Research and optimization of the technology for the synthesis of a modifying additive based on a mixture of hydrosilicates and calcium aluminosilicates
Автор: Loganina V.I., Frolov M.V.
Журнал: Nanotechnologies in Construction: A Scientific Internet-Journal @nanobuild-en
Рубрика: Construction materials science
Статья в выпуске: 3 Vol.14, 2022 года.
Бесплатный доступ
Introduction. To improve the performance properties of lime coatings, modifying additives are introduced into their formulation. The development of a technology for the synthesis of an additive containing calcium hydro- and aluminosilicates, which promote the binding of lime and increase the resistance of the lime composite, is of current interest. Materials and methods. To prepare an additive based on a mixture of hydro silicates and calcium aluminosilicates, liquid sodium glass (GOST 13078), technical (purified) aluminum sulfate (GOST 12966), and quicklime were used. The pozzolanic activity of the materials was determined by the absorption of lime from lime mortar. Results. It has been established that the mineralogical composition of the additive obtained at the 1st stage of synthesis is represented by hydro silicates of the tobermorite group. The mineralogical composition of the additive obtained at the 2nd stage of synthesis is represented by hydro silicates of the tobermorite group, gypsum, and calcium aluminosilicates. The particle size of the additive is from 10 to 40 μm. The pozzolanic activity of the additive obtained at the first stage of synthesis was 238.6 mg/g, and that obtained at the second stage of synthesis was 3.2 times higher and amounted to 762.5 mg/g. The introduction of an additive obtained at the 2nd stage of synthesis into the composition increases the compressive strength of lime composites by 2.87 times. Conclusion. It is proposed to use a modifying additive obtained by a two-stage synthesis technology in heat-insulating DBM. The optimal concentration of the proposed modifying additive is selected, which is 10% by weight of lime.
Modifier, calcium hydro silicates, calcium hydro aluminosilicates, lime, hardening
Короткий адрес: https://sciup.org/142232049
IDR: 142232049 | DOI: 10.15828/2075-8545-2022-14-3-190-197
Текст научной статьи Research and optimization of the technology for the synthesis of a modifying additive based on a mixture of hydrosilicates and calcium aluminosilicates
Original article
H eat-insulating plaster compositions based on lime binder for exterior wall decoration are not widely used, because. It is believed that coatings based on them do not have the required performance properties. In [1–4], in order to improve the moisture resistance, crack resistance, and durability of finishing compositions based on lime binder, it is proposed to introduce various organomineral additives, calcium hydro silicates, and synthetic zeolites into their composition. It has been established that, using specialized modifying additives, it is possible to significantly improve the performance properties of lime coatings [5–7].
It was shown in [8–10] that the introduction of calcium hydro silicates into a lime mixture significantly increases the strength of the resulting composites. In [11–
-
13] it was found that the use of calcium aluminosilicates as a modifying additive makes it possible to significantly accelerate the structure formation of a lime mixture.
The paper proposes to use a mixture of hydro silicates and hydro aluminosilicates of calcium as a modifying additive for the developed heat insulating DBM. As a working hypothesis, it was assumed that when calcium hydro silicates and calcium hydro aluminosilicates are simultaneously introduced into the lime mixture, it is possible to obtain a binder, which makes it possible to obtain composites characterized by improved technical and operational properties.
METHODS AND MATERIALS
Slaked lime (fluff) with an activity of 84%, obtained at the Kamensk enterprise “Atmis-sugar” for technological
CONSTRUCTION MATERIAL SCIENCE purposes, with a true density of 2200 kg/m3, a bulk density of 480 kg/m3, and a specific surface area of 1050 m2/kg, was used as a binder.
To prepare an additive based on a mixture of hydro silicates and calcium aluminosilicates, the following components were used:
– liquid sodium glass (GOST 13078) ;
– technical (purified) aluminum sulfate (GOST 12966 with amendments 1.2);
– quicklime obtained at the Kamensky enterprise "At-mis-sugar" for technological purposes;
– distilled water.
