Secondary metabolites of Schisandra chinensis in homeostasis regulator adaptogen herbal formula for preventive oncology
Автор: Bocharova O.A., Kazeev I.V., Shevchenko V.E., Ionov N.S., Sheichenko O.P., Bocharov E.V., Karpova R.V., Kucheryanu V.G., Lagunin A.A., Filimonov D.A., Kosorukov V.B., Poroikov V.V., Tutelyan V.A., Pyatigorskaya N.V.
Журнал: Cardiometry @cardiometry
Рубрика: Original research
Статья в выпуске: 27, 2023 года.
Бесплатный доступ
The original herbal formula of homeostasis regulator Multiphytoadaptogen (MPhA) for preventive oncology developed by the N.N. Blokhin Center of Oncology containing phytocomponents from Schizandra chinensis has been investigated in vitro, in vivo and in clinical studies. The MPhA multi-target effects are achieved by optimizing the functioning of the nervous, immune and endocrine defense systems that regulate homeostasis under stress. Everything that has been previously studied for MPhA can be considered as preclinical testing, including clinical research, which can be regarded as the pilot studies. This was allowed because MPhA in Russia is registered as a parapharmaceutical agent and therefore standardized according to established requirements. However, due to the high efficiency of MPhA, a detailed study of the chemical composition and standardization of it is required, including the composition of Schisandra chinensis Baill (Schisandraceae) active components, which turned out to be translocated into MPhA as a result of the extraction technology developed. So, for MPhA identification and standardization we detected the secondary metabolites in the herbal formula MPhA as well as in fruits extract of Schisándra chinénsis using high-performance liquid chromatography in combination with mass spectrometry. Chromatography was performed on an ACQUITY UPLC BEH C18 column in a gradient mode. A TSQ Vantage triple quadrupole mass spectrometer with electrospray ionization was used. Lignans Schizandrin and Schizantherin A were identified in the MPhA as well as in Schisándra chinénsis fruits extract obtained by the technology developed. The determined secondary metabolites can be used for standardization and quality testing of the herbal formula MPhA. In addition, we performed in silico analyzes of Schizandrin and Schizantherin A biological activity spectra using computer program PASS (Prediction of Activity Spectra for Substances). Schizandrin and Schizantherin A activities, according the scientific literature and in silico analysis, correspond to the properties studied for MPhA which therefore fits into the concept of a drug - homeostasis regulator adaptogen for preventive oncology.
Herbal formula mpha, schisándra chinénsis, schizandrin and schizantherin a, adaptogen, homeostasis regulator, preventive oncology, standardization, hplc-ms/ms spectroscopy, pass, pharmaexpert, in silico analysis
Короткий адрес: https://sciup.org/148326624
IDR: 148326624 | DOI: 10.18137/cardiometry.2023.27.6374
Текст научной статьи Secondary metabolites of Schisandra chinensis in homeostasis regulator adaptogen herbal formula for preventive oncology
Olga A. Bocharova, Ilia V. Kazeev, Valeriy E. Shevchenko, Nikita S. Ionov, Olga P.Sheichenko , Evgeny V. Bocharov, Regina V. Karpova, Valerian G. Kucheryanu, Alexey A. Lagunin, Dmitry A. Filimonov, Vyacheslav B. Kosorukov, Vladimir V. Poroikov, Victor A. Tutelyan, Natalya V. Pyatigorskaya. Secondary metabolites of Schisandra chinensis in homeostasis regulator adaptogen herbal formula for preventive oncology. Cardiometry; Issue No. 27; May 2023; p. 63-74; DOI: 10.18137/cardiometry.2023.27.6374; Available from:
Adaptogens comprise a category of herbal medicinal and nutritional products promoting adaptability, resilience and survival of living organisms in stress. The multi-target effects of adaptogens are achieved by optimizing the functioning of the nervous, immune and endocrine defense systems – regulatory axes of homeostasis under stress. These effects include triggering intracellular and extracellular adaptive signaling pathways that promote cell survival and body resistance to stress; regulation of metabolism and homeostasis by affecting the expression of stress hormones (cortisol, catecholamines, corticotropin- and gonadotropin-releasing hormones, urocortin, melatonin, heat shock proteins Hsp70, neuropeptide Y etc.) and their receptors. Adaptogens are used as official medicines in the USSR/Russia, as well as in traditional Chinese and Korean medicines, Ayurveda, Campo, and other traditional medical systems [1-11]. This provides a basis for evaluating the use of adapto-gens in the treatment of stress-related and aging-related diseases, including cancer. Adaptogens must be innocuous and cause minimal disorder in the physiological functions of an organism and have nonspecific actions, that is, increase resistance to adverse influences of a wide range of factors with physical, chemical, and biological properties. In addition, they typically possess normalizing actions irrespective of the foregoing pathologic changes direction [12-21].
Classical phytoadaptogens include Panax ginseng, Rhodiola rosea, Aralia mandshurica, Eleutherococcus senticosus, Oplopanax elatus, Schizandra chinensis, etc. The biologically active substances (BAS) of many phy-toadaptogens (from ginseng, rhodiola, eleutherococ-cus, aralia etc.) have been identified, extracted and purified. It was shown that in purified forms some active substances of phytoadaptogens exhibit pronounced toxicity in vitro and in vivo models [1, 22]. Besides that, the use of individual phytoadaptogen extract is often limited by the drug resistance that is developed over the time of administration [9]. In this regard, studies of phytotcomplexes based on the principle of BAS rational combination and providing unique synergistic effects, which cannot be obtained using a separate phytoadaptogen, are scientifically justified and relevant. Moreover, the use of several adaptogens in a particular pharmaceutical composition allows affecting the body without causing addiction. At the same time, both the standardization and the justification problem of the multicomponent phytoadaptogens pharmacological activity taking into account their chemical composition are relevant [16, 18, 23, 24].
