Response of Brassica napus L grains to the interactive effect of salinity and salicylic acid
Автор: Salarizdah Mohammadreza, Baghizadeh Amin, Abasi Forogh, Mozaferi Hossin, Salarizdah Sorayya
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 2 т.8, 2012 года.
Бесплатный доступ
Soil salinity is a serious environmental problem that has negative effect on plant growth, production and photosynthesis. Fresh and dry plant weights decreases with salinity treatments. The very important role of salicylic acid (SA) in response to different stress and modification and decline damages due to stresses has established in different studies. In this research, effect of grain soaking presowing in (0, 1, 1.5, 2 and 5 mM) of salicylic acid (SA) and NaCl (0, 4, 8 and 12 dsm-1) on canola (Brassica napus L) was studied. Increasing of NaCl level reduced the germination percentage(GP), Average velocity of germination (AVG) and growth parameters of 15-day old seedlings in compared to control plants. pretreated of SA in content 1mM significantly increased the germination percentage, and in contents more than of 1mM reduced the germination percentage in seeds under salinity stress. SA in content 1mM increased RWC, root and shoot of fresh weight in the stressed seedlings. Increasing of NaCl level increased Electrolyte leakage and MDA content in the stress seedling. electrolyte leakage and MDA content were markedly reduced under salt stress with SA 1mM than without. It was concluded that SA could be used as a potential growth regulator to improve salt tolerance in canola. Our observations indicate that, although SA is not essential for germination under normal growth conditions, it plays a promotive role in seed germination under high salinity by reducing oxidative damage.
Salicylic acid, germination percentage, average velocity of germination, elative water contents, malondialdehyde, salinity
Короткий адрес: https://sciup.org/14323605
IDR: 14323605
Текст научной статьи Response of Brassica napus L grains to the interactive effect of salinity and salicylic acid
and carotenoids content in maize plants. SA is also known as an important signal molecule for modulating plant responses to environmental stress. (Senaratna et al. 2000). t is now clear that SA provide protection against a number of abiotic stress as heat stress in mustard seedlings (Dat et al. 1998), chilling damage in different plants (Tasgı´n et al.2003), heavy metal stress in barley (Metwally et al. 2003) and drought stress on wheat plants (Singh and Usha, 2003). The present investigation was carried out to study the effect of grain soaking presowing with salicylic acid on the salt stress responses of Brassica napus .
MATERIALS AND METHODS
Grains were sterilized with sodium hypochlorite solution (5%) for 5 min, washed 3 times with sterilized distilled water. Salicylic acid was dissolved in distilled water and the pH was adjusted at 6.5 with KOH (1 N). Preliminary screening the grains were soaked for 12 h in the dark at 220C, either in concentrations (0.5, 1, 1.5, 2 and 5 mM SA) or in distilled water as control. Then many pretreated grains were putted in Petri dishes (9 cm diameter) provided with two filter paper saturated with 20 ml Nacl in concentrations (0, 4, 8 and 12 dsm-1). The final GP and Average velocity of germination (AVG) was recorded after a period of 5 days in grains. n this stage the optimum response of germination in concentration of 1 mM SA was selected. The next stage many pretreated grains in 1mM SA content were putted in Petri dishes saturated with 7 ml Nacl in concentrations (0, 4, 8 and 12 dsm-1) and 20 ml of 50% strength Hoagland’s nutrient solution (Hoagland and Arnon 1950). The pH of the nutrient solution was maintained at pH 6.5 three replicates were prepared for each treatment. The final fresh weights of shoot and root, RWC, Electrolyte leakage and MDA content was recorded after a period of 15 days in seedling. The comparison of mean was done with
LSD test to SPSS 14.o software in probability level of 1٪ for drawing graph, we use Excel 2003 software.
Leaf relative water contents (%):
The leaf relative water contents (RWC) were calculated at the time harvest according to Beadle et al. (1993) using the equation: RWC (%) = [(FW -DW)/ (TW - DW)] 100 Where FW is fresh weight, DW is dry weight, and TW is turgid weight.
Electrolyte leakage measurements:
Approximately 0.2 g of fresh leaves was cut into about 1-cm segments and placed in 5 ml of deionized water at room temperature. After 45 min, the conductivity (C 1 ) was measured and then the samples were incubated in a boiling water bath for 15 min to achieve 100% electrolyte leakage (C 2 ). The results were calculated according to the formula: (%)=(C 1 C 2 )100 ( Dua at el; 2006).