The pozzolanic activity of materials was determined by the method of absorption of lime from lime mortar. When determining the activity of the material, two titrated solutions were used: a saturated solution of lime and 0.05 N. hydrochloric acid solution. To prepare a solution of lime, 10 g of quicklime was placed in a 5-liter bottle and filled with distilled water. The resulting solution was shaken several times a day. After 3 days, the bottle was opened, and a small amount of the solution was filtered off. Then 50 ml of the filtered solution was taken and titrated with 0.05 N. hydrochloric acid solution.
The amount contained in 50 ml of a saturated solution Ca(OH)2 was determined by the formula:
U o = V HCL T, (1)
where VHCL is the amount of 0.05 N hydrochloric acid solution used for titration, ml;
T is titer of 0.05 N hydrochloric acid solution, mg/ml.
The lime solution was saturated until the content Ca(OH)2 in the filtered sample of the solution with a volume of 50 ml Uо reached 53 mg. Mass concentration Ca(OH)2 Cо (mg/l) in a saturated solution was determined by the formula:
C o = U o / V sol , (2)
where Vp–pa is the volume of saturated lime solution selected for titration, equal to 50 ml.
The saturated lime solution thus obtained had a concentration of 1.06 g/l.
The test material was crushed in a porcelain mortar until it passed through a sieve no. A weighed portion of the powder weighing 2 g, previously weighed on an analytical balance, was carefully poured into a previously washed and dried 110 ml container. Then, using a burette, 100 ml of a saturated lime solution was poured into a container. After that, the container was closed and vigorously shaken, making sure that no part of the test powder stuck to the bottom of the container. During the experiment, the container was shaken periodically.
After 2 days from the time of filling the container, 50 ml of the solution was carefully taken from it, without shaking, using a pipette for titration into a 250 ml flask. Then the contents of the flask were titrated after adding 2–3 drops of the methyl orange indicator with a solution of 0.05 N. hydrochloric acid solution.
The amount contained in the solution (mg) Ca(OH)2 Ui was determined by the formula:
U i = V HCL T, (3)
where VHCL is the amount of 0.05 N hydrochloric acid solution used for titration, ml;
T – titer of 0.05 N hydrochloric acid solution, mg/ml.
The amount of lime absorbed by 1 g of the studied powder for the 2nd day Ai (mg) was determined by the formula:
A i = U o –U i . (4)
At the end of the titration, 50 ml of a saturated lime solution was poured into the container using a burette, the contents were shaken for 1 min. After adding 50 ml of a saturated lime solution to the cylinder, the amount contained in 50 ml of the resulting solution Ca(OH)2 UCa(OH) (mg) was determined by the formula
U Ca(OH)2 = (U о +U i )/2. (5)
The experiment was repeated every 2 days, while determining the amount of absorbed lime Ai(mg), formula (4) was transformed into the formula:
A i = U Ca(OH)2 –U i , (6)
where UCa(OH) is the amount Ca(OH)2 contained in 50 ml of the solution obtained in the container after the previous titration, mg.
The activity of materials A (mg) was determined by the amount of Ca(OH)2 absorbed by 1 g of the filler in 30 days:
А = ∑15i = 1 Ai (7)
RESULTS AND DISCUSSIONS
The additive preparation technology was as follows. At the first stage, quick lime was extinguished with water. Then, a liquid glass solution containing 122.4 g/l SiO2 and 47.4 g/l Na2O was poured into this mixture. The resulting pulp was stirred for 15 minutes. Then the precipitate was filtered off, for which the resulting suspension was dehydrated by placing it on filter paper. In the course of the precipitation experiment, the effects of heat treatment on the synthesis of calcium hydrosilicates were investigated. To do this, we changed the temperature conditions under which the reactions for the synthesis of hydrosilicates took
CONSTRUCTION MATERIAL SCIENCE place. The results of the conducted studies are presented in the table 1.