The original herbal formula Multiphytoadaptogen (MPhA) for preventive oncology was developed in the N.N. Blokhin National Medical Research Center
64 | Cardiometry | Issue 27. May 2023
of Oncology [22, 29]. MPhA contains components of 40 official plants extracts, including adaptogens Panax ginseng, Aralia mandshurica, Eleutherococcus senti-cosus, Rhodiola rosea, Oplopanax elatus, Schizandra chinensis . MPhA has been shown to be effective in preventive oncology. As it is known, preventive oncology includes primary (prophylaxis of the occurrence, or chemoprophylaxis), secondary (prophylaxis of relapses and metastases) and tertiary (prophylaxis of chemo-radiation therapy side effects) prophylaxis of cancer diseases [22]. No doubt, antitumor and protective effects are the main properties that medications for preventive oncology should exhibit. Experimental and clinical studies have revealed antimutagenic (which is important for primary cancer prevention), antitumor (essential for secondary prevention), radioprotective, hormone-modulating, antioxidant, neu-roprotective, immunomodulatory, including adhesive and interferon-inducing effects of MPhA (which is important for tertiary cancer prevention) [22, 25-29]. Everything that has been previously studied for MPhA ( in vitro, in vivo and in clinics) can be considered as preclinical testing, including clinical research, which can be regarded as the pilot studies. It was allowed because MPhA in Russia is registered as a parapharmaceutical agent and therefore standardized according to established requirements. However, due to the high efficiency of MPhA, a detailed study of the chemical composition and standardization of it is required.
The effectiveness of the MPhA is due to the complex of BAS included in its composition. To assess the possibilities of quality control and standardization of the MPhA, studies are being performed to determine the BAS of its composition. Thus, using the reverse-phase high-performance liquid chromatography with a UV detector and gas chromatography-mass spectrometry, polyphenolic compounds, essential oils, amino acids were detected in MPhA [ 30-32]. Among the components of MPhA, the main BAS in Ginseng and Aralia – triterpene saponins (ginsenosides Rb1, Rb2, Rc, Rd, Rg1, Rg2, Re, Rf, Ro and aralosides A, В, С) were identified by high-performance liquid chromatography in combination with tandem mass spectrometry (HPLC–MS/MS) [33, 34]. Phenylethanolglycoside (salidroside), phenylpropanoid glycosides (rosavin and rosarin), monoterpene glycoside (rosiri-dine) and flavonoid (rhodionine) were also identified as components of Rhodiola rosea BAS as well as phenol (eleutheroside B) and lignan (eleutheroside E) from
Eleutherococcus senticosus [35, 36]. The compounds identical to ginsenosdes Re and Rd with araloside C were identified in MPhA as components of Oplopánax elátus (triterpene glycosides) [37].
The next stage of the MPhA components analysis using HPLC-MS/ MS is to determine Schisándra chinénsis Baill (Schisandraceae) fruits extract BAS which turned out to be translocated into MPhA as a result of the extraction technology developed. HPLC-MS/ MS method is characterized by specificity and high accuracy, which makes it possible to determine substances in minimal quantities [33-37].
The term adaptogen was introduced in 1958 by the Soviet toxicologist N.V. Lazarev, who applied it to the synthetic stimulant dibazol (2‐phenyl‐imidazol) assuming that adaptogens increase the nonspecific resistance of organisms under conditions of stress resulting in increased endurance, stamina, and performance. This assumption was based on the results of intensive studies of Schisandra chinensis in the USSR during World War II, with the goal of finding an alternative to stimulants used by the German and U.K. army to increase the attention and endurance of pilots. The aim was also to supply the Soviet Armed Forces and Military Industry (soldiers, pilots, sailors, and civilians engaged in the production of weapons and war materials) with easily available natural stimulants, presumably extracts from S. chinensis berry or seeds. The interest in S. chinensis arose from ethnopharma-cological investigations by V.L. Komarov (1895) and V.K. Arsenyev (1903–1907) in far eastern Siberia and northern Manchuria. The berries and seeds were determined to have been used by Nanai hunters (natives of far eastern Siberia and Chinese Manchuria) as a tonic to reduce thirst, hunger, and exhaustion and to improve night‐time vision [1]. The first studies on the stimulating and tonic effects on S . chinensis were published in World War II‐era military journals. During the 1960s and 1970s, other Soviet scientists extende the research of adaptogens to “rejuvenating and invigorating” medicinal plants traditionally used in China, Korea, Japan, Siberia and the far east of the USSR for a variety of pathological conditions including diseases and their symptoms such as hypodynamia, asthenia, shortness of breath, insomnia, impotence, and diabetes etc[1].