Lipid peroxidation measurements:
Lipid peroxidation in leaves was evaluated by the malondialdehyde (MDA) content. Approximately 0.5 g of fresh leaves was homogenized in 5 ml of 5% (w/v) TCA in an ice bath. Then the homogenates were transferred into a tube and centrifuged at 1000g for 10 min at 4°C . Aliquots of the supernatant and 0.5% (w/v) thiobarbituric acid in 20% (w/v) TCA were added into a new tube. This mixture was incubated at 98°C for 40 min, then cooled to room temperature and centrifuged at 8000g for 5 min. The supernatant was subjected to spectrophotometric analysis. MDA content was calculated from the absorbance (A535– A600)using the extinction coefficient of 155/(mM cm). Karabal at el;(2003).
RESULTS AND DISCUSSION
The results of germination percentage(GP) and Average velocity of germination (AVG) Brassica napus grains under SA and salinity treatments has brought at figure (1). n the study, ncreasing of
NaCl levels reduced germination percent in brassica napus. These results are consistent with those of Steppuhn et al;(2001) who showed reduced germination percent in brassica and wheat plants. The seeds pretreated with (0.5, 1, 1.5, 2 and 5 mM) SA solution exhibited lower germination percentage than those of untreated(control) seeds. These results are consistent with those of (Zea mays) (Guan Senaratna et al., 2000). Exogenously applications of salicylic acid helped to increase plant growth significantly in saline conditions (Setevens et al., 2007). Gutieґrrez-Coronado et al. (1998) also reported a similar increase in the growth of shoots and roots of soybean plants under normal conditions in response to salicylic acid treatment. salt stress with oxin and cytokinin reduced growth in wheat seedling. Shakirova, F.rn; et al (2003). SA treatment increased oxin and cytokinin contents in wheat under salt stress. Sakabutdinova at el; (2003). (Singh et al., 2003)found that SA application increased the dry mass of wheat seedlings under water stress. Khodary (2004) has reported that SA increased the fresh and dry weight of shoots and roots of stressed maize plants, which is consistent with our results in brassica plants. The results of RWC, MDA content and electrolyte leakage Brassica napus seedlings under SA and salinity treatments has brought at figure (3).
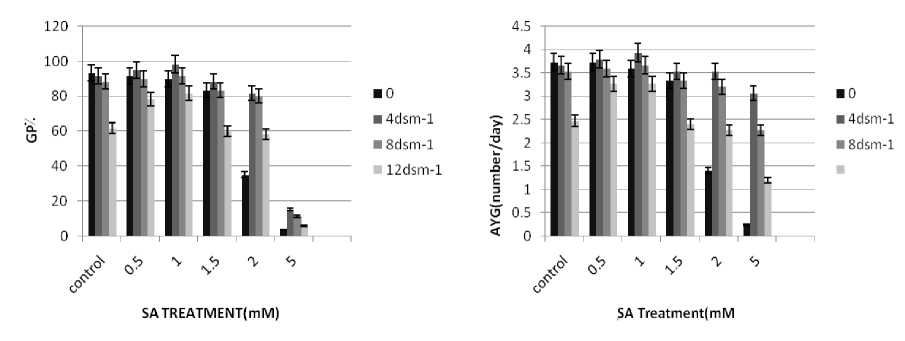
Figure 1 Effect of SA on germination percentage and germination speed mean
SA treatment (niM) controls ImM
SA treatment {mM)
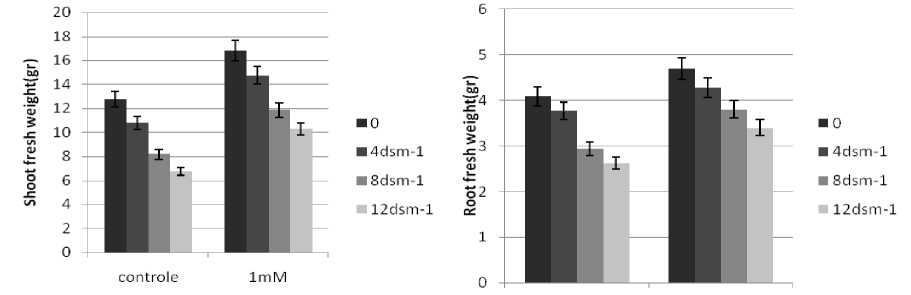
Figure 2 Effect of SA on shoot and root weight in Brassica.
n general, the RWC of leaves declined under salt stress. SA treatments induced an increase in RWC of the stressed seedlings, These observations are consistent with those of Barskosky and Einhellig (1993) who showed a consistently higher leaf diffusive resistance and lower transpiration and water potential in soybean plants with SA application. Because salinity stress enhanced free radicals levels in plants. peroxidation and electrolyte leakage in the stressed seedlings. The data showed that electrolyte leakage and lipid peroxidation increased as the stress level raised. These results agree with those of Bor et al. (2003) who found salt stress increases the lipid peroxidation in the leaves of two beet species. Grains soaking presowing with SA led to a significant decrease in the level of lipid peroxidation.