During the interaction of solutions of lime and water glass, not subjected to heat treatment, no precipitate was observed. Temperature treatment of only the lime mortar leads to the formation of a small amount of sediment, which is practically not amenable to dehydration, which apparently indicates the retention of a significant proportion of unreacted water glass in the suspension. Thermal treatment of all the considered components involved in the additive synthesis reaction at the first stage makes it possible to obtain a significant amount of precipitate. The resulting precipitate lends itself very well to dehydration, which indicates a low content of unreacted liquid glass in it. At the same time, due to the heating of the water that is used to slake the lime, a large number of particles of unreacted lime is not observed in the resulting sediment. Therefore, the following technology for the synthesis of additives at the first stage was subsequently adopted – quicklime was quenched with water heated to 60oС, and the resulting solution was brought to a boil and a liquid glass solution heated to 60oС was poured into it.
At the second stage, the filtered precipitate was treated with a 10% aluminum sulfate solution until the pH of the resulting mixture dropped to 6.5. The choice of this pH value is explained by the fact that at a given pH value, the resulting mixture is neutral and, as a result, further reaction to form calcium hydrosilicates and calcium hydroalu- minosilicates is impossible. The resulting mixture was again filtered. Precipitates formed at the first and second stages were dried at 100–105oС for 12 hours. To assess the feasibility of the adopted two-stage technology for the synthesis of additives, further studies were conducted in parallel for additives formed at the first and second stages.
The additive obtained at the 1st stage of synthesis after drying is a white powder, the true density ρst is equal to 2100 kg/m3, the bulk density ρsat is equal to 380 kg/m3, the specific surface area Ssp measured on the PSKh-12 device by the method of gas filtration through porous bodies is 680 m2/kg;
The additive obtained at the 2nd stage of synthesis after drying is a white powder, the true density ρst is equal to 2140 kg/m3, the bulk density ρsat is equal to 240 kg/m3, the specific surface area Ssp measured on the PSKh-12 device by the method of gas filtration through porous bodies is 1380 m2/kg;
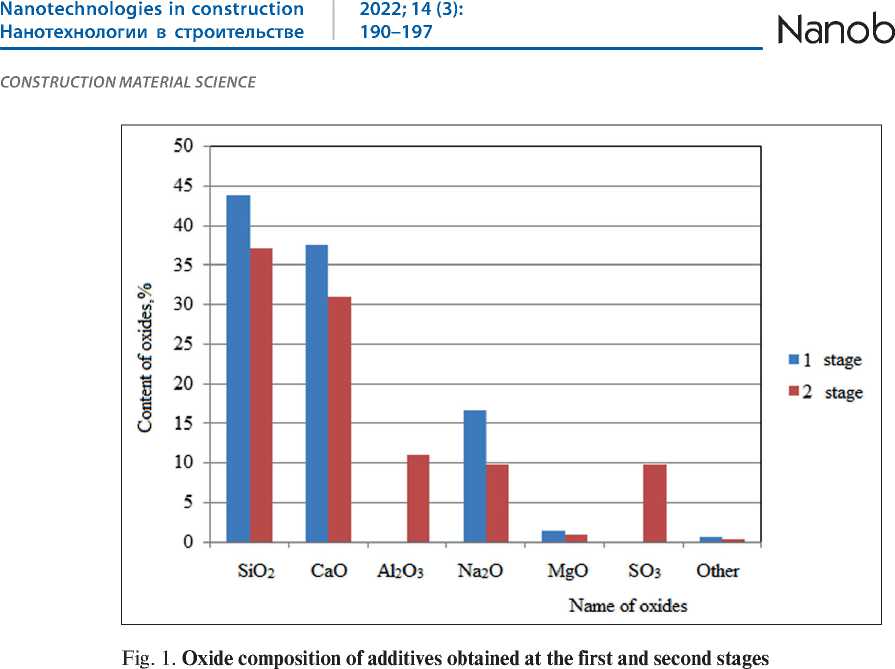
The oxide composition of additives obtained at the first and second stages is shown in the Fig. 1.
