Regulation of the aggregate stability for binary polymer-mineral dispersions
Автор: Valentina A. Poluektova, Nikolay A. Shapovalov, Natalia I. Cherkashina, Elizaveta P. Kozhanova, Sergey A. Starchenko
Журнал: Nanotechnologies in Construction: A Scientific Internet-Journal @nanobuild-en
Рубрика: Application of nanomaterials and nanotechnologies in construction
Статья в выпуске: 3 Vol.15, 2023 года.
Бесплатный доступ
Introduction. In the binary dispersed systems of different origins (mineral and polymer) with particles differing in size by an order of magnitude, heterocoagulation is observed. Regulation of the aggregate stability is crucial in controlling the properties of functional dispersed materials based on such mixed dispersions. This study focuses on the investigation of waterborne mono and binary dispersions of polyvinyl acetate, Portland cement, and chalk by means of static laser light scattering and optical microscopy. Materials and research methods. In order to investigate the action mechanism of the FF modifier based on phloroglucinol furfural oligomers as disperse phase we used chalk (CaCO3 – chalk dispersed technical MTD-2, LLC “Technostroy”, Kopanishchenskoe deposit), Portland cement CEM I 42.5N (JSC “Belgorod Cement”), and polyvinyl acetate (LLC “Kuban-Polymer”). Particle distribution and the aggregate stability of dispersions were studied with an Analysette 22 NanoTecplus device. The particle sizes were determined by optical microscopy with “Axio.Scope.A.1” microscope, and the adsorption of oligomers on the particles of dispersions was analyzed using a UV spectrometer (SPECORD UV). Results and discussion. It has been established that the phloroglucinol furfural modifier contributes to the stabilization of binary polymer-mineral dispersions. Integral and differential distribution curves of polymeric particles have been obtained in a wide range of 0.01–2100 μm. Research has shown the regularity of the modal diameter reduction of adsorption-modified particles with a transition from a narrow to a wider distribution with the absence of large coagulums. Conclusion. The hypothesis has been proposed that the adsorption-solvation factor of aggregate stability makes a significant contribution to the aggregate stability of binary polymer-mineral dispersions. The impact of this factor is different for mono-mineral and binary polymer-mineral dispersions and depends on the hydrophilicity of the surface, increases with the transition from mineral surface to the polymer surface as the Hamaker constant increases.
Binary dispersions, aggregate stability, adsorption, oligomers, nanomodification, differential distribution, modal particle radius
Короткий адрес: https://sciup.org/142238292
IDR: 142238292 | DOI: 10.15828/2075-8545-2023-15-3-258-266
Текст научной статьи Regulation of the aggregate stability for binary polymer-mineral dispersions
Original article
M ixed dispersions are widely used in scientific practice currently [1, 2]. In binary dispersed systems with particles of different nature and differing sizes by an order of magnitude, for example, polymer and cement particles, heterocoagulation is observed [3–5]. As a result of this process, a layer of smaller polymer particles is formed on the surface of larger particles in mineral dispersion [6, 7].
Regulation of the particle size for the dispersed phase and the aggregate stability, determined by the balance of repulsion and attraction forces between particles of hybrid dispersed phase, is a key aspect in controlling the properties of functional dispersed materials based on mixed dispersions [8–11]. One of the most effective ways to regulate the aggregate stability of dispersions is to use chemical modifiers that possess surface activity at the nanoscale – at the phase boundary [12, 13]. However, in binary dispersed systems, manifestations of synergy and
APPLICATION OF NANOMATERIALS AND NANOTECHNOLOGIES IN CONSTRUCTION antagonism are possible due to the presence of several modifying additives in the system. Issues related to the study of such effects are of great practical importance for solving rheotechnological issues in the innovative construction industry – additive technologies [14].
A complex of modifiers possessing water-reducing properties in the dispersed system and allowing the regulation of the mixture’s rheology can lead to the appearance of a number of effects, which are determined as follows: additive action – the components act as individual substances, but the result of their action is summed up; antagonism effect – in the combination, one component reduces the effectiveness of another component action, leading to an increase in their optimal dosages in the presence of both; synergism effect – the components in the combination are capable of enhancing each other’s action, due to the manifestation of activating action, which results in a reduction of the optimal dosages compared to found additive determinations [15, 16].
The aim of this study is to study the aggregate stability of binary polymer-mineral dispersions and to justify theoretically the possibility of regulating their aggregate stability through the nanomodifying effect of an oxyphenol furfural series modifier, in the view of surface phenomena and colloid-chemical regularities at the phase boundary, while considering the specifics of hybrid surfaces.
