Scientific investigation on the difference between the catalytic behaviors of kn-30 and sapo-34 zeolites in the conversion of methanol to light olefins
Автор: Ahmadova R.
Журнал: Science, Education and Innovations in the Context of Modern Problems @imcra
Статья в выпуске: 2 vol.8, 2025 года.
Бесплатный доступ
The presented article decribes regularity of methanol-to-olefins (MTO) process over two type zeolites KN-30 (analog of ZSM-5) and SAPO-34. It was shown that over KN-30 yield of light olefins is low (32%), but this catalyst is stable. As distinct from KN-30, over SAPO-34 high yield (78%) of light olefins was obtained in the same conditions. But this catalyst is less stable than KN-30.
Methanol, light olefins, KN-30, SAPO-34, zeolite
Короткий адрес: https://sciup.org/16010456
IDR: 16010456 | DOI: 10.56334/sei/8.2.66
Текст научной статьи Scientific investigation on the difference between the catalytic behaviors of kn-30 and sapo-34 zeolites in the conversion of methanol to light olefins
The catalytic conversion of methanol to olefins (MTO) has drawn particular attention in recent year,as this process is believed to provide as alternative pathway for the production of ethene and propene. Methanol is commercially produced from synthesis gas generated from natural gas and/or coal [1,2].
In MTO process, the main aim is to increase the selectivity of light olefins. For this purpose many zeolites are being used in this process. ZSM-5 and SAPO-34 are among the most intensively studied catalysts for methanol conversion. The main difference between the two catalysts is the pore and cage structure. SAPO-34 has narrow pores with diameters of 0.3-0.4 nm; however ZSM-5 has larger pores with diameters of 0.51-0.56 nm. Because of narrow pores, generated during the process aromatic intermediates are not able to exit pores. As ZSM-5 has larger pores, larger molecules-aromatics-can escapes. Therefore, because of accumulation of large aromatic species inside the cages of SAPO-34, it deactivates much faster than ZSM-5 [3].
Our main aim was to investigate influence of these catalysts to the selectivity of light olefins, time-on stream stability, and also to study differences in catalytic behavior of industrial KN-30 (analog of ZSM-5) and synthesized with hydrothermal method SAPO-34 in MTO process.
Experimental Part
The industrial catalyst KN-30 was supplied by “Zeosit”, Novosibirsk (S BET =300 m2/g).
The SAPO-34 zeolite was synthesized via hydrothermal method [4]. Required components for synthesizing of zeolite were mixed for 25 h, then transferred into stainless-steel autoclaves and heated at 200⁰C. After heating catalysts washed with distilled water several times, then dried at 120⁰C and at the end calcined at 550⁰C. XRD analysis proved presence of according peaks of pure SAPO-34 phase.
Catalytic tests for conversion of methanol to light olefins were conducted “in situ” in a setup consisting of 15 plug flow fixed-bed quartz tube reactors. The reaction products were analyzed using gas chromatograph equipped with flame ionization (for analysis of hydrocarbons) and thermal conductivity (for analysis of H 2 , CH4, CO and CO 2 ) detectors. The GC analyses were carried out successively, i.e. reac tor by reactor. Catalytic test were conducted at 4500C-, and 0.125 MPa-, for 36 h (in the case of KN-30 zeolite) and 3 h (in the case of SAPO-34) on stream using CH 3 OH:N 2 =60:40 feed with a modified con- tact time of 2.05 mg cat ·min·ml-1 with respect to methanol.
Sci. Educ. Innov. Context Mod. Probl. P-ISSN: 2790-0169 E-ISSN: 2790-0177 Issue 2, Vol. 8, 2025, IMCRA
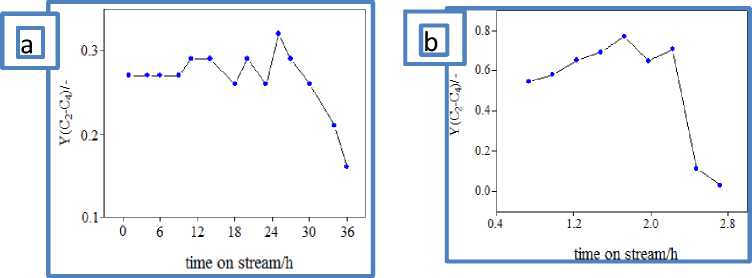
Fig.1. Time-on stream yield of light olefins over catalysts: a) KN-30; b) SAPO-34.
During the process conversion of methanol was complete. As it seems from fig.1 over KN-30 zeo- lite yield of light olefins is ~32% and this catalyst is stable during 36h on stream. With time, yield of light olefins decreases over KN-30 and C 6+ alkanes, some aromatic hydrocarbons (toluene, xylenes) are formed. It can be explained in such way that formed during the process light olefins because of larger pores of zeolite then converted (via secondary reaction such as oligomerization, alkylation with methanol and hydrogen transfer) to other hydrocarbons- alkanes, aromatic hydrocarbons and higher olefins. Over SAPO-34 ~78% (depending on reaction duration 24-35% ethylene and 25-32% propylene) light olefins are obtained, because of narrow pores of this zeolite, higher aromatics and cyclic hydrocarbons are not formed, therefore C 2 -C 3 olefins selectively synthesized over SAPO-34.
In comparison to KN-30, SAPO-34 deactivates rapidly. Amount of carbon deposits was determined from the amounts of CO and CO 2 evolved during temperature-programmed oxidation (TPO) of thecatalyst. Coke amount over KN-30 and SAPO-34 was different (0.121 and 0.238 for 36 and 6 h, respec- tively).