An analysis of the data presented in the Fig. 1 indicates that the composition of the modifying additive obtained at the first stage is dominated by oxides of SiO2, CaO, Na2O. This oxide composition is characteristic of calcium hydrosilicates. In the composition of the modifying additive obtained at the second stage of synthesis, while maintaining a considerable proportion of oxides SiO2, CaO, Na2O, oxides Al2O3, and SO3 additionally appeared.
Table 1
Evaluation of the influence of the parameters of thermal treatment of components on the characteristics of the precipitate obtained at the first stage of synthesis
|
The parameters of thermal treatment |
Sludge characteristics |
||
|
Water for lime slaking |
Lime solution |
Liquid glass solution |
|
|
Is absent |
Is absent |
Is absent |
No sediment |
|
60oС |
Is absent |
Is absent |
No sediment |
|
60oС |
Up to the boil |
Is absent |
A small amount of sludge is observed, the sludge is practically not amenable to dehydration |
|
60oС |
Up to the boil |
40oС |
Sediment formation is observed, the sediment is poorly dehydrated |
|
Is absent |
Up to the boil |
60oС |
Precipitate formation is observed, the sludge is weakly amenable to dehydration, a significant amount of unreacted Ca(OH)2 is observed in the sludge |
|
60oС |
Up to the boil |
60oС |
A significant amount of sludge is observed, the sludge lends itself very well to dehydration |

This suggests that the mineralogical composition of the additive was supplemented with gypsum and calcium hydroaluminosilicates.
To assess the mineralogical composition of the additives obtained at the first and second stages of synthesis, their XPA was conducted. The X-ray pattern of the samples of the additive obtained at the first stage is shown in the Fig. 2. The X-ray pattern of the samples of the additive obtained at the second stage is shown in the Fig. 3.
X-ray analysis showed that the mineralogical composition of the additive obtained at the first stage is repre- sented by minerals of the tobermorite group, Å (d = 3.037, d = 3.201, d = 1.818), portlandite, Å (d = 2.631, d = 4.940, d = 1.681), calcite, Å (d = 2.109, d = 3.034, d = 1.878).