MATERIALS AND METHODS
In this study, a modifier based on phloroglucinol furfural oligomers (hereinafter referred to as FF modifier) was used to modify particles at the nano level in the investigated mono- and binary dispersions. The modifier was synthesized by the authors through polycondensation and was obtained as a brownish-yellow aqueous solution with a concentration of 20% for dry matter; M = 1200 ± 30; ρ = 1210 kg/m3. The following materials were used as the dispersed phases:
– Technical dispersed chalk MTD-2, LLC Technostroy, Kopanishchenskoye deposit; specific surface area Ssp = 8336 cm2/g. Chemical composition, weight %: CaO – 55.42, CO2 – 43.61, Fe2O3 – 0.08, SO3 – traces, H2O – 0.39, insoluble residue – 0.2;
– Portland cement CEM I 42.5N (GOST 31108), LLC Belgorod Cement. Composition, mass %: chemical, CaO – 66.3, SiO2 – 22.5, Al2O3 – 5.0, Fe2O3 – 4.4, MgO – 0.7, SO3 – 0.2; mineralogical, C3S – 64.0, C2S – 16.0, C4AF – 13.0, C3A – 4.0, CaOfree – 0.37.
– Polyvinyl acetate (C4H6O2) n , LLC Kuban Polymer, grade D51S (GOST 18992-80).
The aggregate stability of the investigated binary dispersions was evaluated by the method of static laser light scattering based on the most probable particle radius (modal diameter). The Analysette 22 NanoTecplus device was used, which operates in the range of 10 nm to
2000 µm. Two semiconductor lasers were used for measurement: a green laser with a wavelength of 532 nm and 7 MW is used for small and ultra-small particles, and an infrared laser with a wavelength of 850 nm and 9 MW was used for measuring the sizes of larger particles. The study of nanosized particles requires the registration of light scattered in the opposite direction. For this purpose, a third laser is used, which allows measurements to be carried out by backscattering. In this case, the laser beam is directed at the sample, which is located in front of the detector.
The sizes of particles in aqueous mono- and binary dispersions were determined by the method of optical microscopy (Axio.Scope.A.1 microscope). The analysis was carried out after establishing the adsorption equilibrium and ensuring the amount ratio of the dispersed phase to the dispersion medium. In parallel tests, at least 5 snapshots of each composition of the investigated systems have been obtained with changes in magnification and local changes in the study area. The interpretation of the microscopy results was based on the images with the similar magnification and taking into account the total area of the particles captured by the objective.
We studied the adsorption of modifiers on particles in mono- and binary dispersions by spectrophotometric analysis of the dispersion medium on a SPECORD UV device at a wave number of 50×10–3 cm–1 by reducing the adsorbate concentration in the system after setting the adsorption equilibrium. For this purpose, a series of modifier solutions were prepared by dilution method, and the amounts of adsorbent (mineral, polymer component, or their mixture) were the equal. After 30 minutes of stirring, the investigated systems were centrifuged at 3000 rpm, and the concentration of unadsorbed modifier was determined from the calibration curve. The amount of adsorbate was calculated, and adsorption isotherms graphs were presented for mono- and binary systems.
RESULTS AND DISCUSSION
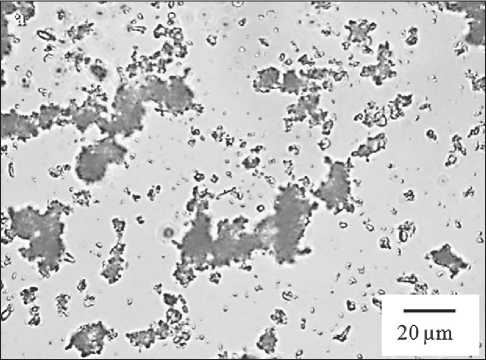
Upon introducing a polymer component into a chalk dispersion, the chalk particles begin to coagulate. Microphotographs of mono- and binary dispersions are presented in Fig. 1a and 1b. The average diameter of particles in the polymer-chalk dispersion increases compared to the control chalk dispersion.
For the monomineral chalk dispersion, the presence of large coagulum with sizes ranging from 10 to 40 µm is characteristic, the percentage content of which is 44%, while the modal diameter of particles in this fraction is approximately 17 µm (Fig. 1a). The introduction of a polymer component and the analysis of the coagulation structure of the binary dispersion (Fig. 1b) has shown an increase in the number of large coagulum with sizes of 10 to 40 µm up to 53%, and the formation of particles
APPLICATION OF NANOMATERIALS AND NANOTECHNOLOGIES IN CONSTRUCTION
*i
20 цш

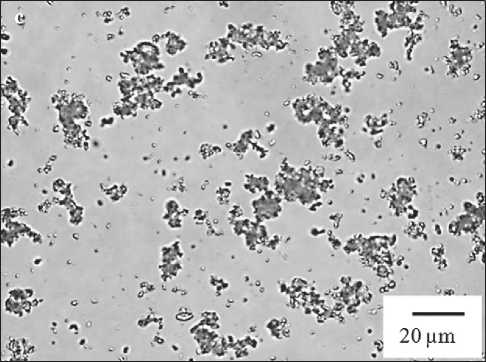
Fig. 1. Microphotographs of mono- and binary dispersions: a – chalk; b – polymer-chalk; c – modified polymer-chalk
ensembles with larger sizes of 40 to 50 µm – 11%. While the modal diameter of large coagulum increases up to 19.5 µm.