Schisándra chinénsis Baill (Schisandraceae) is a perennial, woody, deciduous, climbing vine from the Schisandraceae family. Also, this plant has many folk names, such as “Chinese schizandra”, “Manchurian schizandra “ and “ts-wei-tzu”, which means “berry of five flavors” in Chinese. It grows mainly in China, Japan and Korea. In its wild form on the territory of Russia it is distributed in the Far East, in the Primorsky and Khabarovsk Territories, in the Amur Region and on Sakhalin. It mainly grows on drained, humus-rich soils. The plant is light-loving, so it does not tolerate strong shading [38]. The juice of Schisándra chinénsis fruits contains a large amount of sugars and organic acids (mainly citric, malic, tartaric). There is also a high content of vitamins – ascorbic acid, thiamine and riboflavin. Tocopherol, schizandrin, schizandrol etc. are noted in the seeds. The bark and other parts of the plant contain essential oil. It is highly valued in perfumery for its delicate spicy-lemon aroma. The composition of the essential oil includes sesquiterpene hydrocarbons, aldehydes and ketones. Fatty oil includes α-linoleic, β-linoleic, oleic and near marginal acids [39]. Tincture of Schisándra chinénsis seeds has hepatoprotector and antioxidant actions. It is used for asthenic syndrome, fatigue, in complex therapy with weakening of sexual function, convalescence after somatic and infectious diseases, to improve the body’s performance under increased mental stress [40]. In dermatology, infusions and tinctures of Schisándra chinénsis fruits as a tonic and adaptogen are used for infectious, allergic and viral skin diseases, psoriasis [41, 42].
The aim of our study was to identify biologically active substances of Schisandra chinensis in MPhA by HPLC-MS/MS and to evaluate the biological activity profiles of identified phytocomponents using in silico analysis.
-
2. Materials and Methods
-
2.1 Solutions
-
-
2.2 Chromatographic analysis
Chromatographic analysis conditions: ACQUITY UPLC BEH C18 column (1.7 microns, 2.1 × 100 mm, Waters); mobile phase composition: phase A – 100% water and 0.1% formic acid (FA); phase B – 95% acetonitrile, 5% water and 0.1% FA. For the analysis of extracts, the gradient of the mobile phase supply (in% of phase B) was used: 0-68 min (0-60%), 68-70 min (60100%), 70-75 min (100%), 75-80 min (0%). Samples in the volume of 5 μl were injected into the injector loop with a volume of 25 μl (mobile phase – 20 μl), the flow rate of 450 μl /min.
-
2.3 The calculation of the probable spectra of the biological activity
The biological activity spectra of Schisandra chin-ensis secondary metabolites were calculated using the PASS 2022. PASS 2022 allows predicting 1957 biological activities with an average accuracy of 97%. The PASS algorithm is based on a naive Bayesian classifier and a representation of the structure of chemical compounds in the form of MNA descriptors. The result of prediction is a list of probable activities for each com- 66 | Cardiometry | Issue 27. May 2023
-
3. Results and discussion
Samples of MPhA as well as Schisándra chinénsis extract were prepared using high-quality-certified raw materials. We studied samples of MPhA and Schisán-dra chinénsis extract obtained in the same way (same specific gravity of raw materials, temperature and time regime of extraction, composition of the extractant, etc.). MPhA and Schisándra chinénsis extract were analyzed using a TSQ Vantage triple quadrupole mass spectrometer (Thermo Scientific TSQ series) connected to an Accela HPLC chromatograph equipped with an ACQUITY UPLC BEH C18 column (1.7 μm, 2.1 × 100 mm, Waters).
The MPhA sample was mixed with methanol in a ratio of 1:2 and centrifuged for 5 min at 13000 rpm. The filler liquid was passed through a filter with a pore diameter of 0.22 microns and centrifuged at 13,000 rpm for 1 min. Aliquots (1 ml) of Schisán-dra ex-tract were evaporated on a Concentrator 5301 rotary evaporator (Eppendorf, Germany) at 30 C to dry. The residue was dissolved in 100 μl of methanol and centrifuged at 13,000 rpm for 1 min. The samples were analyzed using a triple quadrupole mass spectrometer TSQ Vantage (Thermo Scientific TSQ series) connected to the Accela HPLC chroma-to-graph.
Ionization was carried out using an electrospray. Ionization conditions: negative polarity, spray capillary voltage 4 kV, gas (creating spray) – 60 psi, flowing gas – 15 rel. units, capillary temperature – 270 °C. The spectra in the full ion scanning mode and in the selected ion monitoring mode (SIM) were taken in the range of 150-1500 Da, the scanning time is 0.1 s.
Mass spectra were obtained by direct injection of a sample through a syringe at a rate of 5 μl/min; the gas pressure in the collision chamber was 0.9 torr. The voltage in the collision chamber was selected for each connection separately.
pound with the corresponding probability estimates Pa and Pi – probabilities of belonging to the classes of “actives” and “inactives”, respectively. All activities for which the calculated values of Pa exceed Pi are considered as probable. The analysis of the integral action and possible drug-drug interactions of chemical compounds established in Schisandra chinensis was performed using the computer program PharmaExpert [29, 43]. The efficiency of the approach using the PASS and PharmaExpert computer programs to analyze the possible biological activities of single phytocomponents and their complexes has been shown in numerous studies [44-50].