X-ray analysis of the additive obtained at the second stage showed that in its mineralogical composition are preserved calcium hydrosilicates, represented by minerals of the tobermorite group, Å (d = 3.049, d = 3.203, d = 1.826, d = 1.676) and a solid solution of CSH(B) in in the form of a weakly crystallized gel, Å (d = 2.181, d = 1.882, d = 1.741). Additionally, gypsum, Å (d = 7.638, d = 4.298) and hemihydrate gypsum, Å (d = 6.063,
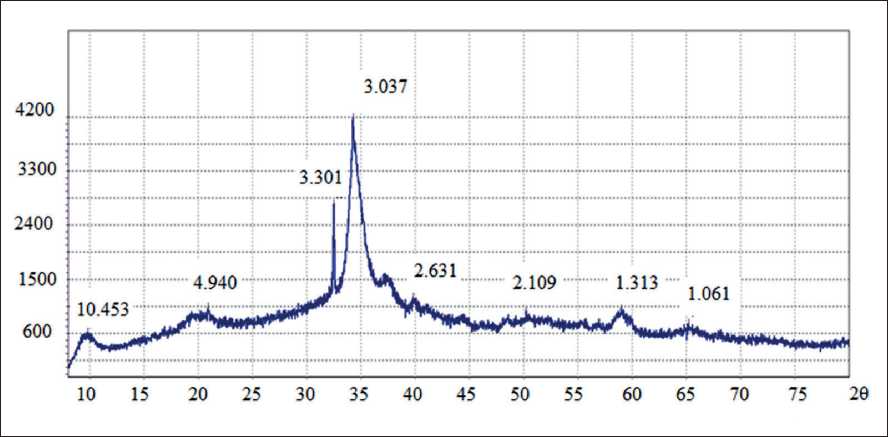
Fig. 2. X-ray diffraction pattern of additive samples obtained at the first stage of synthesis
CONSTRUCTION MATERIAL SCIENCE
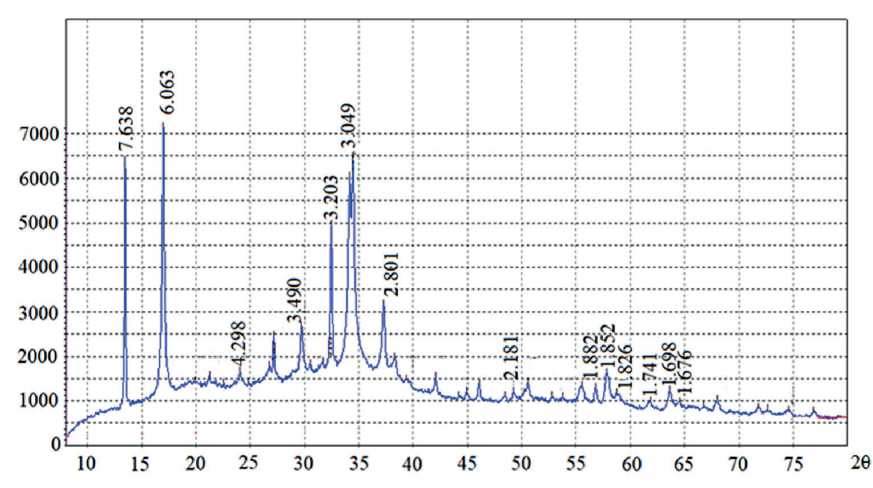
Fig. 3. X-ray pattern of additive samples obtained at the second stage of synthesis
d = 2.801, d = 3.490, d = 1.852, d = 1.698) were found in the mineralogical composition of the additive. Also, taking into account the fact that in the oxide composition of the additive obtained at the second stage of synthesis, a significant content of Al2O3 was found – 10.98, but as a result of X-ray diffraction analysis no minerals containing Al2O3 were found, it can be assumed that the additive contains an X-ray amorphous phase containing calcium hydroaluminosilicates.
The Fig. 4 shows electron microscopic images of the synthesized additives obtained at stages 1 and 2 of the synthesis. Analysis of the data presented in the Fig. 4 indicates that the structure of the additive consists of particles of lamellar and acicular shapes of various sizes from 10 to 40 µm.
Considering that high pozzolanic activity in relation to the binder is one of the main requirements for modifying additives, the pozzolanic activity of additives obtained at the first and second stages of synthesis was evaluated [16–18].
It was found that 30 days after the start of the experiment, the pozzolanic activity of the additive obtained at
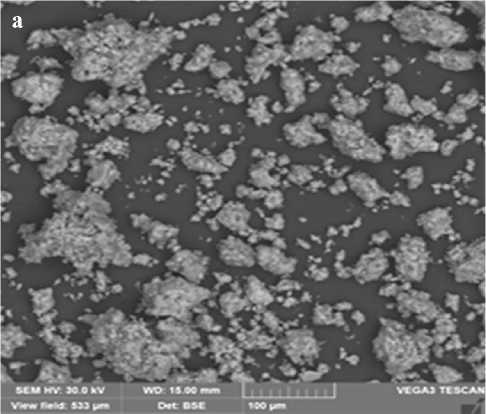
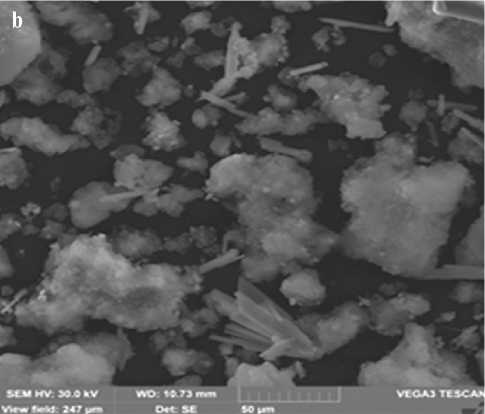
Fig. 4. Electron microscopic picture of the additive: a – obtained at the 1st stage of synthesis; b – obtained at the 2nd stage of synthesis
CONSTRUCTION MATERIAL SCIENCE
Table 2
Strength of composites, MPa
An increase in the pozzolanic activity of the additive obtained at the second stage of synthesis can be explained by the appearance of calcium hydroaluminosilicates in its composition and an increase in the specific surface of the additive [19, 20].
