat capacity of a substrate is lower than the heat capacity of water, therefore lipid layers can be rapidly heated in a microwave oven, faster than water. The selective heating of cellular wall components in the microwave field is one of the important factors distinguishing this type of heating.
This paper proposes a flavoring method consisting of several steps (Figure 2).
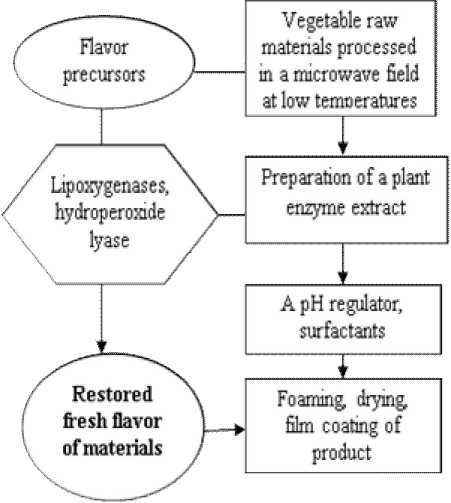
Figure 2. General schematic diagram of the lost flavor recovery
These recommendations are based on the previous studies by this author on the recovery of fresh flavor in heat-treated suspensions of watermelons, cucumbers, sweet peppers, strawberries, and tomatoes. The prepared watermelon suspension containing the 20 g active complex of enzymes is able to restore the lost aroma in 250 gs of the thermally-treated pulp. The reaction time depends on the form of PUFA (micellar, as a detergent complex or as salt) and values of pH determining the degree of carboxyl group dissociation. The reaction time of recovery at optimal combination of all factors is 5-7 minutes.
In the experiments, the flavor-creating complex of enzymes (lipase, lipoxygenase, hydroperoxide lyase) results in the formation of fresh flavors from PUFA cytoplasmic membranes. The enzymatic nature of these processes is confirmed by a small amount of homogenates introduced and a brief reaction time until a persistent flavor.
In order to secure a persistent and pronounced flavor it is necessary to ensure the interphase activation – a large contact surface interaction between the substrate and enzyme. This author used gelatin solutions for the experiments because of their surface-active properties and potential to provide maximum availability of the cytoplasmic membrane substrate to the active centers of enzymes. The probability of a considerable increase of lipase activity in gelatin solution is high enough because molecules of protein in gelatin solution represent natural surface-active nanoparticles possessing properties of nanosystems. Therefore, the final product can be represented by flavored films from gelatin solutions or foam thereof.
For inactivation of enzymes, vegetable homogenates were pre-heated to 85°C; then the possibility to synthesize aromatic components was estimated. The organoleptic analysis demonstrated a substantial difference in samples with active and inactivated enzymes. The researchers were not able to synthesize flavors from precursors upon inactivation of enzymes.