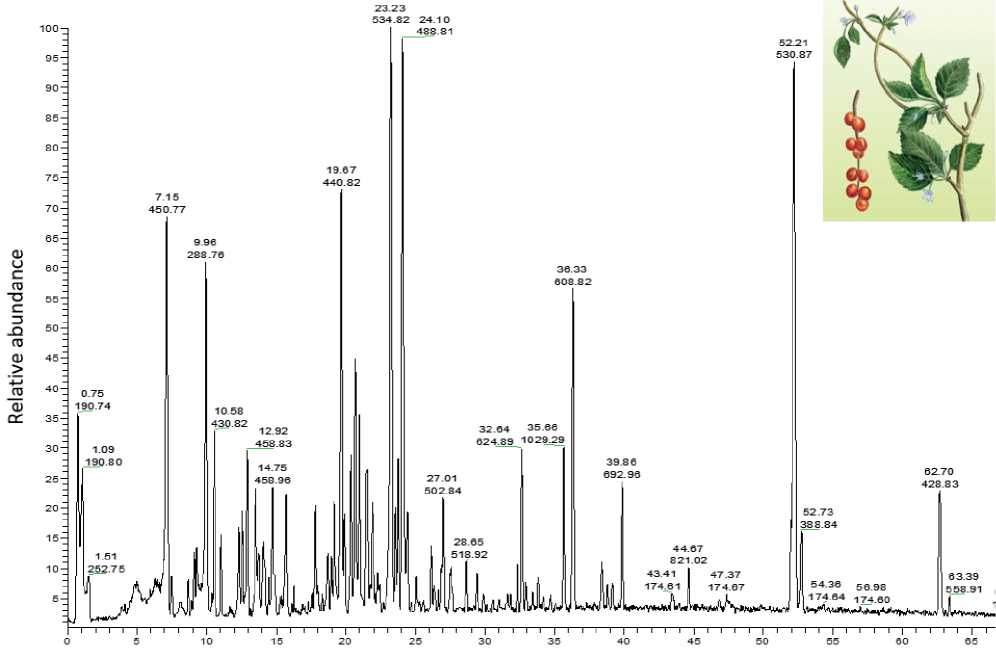
To study the main BAS of Schisándra chinénsis fruit extract that is part of the MPhA, a chromatogram of the extract was obtained taken in the full ion scanning mode. The chromatogram is shown in Figure 1.
Analysis of the literature data on the chemical composition of Schisándra chinénsis fruits has revealed the most important biologically active compounds of this plant. They are Schizandrin A, B, C, Schizandrol A and B, etc. Table 1 shows the molecular weights (MW) and structural formulas of these substances.
Table 2 presents the results of tandem mass spectrometry analysis of Schisandra chinénsis extract (Rt, m/z of pseudomolecular ion and its fragments), as well as the molecular weight of the compound. As follows from Table 2 Schizandrin corresponds to one of the peaks with m/z 430.82 with a retention time Rt = 10.5-11.0 min as well as Schizantherin A corresponds to one of the peaks with m/z 535.0 with retention time Rt = 23.0-23.2.
Thus, the main Schisándra chinénsis BAS which primarily include Shizandrin and Schizantherin A, were identified in the extract of Schisándra chinén-sis . Knowing the retention times corresponding to them and the m/z values of the main molecular ion the MPhA chromatogram was analyzed with respect to the content of Schisándra chinénsis BAS. Figure 2 shows the MPhA chromatogram in the full ion scanning mode.
The analysis of the MPhA chromatogram obtained under the same conditions as the chromatogram of Schisándra chinénsis extract was carried out in the selected ion monitoring mode (SIM) corresponding to the main molecular ions of Schisándra chinénsis (based on the data given in Table 2).
Time (min)
Figure 1. Chromatogram of Schisándra chinénsis extract in the full ion scanning mode.
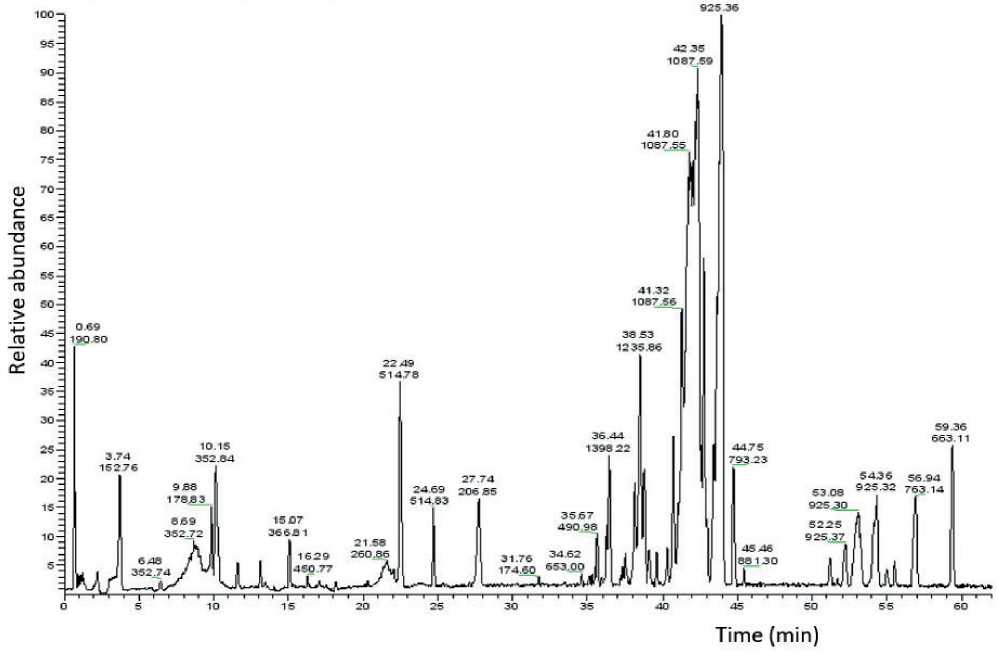
Figure 2. Chromatogram of MPhA in the full ion scanning mode.