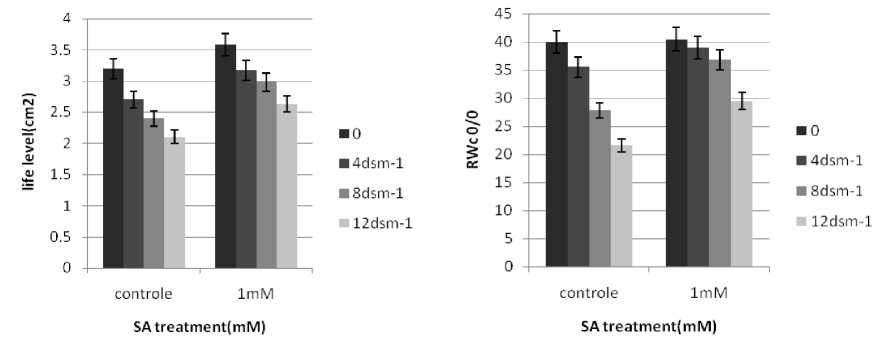
Figure 3 . Effect of SA on relative water content (%) and level leaf Brassic
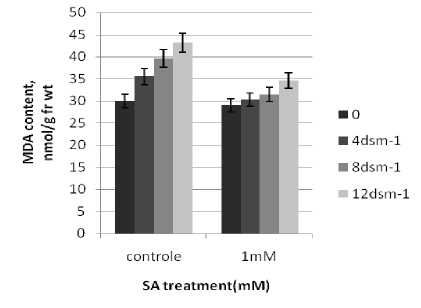
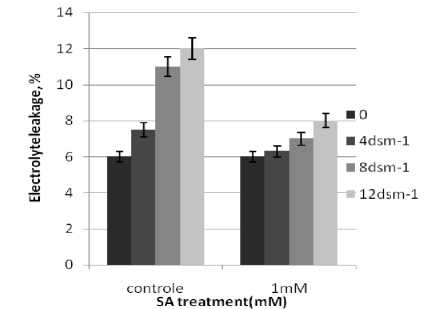
Figure 4. Effect of SA on relative Electrolyte leakage (%) and MDA content Brassica
Список литературы Response of Brassica napus L grains to the interactive effect of salinity and salicylic acid
- Barkosky R.R. and Einhellig F.A. (1993). Effects of salicylic acid on plant water relationship. J. Chem. Ecol. 19: 237-247.
- Bor M., Ozdemir F., Turkan I. (2003). The effect of salt stress on lipid peroxidation and antioxidants in leaves of sugar beet Beta vulgaris L. and wild beet Beta maritima L. Plant Sci. 164: 77-84.
- Beadle C.L., Ludlow M.M., Honeysett L. (1993). Water relations. In: Hall DO, Scurlock JMO, Bolhаr-Nordenkampf HR, Leegood RC, Long SP (eds). Photosynthesis and Production in a Changing Environment, Chapman and Hall, London, England. pp. 113-127.
- Cutt J.R. and Klessig D.F. (1992). Salicylic acid in plants: A changing perspective. Pharmaceu. Technol. 16: 25-34.
- Dat J.F., Foyer C.H. and Scott I.M. (1998). Changes in salicylic acid and antioxidants during induced thermotolerance in mustard seedlings. Plant Physiol. 118: 1455-1461.
- Dempsey DA, Shah J, Klessig DF. (1999). Salicylic acid and disease resistance in plants. Critical Reviews in Plant Sciences 18: 547-575.
- Durrant WE, Dong X. (2004). Systemic acquired resistance. Annual Review of Phytopathology 42: 185-209.
- Duan, H.-G., Yuan, S., Liu, W.-J., Xi, D.-H., Qing, D.-H., Liang, H.-G., and Lin, H.-H. (2006) Effects of Exogenous Spermidine on Photosystem II of Wheat Seedlings under Water Stress, J. Integr. Plant Biol., 48: 920-927.
- Enyedi, A.J., Yalpani, N., Silverman, P,. and Raskin, I. (1992). Signal molecules in systemic plant resistance. Pathojenes and Pests. Cell, 70: 879-886
- Gutierrez-Coronado MA, Trejo-Lopez C, Larque-Saavedra A (1998) Effects of salicylic acid on the growth of roots and shoots in soybean. Plant Physiol Biochem 36: 563-565.
- Ghoulam C.F., Ahmed F. and Khalid F. (2001). Effects of saltstress on growth, inorganic ions and proline accumulation inrelation to osmotic adjustment in five sugar beet cultivars. Environ. Exp. Bot. 47: 139-150.
- Guan L, Scandalios JG. (1995). Developmentally related responses of maize catalase genes to salicylic acid. Proceedings of the National Academy of Sciences USA 92: 5930-5934.