The experiment to determine the pozzolanic activity for the additives obtained in the first and second stages was continued until the amount of calcium hydroxide absorbed by the additives ceased to increase. The additive obtained at the first stage continued to actively absorb Ca(OH)2 for 40 days from the beginning of the experiment, then the absorption rate decreased and after 90 days from the beginning of the experiment, the activity reached 285.0 mg/g. The additive obtained at the second stage continued to actively absorb Ca(OH)2 much longer, up to 90 days, while its activity reached 1280.0 mg/g.
To assess the effectiveness of the use of additives obtained at the first and second stages, the kinetics of the compressive strength development of lime composites obtained with the use of additives was studied. The content of additives obtained at the first and second stages of synthesis was taken equal to 10% of the mass of lime. The water-lime ratio was taken equal to W/L = 1.0. The research results are presented in the Table 2.
When analyzing the experimental data given in the Table 2, it was found that the introduction of the developed additives into the lime composition increases the compressive strength of the resulting lime composites. The introduction of an additive obtained at the first stage of synthesis into the composition increases the compressive strength of the samples after 28 days of air-dry hardening by 54.7% relative to the control samples. It has been established that in lime composites the content of free lime was 49.1%, in composites using an additive obtained at the first stage of synthesis, the content of free lime was 40.2%,
Table 3
Technological and operational properties of the developed heat insulating DBM
|
Parameter |
The value of the indicator for the composition |
||||
|
Composition 1 |
Composition 2 |
Prototype 1 |
Prototype 2 |
Prototype 3 |
|
|
Average coating density, kg/m3 |
580 |
650 |
1100 |
550 |
1550 |
|
Bulk density, kg/m3 |
380 |
440 |
1100 |
550 |
1500 |
|
Compressive strength, MPa |
3.6 |
4.1 |
3.4 |
3.0 |
4.0 |
|
DBM consumption when applying a layer 10 mm thick, kg/m2 |
5.9 |
6.6 |
12.0 |
5.5 |
14.0 |
|
Thermal conductivity coefficient, W/(m•оC) |
0.119 |
0.137 |
0.350 |
0.130 |
0.400 |
|
Vapor permeability coefficient, mg/(m•h•Pa) |
0.157 |
0.150 |
0.100 |
0.110 |
0.100 |
|
Water holding capacity, % |
99.0 |
99.1 |
97.0 |
97.0 |
97.8 |
|
Strength of adhesion to the base, MPa |
0.65 |
0.71 |
0.40 |
0.30 |
0.40 |
|
Frostresistance, brand |
F35 |
F35 |
F35 |
F50 |
F50 |
|
Applicability |
good |
good |
good |
good |
good |
CONSTRUCTION MATERIAL SCIENCE
The introduction of an additive obtained at the second stage of synthesis into the composition increases the compressive strength of the samples after 28 days of air-dry hardening by 187.2% relative to the control samples. It was found that in composites using the additive obtained at the second stage of synthesis, the content of free lime was 28.6%.
The presented data prove the feasibility of the adopted two-stage synthesis technology.