Table 1
The main BAS of Schisándra chinénsis according to the literature
|
Major compound |
МW, g/mol |
Structural formula |
Major compound |
МW, g/mol |
Structural formula |
|
Schizandrin (Schizandrol A) C 24 H 32 O 7 |
432,5 |
0 \ ) О— У \ / |
Schizandrin A (Deoxyschizandrin) C 24 H 32 O 6 |
416,5 |
\ ) Хуо |
|
Schizandrin B C 23 H 28 O 6 |
400,5 |
\ ЛА ) /- /ул \ 1 / ' о^у |
Schizandrol B (Gomisin A) C 23 H 28 O 7 |
416,5 |
\ 0 “нп |
|
Schizandrin C C 22 H 24 O 6 |
384,4 |
°^hX о ‘ ° |
Schisantherin A (Gomisin C) C 30 H 32 O 9 |
536,6 |
\ ЛА ) VaJ |
|
Schisantherin B C 28 H 34 O 9 |
514,6 |
/ УШ 1 X / °s° |
Schisantherin C C 28 H 34 O 9 |
514,6 |
VfT$ ' 0__/ |
Table 2
The tandem mass spectrometry of Schisándra chinénsis extract (Rt, m/z for the main mo-lecular ion, as well as for fragments of the molecular ion) results, as well as the molecular weight according to the literature.
|
Substance |
МW |
Rt, min |
m/z |
|
Schizandrin C24H32O7 0 \ ) 0-_/ \ / |
432,5 |
10,5-11,0 |
430,82 [M-H]- 394,3 384,8 368,5 354,4 340,6 325,5 310,5 296,5 288,69 178,5 151 |
|
Schisantherin A C30H32O9 о ~ип 1 о----- |
536,6 |
23,0-23,2 |
535,0 [M-H]- 516,7 470,9 436,6 418,2 403,8 373,1 354,8 194,5 178,4 142,5 119,1 96,8 |
Pa-Pi
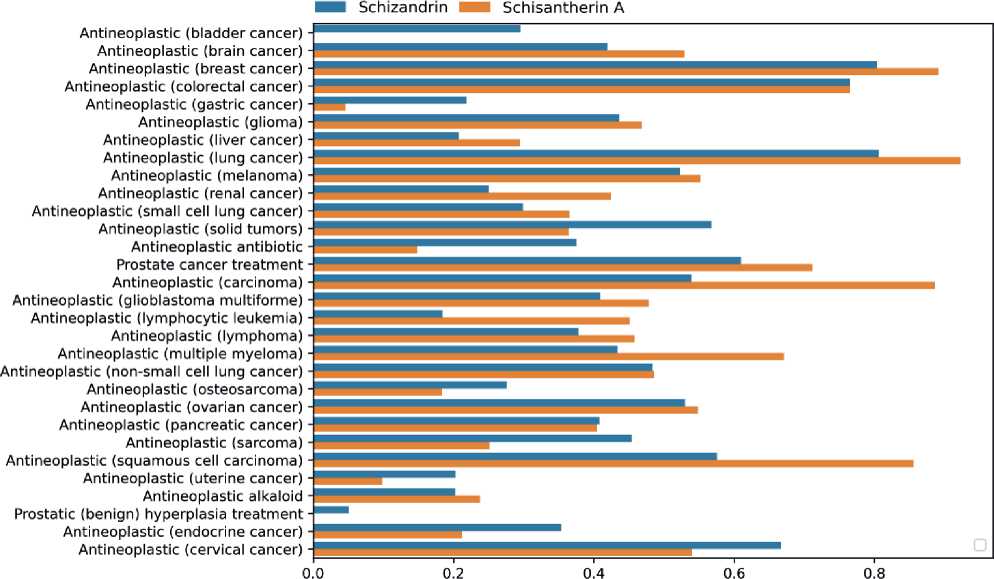
Figure 3. Diagram of positive Pa-Pi values for antitumor effects and compounds Schisantherin A, Schizandrin. Negative Pa-Pi values in this figure are not taken into account and are reduced to zero.
Table 3
Six most probable antitumor effects of Schisantherin A and Schizandrin predicted by PASS.
|
Name |
Antineoplastic effect |
Pa-Pi |
|
Schisantherin A |
Antineoplastic (lung cancer) |
0.92 |
|
Schisantherin A |
Antineoplastic (breast cancer) |
0.89 |
|
Schisantherin A |
Antineoplastic (carcinoma) |
0.89 |
|
Schisantherin A |
Antineoplastic (squamous cell carcinoma) |
0.86 |
|
Schizandrin |
Antineoplastic (lung cancer) |
0.81 |
|
Schizandrin |
Antineoplastic (breast cancer) |
0.8 |
|
Schisantherin A |
Antineoplastic (colorectal cancer) |
0.77 |
|
Schizandrin |
Antineoplastic (colorectal cancer) |
0.77 |
Table 4
The most probable mechanisms of action associated with antineoplastic effects.
|
Mechanism |
Pharmacological Effect |
Compound’s name |
Pa-Pi |
|
Tubulin antagonist |
Antineoplastic (melanoma) Prostate cancer treatment Antineoplastic (ovarian cancer) Antineoplastic (gastric cancer) Antineoplastic (breast cancer) Antineoplastic (lung cancer) Antineoplastic (bladder cancer) Antineoplastic (cervical cancer) Antineoplastic (colorectal cancer) Antineoplastic (multiple myeloma) Antineoplastic (pancreatic cancer) Antineoplastic (renal cancer) Antineoplastic antibiotic Antineoplastic (lymphoma) |
Schisantherin A |
0.82 |
|
Schizandrin |
0.65 |
As a result of the analysis, the desired peaks were found on the MPhA chromatogram and, thereby, the presence of Schizandrine and Schizantherin A in the pharmaceutical composition was confirmed.
It should be emphasized that the identification of individual substances is important to justify the biological activity of a multicomponent medication.
68 | Cardiometry | Issue 27. May 2023
Application of in silico methods makes it possible to obtain additional theoretical substantiation of the identified biological activities of Schisándra chinén-sis extract’s BAS. Since this extract is a part of MPhA herbal formula used in preventive oncology, the antitumor spectra of individual phytocomponents and their combinations were evaluated. With this purpose,
we performed a prediction of 32 pharmacotherapeutic effects and mechanisms of antitumor action for each of the identified phytocomponents using the computer program PASS. The Pa-Pi values diagram of predicted antitumor effects for Schisándra chinénsis secondary metabolites (Schisantherin A, Schizandrin) is shown in Figure 3.