With the adsorption nanomodification with phloroglucinol furfural oligomers of particles in the binary poly-
mer-chalk dispersion (Fig. 1b, 1c), the peptizing of large heterocoagulum 20–50 µm fraction is observed. Thus, in the microphotograph (Fig. 1c), the largest ensembles of particles are represented by the 10–20 µm fraction. At the same time, their content as a result of nanomodification has increased from 27% (Fig. 1b) to 79%, and the modal diameter of large coagulum has decreased from 19.5 µm to 14 µm.
Similar results were obtained in a comparative analysis of cement and polymer-cement dispersions (Fig. 2).
Upon introducing the second component (polymer) into the cement monodispersion, an increase in the percentage content of coagulum of the largest size is also observed, as well as the formation of a coagulation structure of a new size fraction of 60 to 85 µm (Fig. 2b). The modal diameter of particles in the binary polymer-cement dispersion has increased up to 27 µm compared to 20 µm (which is characteristic of the monodispersion cement system) (Fig. 2a).
The nanomodification of particles in the binary polymer-cement system with phloroglucinol furfural oligomers leads to the peptizing of coagulum (Fig. 2c). In the microphotograph, the largest coagulum of particles with a content of about 20% is represented by the size fraction of 10 to 20 µm with a modal diameter of 13 µm.
To investigate the effects of nanomodification on monodispersions of polymer particles, we studied the coagulation structures of polyvinyl acetate particles. The polymer dispersion was diluted with water to match the amount of dispersion medium in the series of studies on various mono- and binary dispersions with the same initial characteristics. Particle nanomodification was carried out with phloroglucinol furfural oligomers at a modifier concentration of 0.2% of dry substance from the mass of the dispersion phase (Fig. 3).
Comparative analysis of microscopic studies has allowed us to record the slowing down coagulation process as a result of adsorption nanomodification at the phase boundary by phloroglucinol furfural oligomers. At the same time, a decrease in the modal particle size of the polyvinyl acetate monodispersion to 5 µm was observed. The formation of large coagulum, which is characteristic of the unmodified monodispersion with a modal particle size of 30 µm (Fig. 3a), was not recorded (Fig. 3b).
