Research of separation of extraction and anthocyanins in prepa-ration of red table wines
Автор: Panahov T., Sharifzadah, J.
Журнал: Science, Education and Innovations in the Context of Modern Problems @imcra
Статья в выпуске: 6 vol.8, 2025 года.
Бесплатный доступ
The extraction stage in wine materials prepared from madarasa grape sort and separation process of anthocyanins was worked out practically and obtained results were given in the article. The separation stages of extraction and anthocyanins were studied in scientific-researches conducted by the purpose of improvement of preparation tech-nolgies of table wines from local red grape sorts grew in Azerbaijan.
Extraction, chromatography, elution, filter, anthocyanin, identification
Короткий адрес: https://sciup.org/16010746
IDR: 16010746 | DOI: 10.56334/sei/8.6.07
Текст научной статьи Research of separation of extraction and anthocyanins in prepa-ration of red table wines
RESEARCH ARTICLE Research of separation of extraction and anthocyanins in preparation of red table wines Tariyel Panahov \ \ \ \ Doctor Vine-growing and and wine-making Scientific-Research Institute, АZ0118 Baku city, Azerbaijan Email: Jahangir Shafizada Doctor Vine-growing and and wine-making Scientific-Research Institute, АZ0118 Baku city, Azerbaijan Email: Doi Serial Keywords \ Extraction, chromatography, elution, filter, anthocyanin, identification. Abstract The extraction stage in wine materials prepared from madarasa grape sort and separation process of anthocyanins was worked out practically and obtained results were given in the article. The separation stages of extraction and anthocyanins were studied in scientific-researches conducted by the purpose of improvement of preparation tech-nolgies of table wines from local red grape sorts grew in Azerbaijan. Citation Panahov T., Sharifzadah, J. (2025). Minority Literature Translation and the Problematic Interplay of Cultural Transfer. Science, Education and Innovations in the Context ofModern Problems, 8(5), 73-77; doi:10.56352/sei/8.6.07. Licensed © 2025 The Author(s). Published by Science, Education and Innovations in the context of modern problems (SEI) by IMCRA - International Meetings and Journals Research Association (Azerbaijan). This is an open access article under the CC BY license . Received: 26.01.2025 Accepted: 27.04.2025 Published: 20.05.2025 (available online)
The coloring substances were extracted with the support of 70% ethanol and 0,1% salt acid from fermented moonshine of Madrasa grape (the moonshine of product technically grew in 2012 was kept in freeze after fermentation for 4 days). By adding 0,25 dm3 alcohole to 64 gr. chopped moonshine, the extraction was performed by mixing periodically in 50±1oC. After one hour, the extract was pulled out and the operation was continued. Extract shares are filtered after combining, they wee steamed in steaming up to 40±2oC-d ə 0,060...0,065 dm3.
The research was implemented on paper by the method of one-size increasing chromotography. The washing was carried out for 3 ...5 hours by salt acid in 1:4 relation and then with water distilled up to neutral reaction.
The system of following fluids are used as movable phase: System No.1 – n-butanol: acetic acid: water (40:12:29); System No.2 - n-butanol: acetic acid: water (4:1:5)- upper layer; system No.3- water: thickened salt acid (97:3); System No.4 – acetic acid: salt acid: salt acid: water (15:3:82). System No.1 was used for separation of extract.
The chromotograms dried in drying cabinet after separation of extract were looked in the light, the zones were signed. Especially clear selected areas were pulverized by the purpose of eluation cutting separately and it emulated quickly with oxidizing ethanol (pH 1-2) with salt acid in 5±1oC temperature in the darkness. By rescheduling the eluate from porous filter, it was steamed under vacuum in 40±2oC temperature up to 3...4 ml. The obtained extract chromatographied again in the same fluid, then the necessary zone was eluted again, it was thickened and chromatographied, then the competentness of anthocyanins in chromatography were defined.
Depending on separation condition, there are different zones up to six in chromatography of initial extract. One of them became in dominant condition for the strength of color (is selected). After conduction of third rechromatography of such zone, 35 mg pure fraction of special (major) anthocyanin was obtained. This corresponds to 0,146% dry substance of moonshine [90].
Three main system ( № 2, № 3 and № 4) were used to identificate the structure of anthocyanin. The literature information about obtained Rf substance and malvidine – 3 – 0- glucoside were described in Table 1 [33].
Table 1
Rf×100 Anthocyanin marks of Madrasa grape in different solving systems and literature informations for malvidine – 3 – 0- glucoside
|
Systems |
Zones |
|
|
Example |
malvidine – 3 – 0- glucoside |
|
|
1 № -li |
32 |
33 |
|
2 № -li |
38 |
38 |
|
3 № -li |
05 |
06 |
|
4 № -li |
28 |
29 |
As it is known, depending on diversity of enviroment and working with specific reagents, changing of the colors of anthocyanins, also, is considered the identification sign [73]. The color of selected zone and during the chromatography, the literature informations of air and ammonia steam were reflected in table 2.