As can be seen in Figure 3, at the threshold Pa>Pi for Schisantherin A 30 and for Schizandrin 32 antitumor effects are predicted 12 antitumor effects are predicted for compound Schisantherin A and 11 for compound Schizandrin at a Pa-Pi>0.5 threshold. Six most probable antitumor effects are presented in table 3.
As shown in Table 3, Antineoplastic (lung cancer) is predicted with the highest Pa-Pi value for Schisan-therin A (Pa-Pi=0.92). Antineoplastic (lung cancer) is predicted at Pa-Pi=0.81 for Schizandrin. Table 3 demonstrates that the first four antineoplastic effects are predicted for Schisantherin A with the highest Pa-Pi values. Antineoplastic (colorectal cancer) are predicted for both compounds with equal Pa-Pi values 0.77
Using PharmaExpert we detected the most probable synergistic/additive mechanisms of the antineoplastic effects. 30 mechanisms of action associated with antitumor effects are predicted at the Pa>-Pi threshold for Schisantherin A and Schizandrin. Six of them are predicted at a Pa-Pi>0.5 threshold. Data of the mechanisms of antitumor action predicted for the compounds under study at the Pa-Pi>0.5 threshold are presented in Table 4.
As can be seen from the data presented in Table 3, the three mechanisms of action predicted with the highest probability are Tubulin antagonist (Schisan-therin A, Pa-Pi = 0.82), Antimitotic (Schisanther-in A, Pa-Pi = 0.78), Apoptosis agonist (Schizandrin, Pa-Pi = 0.72).
An analysis of the literature devoted to the study of the identified compounds from Schisándra chinénsis biological activity gave the following results. Schizan-drin (Schizandrol A) and Schisantherin A (Gomisin C) exhibit antitumor, neuroprotective, immunomodulatory, antioxidant as well as with therapeutic potential to overcome multidrug resistance in cancer [51-61].
|
Mechanism |
Pharmacological Effect |
Compound’s name |
Pa-Pi |
|
Antimitotic |
Prostate cancer treatment Antineoplastic (ovarian cancer) Antineoplastic (breast cancer) Antineoplastic (lung cancer) Antineoplastic (cervical cancer) Antineoplastic (colorectal cancer) Antineoplastic (multiple myeloma) Antineoplastic (pancreatic cancer) Antineoplastic (renal cancer) Antineoplastic (lymphoma) |
Schisantherin A |
0.78 |
|
Schizandrin |
0.55 |
||
|
Apoptosis agonist |
Antineoplastic (melanoma) Prostate cancer treatment Antineoplastic (gastric cancer) Antineoplastic (breast cancer) Antineoplastic (lung cancer) Antineoplastic (bladder cancer) Antineoplastic (cervical cancer) Antineoplastic (colorectal cancer) Antineoplastic (multiple myeloma) Antineoplastic (pancreatic cancer) Antineoplastic (renal cancer) Antineoplastic (liver cancer) Antineoplastic (osteosarcoma) Antineoplastic (lymphoma) Prostatic (benign) hyperplasia treatment |
Schizandrin |
0.72 |
|
Schisantherin A |
0.65 |
||
|
Caspase 3 stimulant |
Prostate cancer treatment Antineoplastic (breast cancer) Antineoplastic (lung cancer) Antineoplastic (bladder cancer) Antineoplastic (renal cancer) |
Schisantherin A |
0.64 |
|
Schizandrin |
0.50 |
||
|
Transcription factor NF kappa B inhibitor |
Prostate cancer treatment Antineoplastic (gastric cancer) Antineoplastic (breast cancer) Antineoplastic (lung cancer) Antineoplastic (bladder cancer) Antineoplastic (colorectal cancer) Antineoplastic (multiple myeloma) Antineoplastic (renal cancer) Antineoplastic (lymphoma) Antineoplastic (solid tumors) |
Schisantherin A |
0.68 |
|
Schizandrin |
0.68 |
||
|
P-glycoprotein inhibitor |
Antineoplastic (melanoma) Antineoplastic (ovarian cancer) Antineoplastic (lung cancer) Antineoplastic (multiple myeloma) Antineoplastic (pancreatic cancer) Antineoplastic (liver cancer) |
Schisantherin A |
0.62 |
|
Schizandrin |
0.60 |
At the same time, it should be noted that the MPhA original herbal formula provides antiproliferative activity against ovarian and cervical adenocarcinomas as well as human hypernephroma in our in vitro studies. Experiments in vivo on high-cancer CBA mice-males have demonstrated potent MPhA effect against hepatocellular carcinoma. In vivo a 100% antimetastatic effect was found on Lewis lung carcinoma. Antitumor activity in stage four advanced gastric cancer has been shown in the clinics. Chemopreventive (oncoprophy-laxis) effect was demonstrated in CBA mice as well as in the clinics for the treatment of precancerous oral leukoplakia. An evidential therapeutic effect of MPhA in relation to age-related pathologies – benign prostatic hyperplasia and Parkinson’s disease – was obtained in clinical studies. In addition, neuroprotective antioxidant, antimutagenic, radioprotective, immunomodulating MPhA action have been confirmed [22, 25-29].
In other words the activities of Schizandrin and Schizantherin A, according to the scientific literature and in silico analysis, correspond to the properties we studied for MPhA which fully fits into the concept of
Issue 27. May 2023 | Cardiometry | 71
a medication for preventive oncology. Of course, antitumor and protective effects are the main properties that medications for preventive oncology should have.