The results of microscopy are in good agreement with the data obtained on a laser particle size analyzer in a broad range. There were large agglomerates in the polyvinyl acetate monodispersion, while their complete absence was observed in the dispersion nanomodified with phloroglucinol furfural oligomers.
The differential distribution curves of nanomodified polyvinyl acetate monodispersion particles in a wider range, obtained from Analysette 22 laser particle size analyzer, have shown that at the initial time point, the process is characterized by a wider distribution and a slight
APPLICATION OF NANOMATERIALS AND NANOTECHNOLOGIES IN CONSTRUCTION
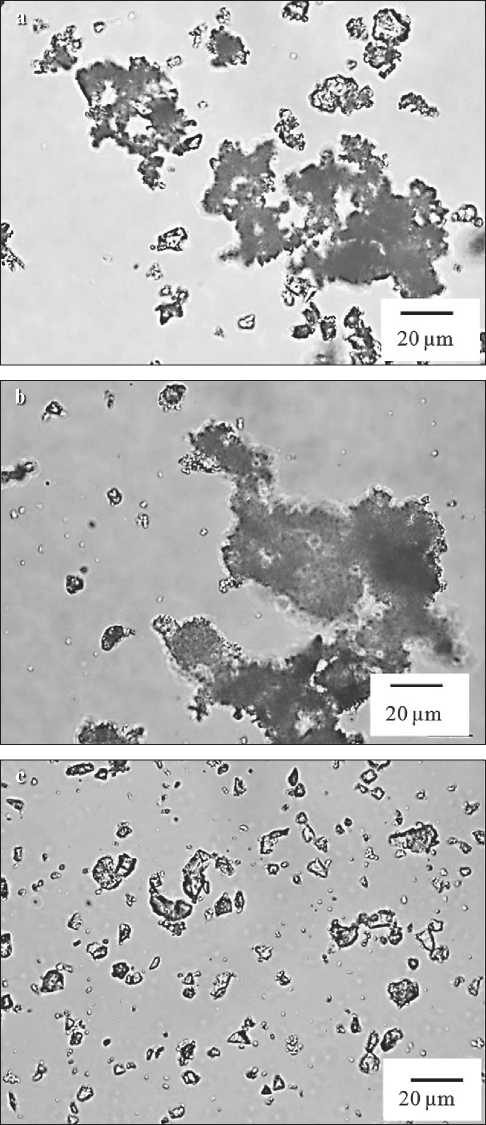
Fig. 2. Microphotographs of mono- and binary dispersions: a – cement; b – polymer-cement; c – modified polymer-cement decrease in the modal particle diameter compared to the unmodified monodispersion. Thus, the modal particle diameter of the polymeric monodispersion is 6.8 µm, while in the modified one it is 5.9 µm, which means that the modal particle size has decreased by only 13%. Research
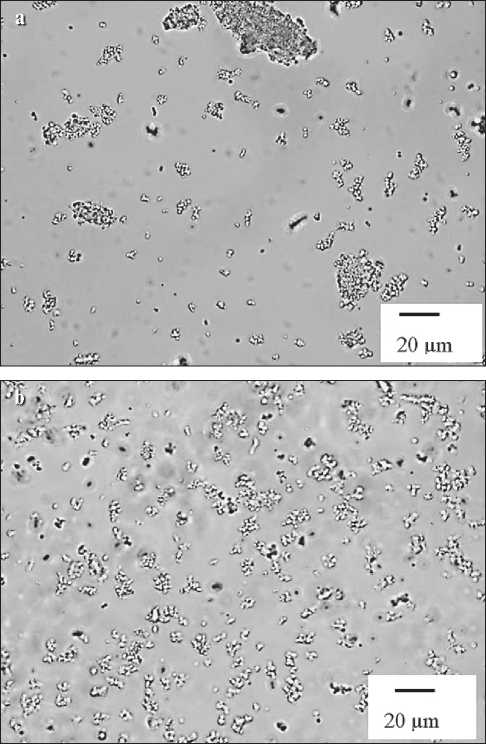
Fig. 3. Microphotographs of the polymeric monodispersion with an increased content of the dispersion medium in 25 times: a – unmodified; b – modified with phloroglucinol furfural oligomers data have confirmed the presence of large aggregates (50–60 µm) in the control polymeric monodispersion, which are visually determined by optical microscopy; and complete absence of large aggregates in the nanomodified polymeric dispersion is observed [17] (Fig. 4).
However, it should be noted that the investigated binary dispersions also contain an additional polymeric phase boundary modifier – polyvinyl alcohol (PVA), which is added at the production stage of the polyvinyl acetate dispersion. It is present, as a result, in all investigated binary dispersions. The authors [18] have studied previously the process of PVA molecule desorption in the presence of the FF modifier. The research was carried out using the addition method with a developed refractometric analysis technique. It was found that the introduction of phloroglucinol furfural oligomers led to an increase in the number of desorbed PVA molecules compared to the value of desorption during simple dilution of the polymeric dispersion. The difference in desorption values was 39%.
APPLICATION OF NANOMATERIALS AND NANOTECHNOLOGIES IN CONSTRUCTION
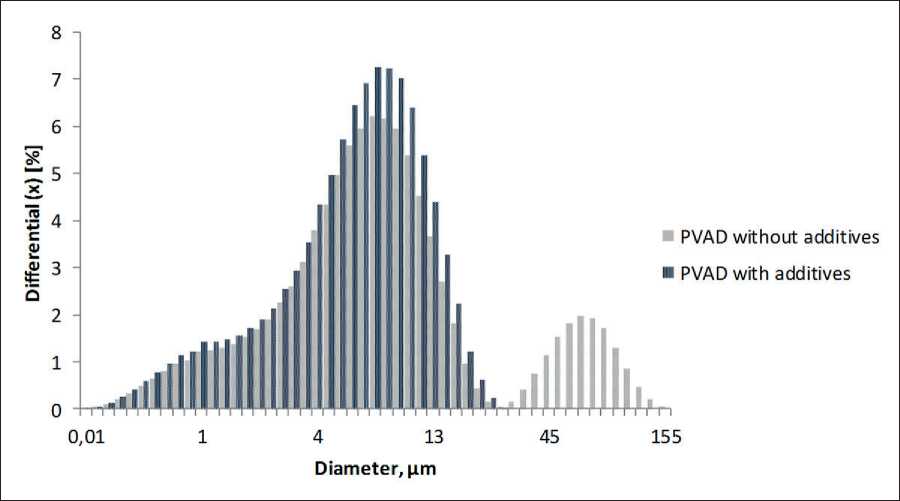
Fig. 4. Differential distribution curves of the control polyvinyl acetate monodispersion (PVAD) without additives and the modified PVAD monodispersion particles
At the same time, there is a joint competitive adsorption of molecules of two modifiers.