Table 2
Color of deparated substance and malvidine – 3 – glucoside depending on different conditions
|
Condition |
Anthocyanins |
|
|
Example |
Malvidine – 3 glucoside |
|
|
Duirng chromatography |
Pink |
Pink |
|
Air |
Violet |
Violet |
|
In Ammonia (NH 3 ) steam |
Blue |
Blue |
The anthocyanin separating while identification contains malvidine–3–0–glucosine (figure 1).
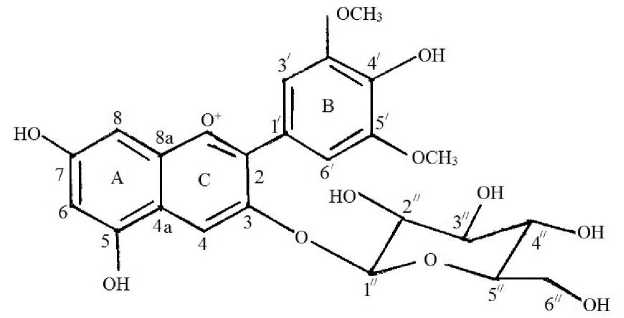
Figure.1. Malvidine-3-0-glucoside.
Acidic hydrolysis were implemented in following figure: it was mixed by adding 7 drip 6 n. HCl to the 1 ml anthocyanin fluid and the example was took after keeping 10 minutes in hot water. The example was separated by system No.1 by frictioning to chromatogram. Rf 1 =0,34 zone was in cotrol chromatogram by completing chromatography in the system. There are two zones Rf i =0,33 v a Rf 2 =0,64 in hydrolyzed chromatogram. It is seemed that the upper zone corresponds to hydrolyzed malvidine (anthocyanin) [31].
The ultraviolet and seemed spectroscopy were used for the identification of substance. The spectral characteristic wrote in standart solvent of anthocyanin and literature information for malvidine-3-0-glucoside was described in table 3.
Table 3
Spectral characteristic of anthocyanin and literature information
|
Anthocyanins |
Characteristics |
||||
|
In maximum seemed sphere of spectrum (C 3 OH+0,1%HCl), nm |
In maximum seemed sphere of spectrum (C 2 H 5 OH+0,01%HCl), nm |
Change by adding AlCl 3 , nm |
A, UB |
A 440 2 1 , max gor |
|
|
A, ■ 2 x, max gor |
|||||
|
Separated anthocyanin |
537 |
545 |
0 |
1,27 |
0,29 |
|
malvidine -3-0-glucoside |
537±2 |
545±1 |
0 |
1,20±20 |
0,30 |
Written spectrums of anthocyanins separated in the range of 200- 750 nm are issued in fugure 1 and figure 2. While adding AlCl 3 to anthocyanin fluid, the change was not observed in the spectrum of substance. But this shows that there is not ortho-hydroxide group in B ring. This certifies the structure of above mentioned pigment.
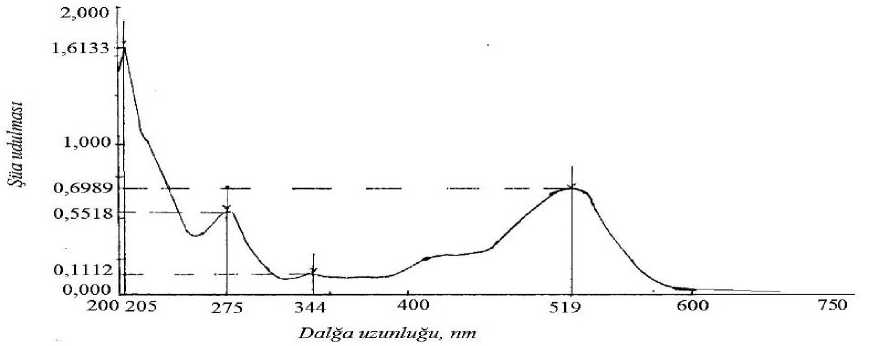
Figure. 2. Spectrum and ray swallowing maximums of anthocyanins separated in Calium choride (KCl) pH 1,0 buffer solution
(ray swallowing, wave length, nm)
There is a peak with 493 m/z (0,35) [M]+ mar kin the positive area of spectrum of masses during identification of anthocyanins separated by the method of fluid chromato-massive-spectroscopy method.
This corresponds with both molecular mass and literature information [10] on malvidine -3-0-glucoside.