-
4. Conclusions
For standardization and identification of Multi-phytoadaptogen for preventive oncology the tandem HPLC/MS-MS mass spectrometry method has been successfully applied to determine secondary metabolites from Schisándra chinénsis – lignans Schizandrine and Schizantherin A as components of the original herbal formula. Lignans Schizan-drin and Schizantherin A were identified translocated into Multiphytoadaptogen as well as into Schisándra chinénsis fruits extract by the technology developed.
Chromatograms and spectra obtained during the research can be used for standardization and identification of Multifitoadaptogen. The results are also important for justification the biological activity of the Multiphytoadaptogen pharmaceutical composition, taking into account the lignans group components of the Schisándra chinénsis BAS.
The results of the computational estimation correspond to the data obtained for Mul-tiphytoadaptogen in vitro, in vivo and in clinical studies. However, in silico analysis of predicted Schisándra chinénsis phytocomponents antitumor properties made it possible to identify some additional probable antineoplastic effects. The variety of the most potential mechanisms for achieving the pharmacological effect positively characterizes the pleiotropy of individual secondary metabolites and their complexes. The information obtained about the most likely additive/synergistic mechanisms of action justifies the use of extracts in one mixture and also reveals the further prospects for investigations.
So, the possibility of complex phytopreparations standardization and identification which include lig-nans, secondary metabolites from Schisándra chinén-sis is demonstrated. The latter can be included in the regulatory documentation when registering a drug based on MPhA.
The results of the research also suggest that the activity of Schizandrin and Schizantherin A, according to the scientific literature and in silico analysis, corresponds to the properties earlier obtained for MPhA, which completely fits into the concept of a drug – homeostasis regulator adaptogen for preventive oncology.
Список литературы Secondary metabolites of Schisandra chinensis in homeostasis regulator adaptogen herbal formula for preventive oncology
- Panossian, A.; et al. Evolution of the adaptogenic concept from traditional use to medical systems: Pharmacology of stress‐ and aging‐related diseases. Medicinal Research Reviews 2021, 41, 630-703.
- Panossian, A., Efferth, T. Network pharmacology of adaptogens in the assessment of their pleiotropic therapeutic activity. Pharmaceuticals 2022, 15, 1051.
- Panossian, A.; Wikman, G. Evidence‐based efficacy of adaptogens in fatigue, and molecular mechanisms related to their stress‐protective activity. Curr. Clin. Pharmacol. 2009, 4, 198‐219.
- Panossian, A. Understanding adaptogenic activity: specificity of the pharmacological action of adaptogens and other phytochemicals. Ann. N. Y. Acad. Sci. 2017, 1401, 49‐64.
- Panossian, A.; Wikman, G.; Sarris, J. Rosenroot (Rhodiola rosea): traditional use, chemical composition, pharmacology and clinical efficacy. Phytomedicine. 2010; 17:481‐493.
- Panossian, A. Adaptogens in mental and behavioral disorders. Child Adolesc. Psychiatr. Clin. North Am. 2013, 36, 49‐64.
- Shikov, A.N.; Pozharitskaya, O.N.; Makarov, V.G. Aralia elata var. mandshurica (Rupr. & Maxim.) J. Wen: An overview of pharmacological studies. Phytomedicine 2016, 23, 1409-1421.
- Shikov, A.N.; et al. Oplopanax elatus (Nakai) Nakai: chemistry, traditional use and pharmacology. Chin. J. Nat. Med. (Amsterdam, Neth.) 2014, 12, 721-729.
- Gerontakos, S.; et al. Findings of Russian literature on the clinical application of Eleutherococcus senticosus (Rupr. & Maxim.): A narrative review. Journal of Ethnopharmacology 2021, 278, 114274.
- Wiegant, F.A.C.; Limandjaja, G.; de Poot, S.A.H. 23. Plant Adaptogens Activate Cellular Adaptive Mechanisms by Causing Mild Damage. Adaptation Biology and Medicine: Volume 5, 2008, 319.
- Panossian, A.; Wagner, H. Stimulating effect of adaptogens: an overview with particular reference to their efficacy following single dose administration. Phytother Res. 2005, 19, 819‐838.
- Wiegant, F.A.; et al. Plant adaptogens increase lifespan and stress resistance in C. elegans. Biogerontology 2009, 10, 27-42.
- Kaur, P.; et al. Immunopotentiating significance of conventionally used plant adaptogens as modulators in biochemical and molecular signalling pathways in cell mediated processes. Biomed. Pharmacother. 2017, 95, 1815‐1829.
- Melnikov, V.N. A quantitative method for estimating the adaptedness in a physiological study. Theor. Biol. Med. Modell. 2019, 16, 1-9.
- Dhabhar, F.S. The short‐term stress response—mother nature’s mechanism for enhancing protection and performance under conditions of threat, challenge, and opportunity. Front. Neuroendocrinol. 2018, 49, 175‐192.
- Bocharova, O.A; et al. Phytoadaptogens in biotherapy of tumors and geriatrics (Part 1). Ross. Bioterapevticheskii Zh. 2020, 19(2), 13-21.
- Bocharova, O.A.; et al. Phytoadaptogens in biotherapy of tumors and geriatrics (Part 2). Ross. Bioterapevticheskii Zh. 2020, 19(3), 12-20.
- Todorova, V.; et al. Plant adaptogens – history and future perspectives. Nutrients. 2021, 13(8), 2861. DOI: 10.3390/nu13082861.
- Panossian, A.; Brendler, T. The Role of Adaptogens in Prophylaxis and Treatment of Viral Respiratory Infections. Pharmaceuticals 2020, 13, 236.