Dispersion analysis on a Microtrac S3500 laser diffractometer in the small range up to 6.5 µm of nanomodified polyvinyl acetate monodispersions has shown that with an increase in the FF modifier concentration (Fig. 5), a decrease in the modal particle diameter has been observed, while at the same time there is a transition from a narrow to a wider distribution after a certain concentration of the FF modifier. This allows us to fix the concentration limit of oligomers, above which coagula- tion of primary particles is observed, probably due to the bridging effect of interaction (formation of associates) of adsorbed modifier molecules.
In previous research [19–21], some authors have found that oxyphenolfurfural oligomers, when adsorbed on the surface of monodispersion particles, form a nanoscale monomolecular layer. In this article, adsorption isotherms of oligomeric molecules on the surface of binary dispersion particles have been obtained (Fig. 6).
Analysis of the adsorption isotherms of the nanomodifier FF on the particles surface in monodispersions of
а) Dm = 5.9 µm
b) Dm = 5.6 µm
c) Dm = 4.9 µm
d) Dm = 3.4 µm
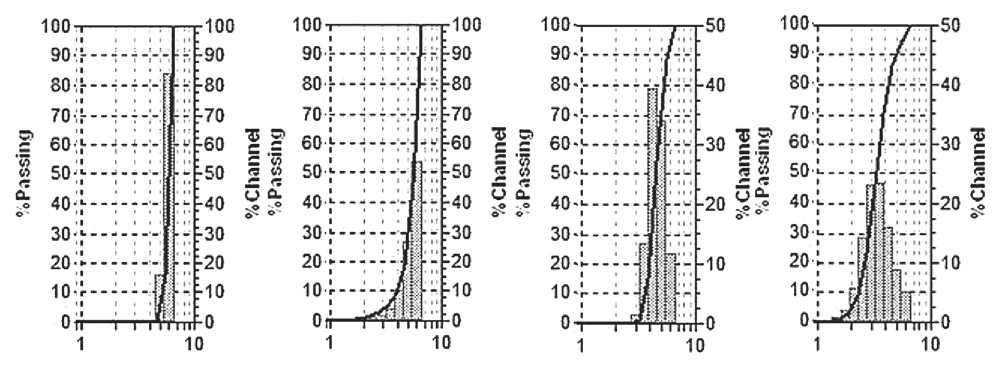
Fig. 5. Dispersion analysis of modified PVAD dispersions: a) 0% modifier; b) 0.01%; c) 0.2%; d) 0.3%
APPLICATION OF NANOMATERIALS AND NANOTECHNOLOGIES IN CONSTRUCTION
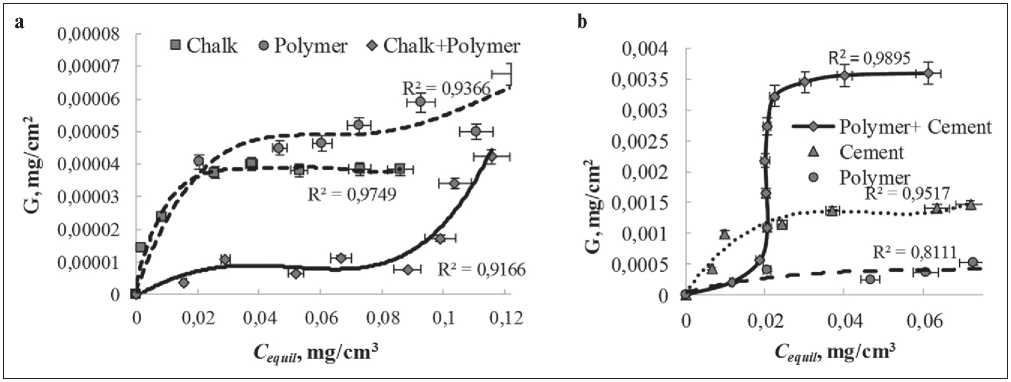
Fig. 6. Adsorption isotherms of the FF modifier on the particles of mono and binary systems (polymer-mineral ratio 0.1)
mineral nature allows us to attribute them to the Langmuir-type isotherms (by the characteristic convex initial segment). However, the obtained adsorption isotherms on the particles of monodispersions polymer nature and on binary polymer-mineral dispersions have a clearly pronounced S-shaped form (Fig. 6). Multiple changes of the solvent have shown that physical adsorption is observed in all studied mono and binary systems.