Using the developed modifying additive, compositions of heat insulating DBM for finishing aerated concrete have been developed.
The table 3 shows the main operational and technological properties of heat-insulating compositions for finishing aerated concrete based on the developed DBM formulations.
Lightweight cement plaster “Knauf GRUNBAND” manufactured by LLC “Knauf GIPS” was chosen as prototype 1, as prototype 2 heat-insulating plaster for aerated concrete “UMKA UF-2” manufactured by LLC “Eco-termogroup”, as prototype 3 – plaster “Lime-cement” for finishing aerated concrete company “Bolars”.
An analysis of the data given in the Table 3 showed that the developed compositions of heat-insulating DBM for finishing aerated concrete have a number of advantages compared to analogues: high vapor permeability, high adhesion strength to the surface of aerated concrete, high water-holding capacity.
CONCLUSION
It is proposed to use a modifying additive obtained by a two-stage synthesis technology in heat-insulating DBM. The chemical and mineralogical composition of the additives obtained at the first and second stages of the synthesis of the modifying additive has been determined. It has been established that the oxide composition of the additive obtained at the 1st stage of synthesis is dominated by oxides: SiO2, CaO, Na2O. The mineralogical composition of the additive obtained at the 1st stage of synthesis is mainly represented by hydrosilicates of the tobermorite group. In the oxide composition of the additive obtained at the 2nd stage of synthesis, while maintaining a significant proportion of the oxides SiO2, CaO, Na2O, oxides of Al2O3 and SO3 were additionally found. The mineralogical composition of the additive obtained at the 2nd stage of synthesis is represented by hydrosilicates of the tober-morite group, gypsum, and calcium aluminosilicates.
The expediency of the adopted two-stage technology for the synthesis of the modifying additive is proved. The pozzolanic activity of additives obtained after stages 1 and 2 was determined by the method of lime absorption from lime mortar. It has been established that the additive obtained after the 1st stage of synthesis is characterized by pozzolanic activity of 238.6 mg/g. The pozzolanic activity of the additive obtained after the 2nd stage of synthesis is 3.2 times higher and amounts to 762.5 mg/g. Regularities of hardening of lime mortars in the presence of additives obtained at the 1st and 2nd stages of synthesis have been established. It was revealed that the introduction of an additive obtained at the 1st stage of synthesis into the composition increases the compressive strength of lime composites by 1.55 times. The introduction of an additive obtained at the 2nd stage of synthesis into the composition increases the compressive strength of lime composites by 2.87 times. The optimal concentration of the proposed modifying additive was selected, which is 10% by weight of lime.
Список литературы Research and optimization of the technology for the synthesis of a modifying additive based on a mixture of hydrosilicates and calcium aluminosilicates
- Loganina V.I., Petukhova N.A., Gorbunov V.N., Dmitrieva T.N. Prospects for the manufacture of organo-mineral additives based on domestic raw materials. News of higher educational institutions. Construction. 2009; No. 9 (609):36-39.
- Loganina V.I., Makarova L.V. Plaster compositions for restoration work using colored fillers. Regional architecture and construction. 2009; No. 1: 38-40.
- Loganina V.I., Kislitsyna S.N., Zhernovsky I.V., Sadovnikova M.A. Lime finishing compositions with the use of synthesized aluminosilicates. Bulletin of the Belgorod State Technological University named after V.G. Shukhov. 2014; No. 2: 55-57.