- Hussain K, Ashraf M, Ashraf MY (2008). Relationship between growth and ion relation in pearl millet (Pennisetum glaucum (L.) R. Br.) at different growth stages under salt stress. Afr. J. Plant Sci. 2(3): 23-27.
- Hasegawa PM, Bressan RA, Zhu JK, Bohnert HJ (2000). Plant cellular and molecular responses to high salinity. Ann. Rev. Plant Physiol. Plant Mol. Biol. 51: 463-499.
- Harper J.P. and Balke N.E. (). Characterization of the inhibition of K+ absorption in oat roots by salicylic acid. Plant Physiol.68: 1349-1353
- Hussain K, Majeed A, Nawaz K, Nisar FK, Khan F, Afghan S and Ali K (2010). Comparative study for salt stress among seed, root stock and direct regenerated violet (Viola odorata L.) seedlings in relation to growth, ion contents and enzyme activities. Afr. J. Biotechnol. 9(14): 2108-2117.
- Karabal, E., Yucel, M., and Oktem, H.A., (2003). Antioxidant Responses of Tolerant and Sensitive Barley Cultivars to Boron Toxicity, Plant Sci., 164: 925-933.
- Khan W., Prithiviraj B. and Smith D. (2003). Photosynthetic responses of corn and soybean to foliar application of salicylates. J. Plant Physiol. 160: 485-492.
- Khodary S.E.A. (2004). Effect of salicylic acid on the growth, photosynthesis and carbohydrate metabolism in salt-stressed maize plants. Int. J. Agri. Biol. 6: 5-8.
- Khatoon T, Hussain K, Majeed A, Nawaz K, Nisar MF (2010). Morphological variations in Maize (Zea mays L.) under different levels of NaCl at germinating stage. World Appl. Sci. J. 8(10): 1294-1297.
- Kang H.-M. and Saltveit M.E. (). Chilling tolerance of maize, cucumber and rice seedlings leaves and roots are differentially affected by salicylic acid. Physiol. Plant. 115: 571-576.
- Larqueґ-Saaveda A. (1979). Stomatal closure in response to salicylic acid treatment. Z. Pflanzenphysiol. 93: 371-375.
- Dat J.F., Foyer C.H. and Scott I.M. (1998). Changes in salicylic acid and antioxidants during induced thermotolerance in mustard seedlings. Plant Physiol. 118: 1455-1461.
- Metwally A., Finkmemeier I., Georgi M. and Dietz K.-J. (2003). Salicylic acid alleviates the cadmium toxcity in barley seedlings. Plant Physiol. 132: 272-281
- Nishimura N, Kitahata N, Seki M, Narusaka Y, Narusaka M, Kuromori T, Asami T, Shinozaki K, Hirayama T. (2005). Analysis of ABA hypersensitive germination2 revealed the pivotal functions of PARN in stress response in Arabidopsis. Plant Journal 44: 972-984.
- Rajasekaran, L.R., A. Stiels and C.D. Caldwell, (2002). Stand establishment in processing carrots: Effects of various temperature regimes on germination and the role of salicylic acid in promoting germination at low temperatures. Canadian j. of Sci., 82: 443-500
- Raskin, I. (1992). Role of salicylic acid in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol, 43: 439-49
- Senaratna T., Touchell D., Bumm E. and Dixon K. (2000). Acetyl salicylic acid (asprin) and salicylic acid induce multiple stress tolerance in bean and tomato plants. Plant Gowth Regul. 30: 157-161.
- Sakhabutdinova A, Fatkutdinova DR, Bezrukova MV, Shakirova FM (2003). Salicylic acid prevents the damaging action of stress factors on wheat plants. Bulg. J. Plant. Physiol. 21: 314-319.
- Singh B. and Usha K. (). Salicylic acid induced physiological and biochemical changes in wheat seedlings under water stress. Plant Growth Regul. 39: 137-141
- Stevens, J., T. Senaratna and K. Sivasithamparam. (2006). Salicylic acid induces salinity tolerance in tomato (Lycopersicon esculentum cv. Roma): Associated changes in gas exchange, water relations and membrane stabilisation. J. Plant Growth Regul. 49(1):77-83.
- Tasgin E., Atici O. and Nalbantog lu B. (2003). Effect of salicylic acid on freezing tolerance in winter wheat leaves. Plant Growth Regul. 41: 231-236.
- Xie Z, Zhang ZL, Hanzlik S, Cook E, Shen QJ. 2007. Salicylic acid inhibits gibberellin-induced alpha-amylase expression and seed germination via a pathway involving an abscisic-acid-inducible WRKY gene. Plant Molecular Biology 64: 293-303.
- Yalpani N, Enyedi AJ, Leуn J, Raskin I (1994). Ultrapearl millet light and ozone stimulate accumulation of salicylic acid and pathogenesis related proteins and virus resistance in tobacco. Planta, 193: 373-376