According to the S-shaped form of the obtained adsorption isotherms, the following conclusion can be drawn. The interaction energy of the adsorbate-adsorbate in the binary system between the FF and the polymeric stabilizer PVAC, introduced at the suspension polymerization stage, on the one hand, and with an increased content in the cement monodispersion due to the surface hydrolysis of acetate groups in an alkaline environment of the cement gel, on the other hand, is higher than the interaction energy of the adsorbate-adsorbent. This is due to the fact that oligomers of the modifier molecules are adsorbed, rather than individual molecules. However, we should not exclude the possibility that at FF concentrations lower than those required for complete filling of the adsorption monolayer, heterocoagulation of binary particles occurs, which also affects the shape of the adsorption isotherm.
Thus, there are two substances capable of adsorption in the dispersion medium of a binary system: molecules of the nanomodifier FF and the polymeric stabilizer PVAC. Phloroglucinol furfural oligomers are ionogenic surfactants at the solid-liquid interface with hydrophilic oxy groups and hydrophobic aromatic and furan rings. Polyvinyl alcohol is a non-ionic surfactant with hydrophilic OH–-groups and a hydrophobic hydrocarbon skeleton. Attractive interaction in the adsorbate-adsorbate system determines the manifestation of one of the synergism or antagonism effects considered in the introduction, depending on the nature of the binary system.
Analysis of the adsorption isotherms of the adsorbent complex on the hybrid surface in a binary system has shown that in the case of a polymer-chalk dispersion, the experimental value of Gmаx is less than the value (Fig. 6a) calculated according to the additivity rule, and in the binary polymer-cement system, on the contrary, the experimental Gmаx is greater than the additive value of adsorption (Fig. 6b). This with a high degree of probability indicates the attractive interaction in the adsorbateadsorbate system. With the Frumkin equation and analysis of typical isotherms, an estimate is given for the degree of this interaction in accordance with the equilibrium G and maximum adsorption Gmаx values, g/m2.
θ = G/Gmax, b•C = (θ/1–θ)•e–2aθ, where C is the equilibrium concentration of the adsorbate, g/m3; b is the adsorption equilibrium constant; a is the attraction constant.
In the case of a > 0, the isotherm takes on an S-shaped form, which is characteristic of attraction between adsorbed particles. With a < 0, the isotherm has a logarithmic form, indicating a repulsive interaction between adsorbed particles. When a = 0, a compensation of attraction and repulsion effects is observed, and formally, the Langmuir adsorption isotherm is satisfied.
In case of the adsorption of FF nanomodifier molecules on mineral particles, the attraction constant is a < 1, which is characteristic of the predominance of repulsion between the adsorbed particles. When the competitive adsorption of FF and PVAC on monodisperse polymer particles exists, then a = 0. When adsorption on the hybrid surface of binary polymer-chalk and polymer-cement dispersions takes place, then a > 0 according to the typical isotherms (Fig. 7), calculated with the equation presented in [22].
The value of the attraction constant a for adsorbates during their adsorption in monodisperse systems is prac-
APPLICATION OF NANOMATERIALS AND NANOTECHNOLOGIES IN CONSTRUCTION
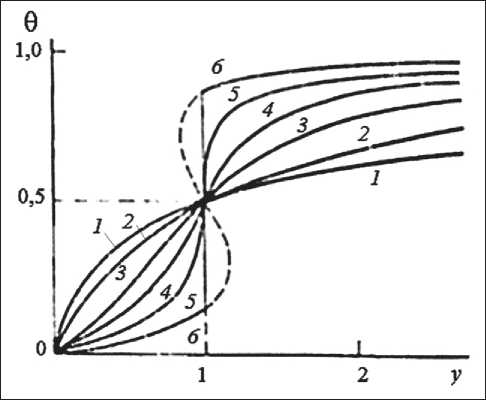
Fig. 7. Adsorption isotherms calculated with the Frumkin equation at various values of the attraction constant:
1 – a = –1; 2 – a = 0; 3 – a = 1; 4 – a = 1.5; 5 – a = 2;
6 – a = 2.5
tically zero. When studying the adsorption process of the adsorbate complex in binary systems, an increase in attractive interaction is observed, which is probably due to spatial difficulties during their joint competitive adsorption. The increase in the value of the constant a to 2.5 indicates the increase in the interaction energy between the adsorbates and explains experimental values of Gmаx , which are higher than calculated values according to the additivity rule. Interaction in the adsorbate-adsorbate system taking into account the presence of different hydrophilic groups leads to the adsorption of associates in the form of chains. As a result, in the binary polymer-cement system, a synergistic effect is manifested, which slows down (in the studied time range, it is completely absent) the coagulation and heterocoagulation of particles in binary systems due to the complex action of the FF and PVAC modifiers (Fig. 4), resulting in the aggregate stability that system acquires.