- Grishina A.N., Korolev E.V., Gladkikh V.A. Hydration of Cement in the Presence of Biocidal Modifiers Based on Metal Hydrosilicates. Materials. 2022;15(1):29. https://doi.org/10.3390/ma15010292
- Scherb S., Maier M., Beuntner N., Thienel K.-C., Neubauer J. Reaction kinetics during early hydration of calcined phyllosilicates in clinker-free model systems. Cement and Concrete Research.2021; 143: 106382. https://doi.org/10.1016/j.cemconres.2021.106382
- Kadri E.-H., Kenai S., Ezziane K., Siddique R., De Schutter G. Influence of metakaolin and silica fume on the heat of hydration and compressive strength development of mortar. Applied Clay Science. 2011; 53 (4): 704-708. https://doi.org/10.1016/j.clay.2011.06.008
- Strokova V.V., Nelubova V.V., Rykunova M.D. Resistance of cement stone in sanitation solutions. Magazine of Civil Engineering.2019; 90 (6): 72-84. https://doi.org/10.18720/MCE.90.7
- Valentina Loganina, Kristina Sergeeva, Roman Fediuk, Sergey Klyuev, Nikolai Vatin and YuriyVasilev. Modified Lime Binders for Restoration Work. Buildings 2021;11: 98. https://doi.org/10.3390/buildings11030098
- Laniesse P., Cau Dit Coumes C., Le Saout G., Mesbah A. Understanding the setting and hardening process of wollastonite-based brushite cement. Part 2: Influence of the boron and aluminum concentrations in the mixing solution. Cement and Concrete Research.2021; 140:106288. https://doi.org/10.1016/j.cemconres.2020.106288
- Grishina, A., Korolev, E. Chemical composition of silicate modifier for composite biocidal binder (2016) AIP Conference Proceedings.2016; 1772: 020003. https://doi.org/10.1063/1.4964525
- Loganina V.I., Tarasov R.V., Makarova L.V., Sadovnikova M.A. Composition limy binder with the use of the synthesized alumiosilicates for dry construction blends. Advanced Materials Research.2014;977:34-37. https://doi.org/10.4028/www.scientific.net/AMR.977.34
- Loganina, V., Zhegera, K., Fediuk, R., Zayakhanov, M., Liseitsev, Y. Amorphous Aluminosilicates as a Structure-Forming Additive in Cementitious Systems. Journal of Materials in Civil Engineering. 2020;32(5):06020004. https://doi.org/10.1061/(ASCE)MT.1943-5533.0002995
- Galkin Yu. Yu., Udodov U.S., Vasil’eva L.V. The phase composition and properties of aluminate cements after early loading. Magazine of Civil Engineering. 2017; 75 (7): 114-122. https://doi.org/10.18720/MCE.75.11
- Loganina V., Frolov M., Fediuk R. Developed heat-insulating dry mortar mixes for the finishing of aerated concrete walls. Magazine of Concrete Research.2021;73(17):890-903. https://doi.org/10.1680/jmacr.19.00446
- Pachta V. The role of glass additives in the properties of lime-based grouts. Heritage. 2021;4(2):906-916. https://doi.org/10.3390/heritage4020049
- Grishina A.N., Eremin A. V. Effect of barium hydrosilicates on the early hydration rate of Portland cement. Inorganic Materials. 2016;52 (9):973-977. https://doi.org/10.1134/S0020168516090077
- Loganina V.I., Davydova O.A., Simonov E.E. Influence of diatomite activation on the properties of lime compositions. News of higher educational institutions. Construction. 2011; No. 3 (6):20-23.
- Centauro I., Cantisani E., Grandin C., Salvini A., Vettori, S. The Influence of Natural Organic Materials on the Properties of Traditional Lime-Based Mortars. International Journal of Architectural Heritage. 2017; 11 (5): 670-684. https://doi.org/10.1080/15583058.2017.1287978
- Elert K., García Sánchez R.M., Benavides-Reyes C., Linares Ordóñez F. Influence of animal glue on mineralogy, strength, and weathering resistance of lime plasters. Construction and Building Materials. 2019; 226: 625-635. https://doi.org/10.1016/j.conbuildmat.2019.07.261
- Brzyski P. The influence of gum arabic admixture on the mechanical properties of lime-metakaolin paste used as binder in hemp concrete. Materials. 2021;14(22):6775. https://doi.org/10.3390/ma14226775