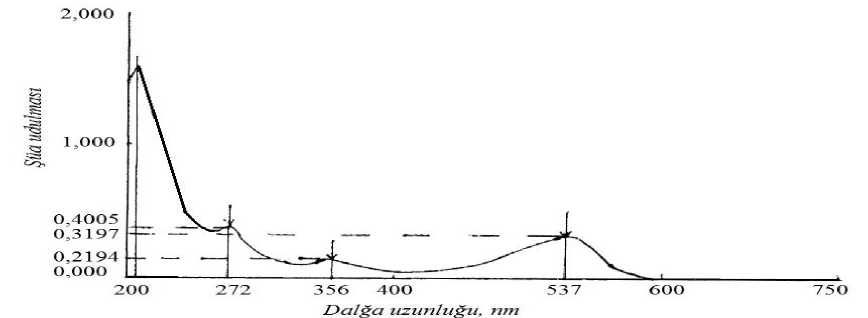
Figure. 3. Spectrum and ray swallowing maximums of anthocyanin in methanol fluid with 0,01% salt composition separated in Calium choride (KCl) pH 1,0 buffer solution (ray swallowing, length of wave, nm)
There is 509 m/z (0,40) [M+OH-H]- ion in negative sphere. This is created by monoglucoside of malvidin. The signals of nuclear magnate resonance spectrum of the substance will be corresponded with literature information and then they described in table 3.15 and table 3.16.
All signals of 1H spectrum of separated anthocyanin are correlated by the signals of 13C spectrum. The dependence of spectrum from heteronuclear correlation (examples C2-H4; C3-H4; C7-H8; C3`-H3`; C5`-H5`; C4`-H2`; C4`-H6`) corresponds to literature information [10].
Table 4
The comparison of chemical sliding of “13C ЯМР ” spectrum of anthocyanin of Madrasa sort and literature information for maldivine -3-0-glucoside.
|
Sign of situation |
For V.Atanasov |
For T.Mas |
Presented example |
|
1 |
2 |
3 |
4 |
|
2 |
162,48 |
164,1 |
163,7 |
|
3 |
145,06 |
146,2 |
145,5 |
|
4 |
136,55 |
137,5 |
137,1 |
|
4a |
113,26 |
114,2 |
113,2 |
|
5 |
158,77 |
160,3 |
158,9 |
|
6 |
103,00 |
104,0 |
103,3 |
|
7 |
170,10 |
172,2 |
170,2 |
|
8 |
94,89 |
95,9 |
95,2 |
|
8a |
157,13 |
158,6 |
158,6 |
|
1` |
119,96 |
120,3 |
118,8 |
|
2`,6` |
109,96 |
111,2 |
110,5 |
|
3` |
149,51 |
150,6 |
149,6 |
|
4` |
145,87 |
147,3 |
145,8 |
|
5` |
149,51 |
150,6 |
149,6 |
|
OCH 3 |
56,50 |
57,6 |
57,2 |
|
1`` |
103,25 |
104,5 |
103,7 |
|
2`` |
74,52 |
75,5 |
74,8 |
|
3`` |
76,82 |
79,3 |
78,0 |
|
4`` |
69,76 |
71,6 |
71,0 |
|
5`` |
78,12 |
78,8 |
78,7 |
|
6A`` |
61,54 |
62,7 |
62,0 |
|
6B`` |
61,54 |
62,7 |
62,1 |
C a dv a l 5
The comparison of chemical sliding of “1H ЯМР ” spectrum of anthocyanin of Madrasa sort and literature information for maldivine -3-0-glucoside.
|
Sign of situation |
For V.Atanasov |
For T.Mas |
For A.V.Ptitsın |
For E.Paley |
For A.Cheminat |
Presented example |
|
1 |
2 |
3 |
4 |
5 |
6 |
7 |
|
4 |
8,94 |
8,91 |
8,95 |
8,94 |
8,80 |
9,00 |
|
6 |
6,72 |
6,58 |
7,18 |
6,60 |
6,59 |
6,65 |
|
8 |
7,02 |
6,83 |
6,95 |
6,83 |
6,86 |
6,94 |
|
2`,6` |
7,93 |
7,84 |
8,15 |
7,87 |
7,71 |
7,95 |
|
OCH 3 |
3,90 |
3,94 |
3,79 |
3,91 |
3,87 |
3,97 |
|
1`` |
5,35 |
5,30 |
5,39 |
5,25 |
- |
5,32 |
|
2`` |
3,45 |
3,62 |
3,35 |
3,30 |
- |
3,62 |
|
3`` |
3,40 |
3,55 |
3,25 |
3,30 |
- |
3,54 |
|
4`` |
3,25 |
3,43 |
3,10 |
3,30 |
- |
3,39 |
|
5`` |
3,48 |
3,55 |
3,95 |
3,30 |
- |
3,55 |
|
6A`` |
3,73 |
3,91 |
4,10 |
3,84 |
- |
3,65 |
|
6B`` |
3,52 |
3,72 |
3,80 |
3,65 |
- |
3,88 |
The structure formula of pigment of Madrasa grape was defined: : malvidine-3-0-glucoside; 3-0— P —D gluczyloxy -4', 5, 7-trihydroxy -3`, 5`- demetoksiflavilium.