In the binary polymer-chalk system, an antagonistic effect has been determined during the adsorption of the FF and PVAC modifier complex, which can be explained by the relatively equivalent interaction between the adsorbates and in the adsorbate-adsorbent system. According to the results of microscopy (Fig. 1), incomplete prevention of particle coagulation has been visually observed during nanomodification with phloroglucinol furfural oligomers, while for monodispersions, this amount of modifier is optimal for the peptization of chalk particles into primary particles.
The aggregate stability of binary dispersions is due to the presence of hydrophobic and hydrophilic ionogenic and non-ionogenic groups in the FF and PVAC modifier, which leads to the joint action of various factors of aggregate stability. As the research results have shown, the aggregate stability of mono- and binary dispersions is different and probably depends on the hydrophilicity of the hybrid polymer-mineral surface. The action of adsorption-solvation is due to the hydrophilization of the surface, which leads to a decrease in the Hamaker constant. As the Hamaker constant increases, which is typical for the transition from a mineral surface to a polymer surface for monodispersions, the contribution of the adsorption-solvation factor to the aggregate stability of binary systems with a modifier complex will increase.
CONCLUSION
On a laser diffractometer in the range of 0.8 nm to 6500 nm, we have found that the dispersion of polyvinyl acetate loses its aggregate stability as the amount of dispersion medium increases. The modal diameter of particles increases from 4.5 to 6.0 µm. Microscopic analysis has clearly shown the formation of large polymer particle ensembles up to 40 µm in diameter. Analyzing the obtained results, one can disagree with the statement made in [23] that the polyvinyl acetate dispersion is diluted with water in any ratio and mixes well with cement without any signs of coagulation. Heterocoagulation of binary dispersions is observed.
It is proved that the phloroglucinol furfural modifier contributes to the stabilization of binary polymermineral dispersions. According to the differential curves of particles distribution in a wide range from 10 nm to 2100 µm, a pattern of reducing the modal diameter of adsorption-nanomodified polymer particles in the investigated concentration limits has been established, also complete absence of large coagula, and a transition to a wider distribution. Regularities of the interaction of complex modifier components and their influence on the properties of binary dispersions have been obtained.
New patterns of the interaction of complex modifier components and their influence on the properties of binary dispersions have been obtained. In the polymerchalk system, an antagonistic effect has been observed in the presence of an additive complex; effect is caused by the relatively equal action of forces both in the adsorbateadsorbent system and in the adsorbate-adsorbate system. In the polymer-cement system, on the contrary, a synergies effect has been observed that significantly slows down the coagulation processes through the joint action of the modifier complex, resulting in the binary system acquiring aggregate stability.
We hypothesized that the adsorption-solvation factor contributes significantly to the aggregate stability of binary polymer-mineral dispersions. The degree of its action is different for mono- and binary polymer-mineral dispersions and depends on the hydrophilicity of the dispersed phase surface. The role of this factor increases with increase in Hamaker constant.
APPLICATION OF NANOMATERIALS AND NANOTECHNOLOGIES IN CONSTRUCTION
Список литературы Regulation of the aggregate stability for binary polymer-mineral dispersions
- Shenoy S.S., Sadowsky R., Mangum J.L., Hanus L.H., Wagner N.J. Heteroflocculation of binary latex dispersions of similar chemistry but varying size. Journal of Colloid and Interface Science. 2003; 2(268): 380–393. – https://doi.org/10.1016/j.jcis.2003.08.039
- Tinkler J.D., Scacchi A., Kothari H.R., Tulliver H., Argaiz M., Archer A.J., Martín-Fabiani I. Evaporation-driven self-assembly of binary and ternary colloidal polymer nanocomposites for abrasion resistant applications. Journal of Colloid and Interface Science. 2021; (581): 729–740. – https://doi.org/10.1016/j.jcis.2020.08.001
- Blinov A.V., Maglakelidze D.G., Rekhman Z.A., Yasnaya M.A., Gvozdenko A.A., Golik A.B., Blinova A.A., Kolodkin M.A., Alharbi N.S., Kadaikunnan S., Thiruvengadam M., Shariati M.A., Nagdalian A.A. Investigation of the Effect of Dispersion Medium Parameters on the Aggregative Stability of Selenium Nanoparticles Stabilized with Catamine AB. Micromachines. 2023, 14: 433. – https://doi.org/10.3390/mi14020433
- Horszczaruk E., Łukowski P., Seul C. Influence of Dispersing Method on the Quality of Nano-Admixtures Homogenization in Cement Matrix. Materials. 2020; 13: 4865. – https://doi.org/10.3390/ma13214865
- Sosa M.E., Villagrán-Zaccardi Y.A., Peralta J.P. and Zega C.J., Efficiency of cement-admixture systems in `mortars with binary and ternary Portland cements. DYNA. 2018; 85 (204):134–142.
- Li H., Qiu Y. Dispersion, sedimentation and aggregation of multi-walled carbon nanotubes as affected by single and binary mixed surfactants. Royal Society Open Science. 2019; 6: 190241. – https://doi.org/10.1098/rsos.190241
- Lange A., Plank J. Contribution of non-adsorbing polymers to cement dispersion. Cement and Concrete Research. 2016; 79: 131–136. https://doi.org/10.1016/j.cemconres.2015.09.003
- Gonzatti G.K., Netz P.A., Fiel L.A., Pohlmann A.R. Colloidal Dispersion Stability: Kinetic Modeling of Agglomeration and Aggregation. Journal of the Brazilian Chemical Society. 2015; 26(2): 373–380. – https://doi.org/10.5935/0103-5053.20140290
- Domnichenko R.G., Vostrikova G.Yu., Nikulin S.S. Production of Combined Epoxyacrylate Dispersion. Bulletin of VGUIT. 2021; 83 (1): 278-283. – https://doi.org/10.20914/2310-1202-2021-1-278-283
- Brinke A.J.W., Bailey L., Lekkerkerker H.N.W., Maitland G.C. Rheology modification in mixed shape colloidal dispersions. Part I: pure components. Soft Matter. 2007: 1145–1162. – https://doi.org/10.1039/B704742H
- Opanasenko O.N., Krutko E.T., Luksha O.V., Yakovets N.V. Structuring in mixed dispersions based on bitumen and epoxy emulsions. Proceedings of BSTU. 2011; 4: 123-125.
- Jędrzejczak P., Ławniczak Ł., Ślosarczyk A., Klapiszewski Ł. Physicomechanical and Antimicrobial Characteristics of Cement Composites with Selected Nano-Sized Oxides and Binary Oxide Systems. Materials. 2022; 15, 661. https://doi.org/10.3390/ma15020661
- Shcherban’ M.G., Sosna M.Kh., Gogolishvili O.Sh., Anikushin B.M., Kornilitsina E.V. Dynamic aggregative stability of highly concentrated dispersion. Vestnik PGU. Chemistry. 2018; 8(3): 360-368. – https://doi.org/10.17072/2223-1838-2018-3-360368
- Marchon D., Kawashima S., Bessaies-Bey H., Mantellato S., Ng S. Hydration and rheology control of concrete for digital fabrication: Potential admixtures and cement chemistry. Cement and Concrete Research. 2018; 112: 96–110. https://doi.org/10.1016/j.cemconres.2018.05.014
- Klein B., Pawlik M. Rheology modifiers for mineral suspensions. Mining, Metallurgy & Exploration. 2005; 22: 83–88. – https://doi.org/10.1007/BF03403119
- Kosukhin M.M., Kosukhin A.M. The Role of Surface Phenomena in Modified Cement Dispersions at Studying Poly-Functional Modifiers’ Mechanism of Action. Solid State Phenomena. 2020; 299:1038–1043. – https://doi.org/10.4028/www.scientific.net/ssp.299.1038
- Poluektova V.A., Shapovalov N.A., Yastrebinsky R.N. Influence of Adsorption Modification on the Aggregate Stability of Polyvinyl Acetate Dispersion. Bulletin of Technological University. 2020; 11(23): 63-68.
- Poluektova V.A., Shapovalov N.A., Kozhanova E.P. Study of Competitive Adsorption of Modifiers on Particles of Polymer-cement System for Additive Technologies. Sorption and Chromatographic Processes. 2019; 3(19): 315-324. https://doi.org/10.17308/sorpchrom.2019.19/748
- Poluektova V.A. Electrokinetic Properties and Aggregative Stability of Polymer–Mineral Dispersions for 3D Printing in Building. Russian Journal of Physical Chemistry A. 2019; 9(93): 1783–1788. https://doi.org/10.1134/S0036024419090164
- Poluektova V.A., Kozhanova E.P., Kudina A.E. Adsorption of phloroglucinfurfural oligomers on the surface of polymer-mineral dispersions. Vestnik BSTU named after V.G. Shukhov. 2017; 10:116–122. https://doi.org/10.12737/article_59cd0c61195958.39964053
- Shapovalov N.A., Poluektova V.A. Some aspects of nanomodification of mineral dispersions by oligomers based on trifunctional oxyphenyl // Nanotechnologies in Construction: A Scientific Internet-Journal. 2016; 6(8): 43–57. https://doi.org/10.15828/2075-8545-2016-8-6-43-57
- Electronic processes in solutions of organic compounds: Textbook. Ed. by B.B. Damaskin. Moscow: Publishing House of Moscow University; 1985.
- Popov K.N. Polymer and Polymer-cement Concretes, Solutions and Mastics. Moscow: Higher School Publishing; 1987.