- Panossian, A.; Seo, E.J.; Efferth, T. Novel molecular mechanisms for the adaptogenic effects of herbal extracts on isolated brain cells using systems biology. Phytomedicine 2018, 50, 257-284. DOI: 10.1016/j.phymed.2018.09.204.
- Shin, D.; et al. Effects of Schisandrae Fructus on menopause symptoms in ovariectomized mice. Journal of Korean Medicine 2016, 37, 39-46.
- Bocharova, O.A.; et al. Research of new phytoadaptogens and possibilities of herbal formulas application. Ross. Bioterapevticheskii Zh. 2020, 19, 35.
- Panossian, A.; Wikman, G.; Sarris, J. Rosenroot (Rhodiola rosea): traditional use, chemical composition, pharmacology and clinical efficacy. Phytomedicine 2010, 17, 481-493.
- Shikov, A.N.; et al. Medicinal plants of the Russian Pharmacopoeia; their history and applications. J. Ethnopharmacol. 2014, 154, 481. DOI: 10.1016/j.jep.2014.04.007.
- Bocharova, O.; et al. The first in vitro and in vivo trials of the phytomixture for anticancer treatment. Farmacevtski Vestnik 1997, 48, 414-415.
- Pozharitskaya, M.M.; et al. Modern aspects of the pathogenesis and treatment of oral mucosa leukoplakia. Methodological guide for doctors; SEI HESMC: Moscow, Russia, 2004; p 46
- Bocharova O.A.; Baryshnikov, A.Yu. Phytoadaptogens in oncology; ZooMedVet: Moscow, Russia, 2004, p 138
- Alperina, E.L.; Bocharov, E.V.; Bocharova, O.A. Actual problems of neuroimmunopathology; Genius Media: Moscow, Russia, 2012, p 423.
- Bocharova, O.A.; et al. Computer-Aided Evaluation of Polyvalent Medications’ Pharmacological Potential. Multiphytoadaptogen as a Case Study. Mol. Inf. 2022 41, 2200176.
- Sheychenko, V.I.; et al. Analytical capabilities of the NMR method for determining the components of Phitomix-40. Factory laboratory. Diagnostics of materials. 2006, 72(8), 15-23.
- Sheychenko, O.P.; et al. Possibility of using electronic absorption spectra for standardization of the multicomponent preparation “Phitomix-40”. Problems of Biol. Med. Pharm. Chem. 2007, 5(2), 20-25.
- Sheychenko, O.P.; et al. Investigation of a complex phytoadaptogen by HPLC. Problems of Biol. Med. Pharm. Chemistry 2012, 10, 52-59.
- Kazeev, I.V.; et al. Tandem mass spectrometry for the analysis of ginsenosides in a phytoadaptogenic composition with antitumor properties. Theor Found Chem. Eng. 2021, 55(6), 780-792.
- Kazeev, I.V.; et al. Tandem mass spectrometry in the technology of determining aralosides of phytoadaptogene compositions. Theor Found Chem. Eng. 2020, 54(6), 1242-1246.
- Bocharova, O.A.; et al. A Potential Method for Standardization of Multiphytoadaptogen: Tandem Mass Spectrometry for Analysis of Biologically Active Substances from Rhodiola rosea. Pharm Chem J. Eng. 2022, 56(1), 78-84.
- Bocharova, O.A.; et al. Eleutherosides Definition by Tandem Mass Spectrometry Technology in Assay of Multiphytoadaptogen for Preventive Oncology. Pharm Chem J. Eng. 2022, 56(6), 806-814.
- Kazeev I.V., et al. Secondary metabolites of Oplopanax elatus: possibilities of standardization of a multifitoadaptogen for preventive oncology// Pharm Chem J. 2023. 57(1), 29-36.
- Novikov, V.S.; Gubanov, I.A. Populyarnyj atlas-opredelitel’. Dikorastushchie rasteniya. 5th ed.; Prosveshchenie/Drofa: Moscow, Russia 2008, pp. 65-66. (in Russian)
- Shikov, A.N.; et al. Traditional and current food use of wild plants listed in the Russian Pharmacopoeia. Front. Pharmacol. 2017, 8, 841.
- Makarov, V.G.; et al. Effect of polyphenol preparation Licol on cebtral nervous systeme. Psychopharmacol. Biol. Narcol. 2004. 4, 601-607.
- Shikov, A.N.; et al. Medicinal Plants from the 14th edition of the Russian Pharmacopoeia, recent updates. J. Ethnopharmacol. 2021, 268, 113685.
- Kosman, V.M.; et al. Pharmacokinetics of lignans from Schisandra chinensis. Review on Clinical Pharmacology and Medicinal Therapy 2015, 13, 3-21
- Poroikov, V.V. Computer-aided drug design: From discovery of novel pharmaceutical agents to systems pharmacology. Biochemistry (Moscow), Supplement Series B: Biomedical Chemistry. 2020, 14, 216-227. DOI: 10.1134/S1990750820030117
- Patil, K.R.; et al. Pentacyclic Triterpenoids Inhibit IKKβ Mediated Activation of NF-κB Pathway: In Silico and In Vitro Evidences. PLoS One. 2015, 10, e0125709.
- Gawande, D.Y., Goel, R.K. Pharmacological validation of in-silico guided novel nootropic potential of Achyranthes aspera L. J. Ethnopharmacol. 2015, 175, 324-334. DOI: 10.1016/j.jep.2015.09.025
- Foudah, A.I.; et al. Phytochemical Screening, In Vitro and In Silico Studies of Volatile Compounds from Petroselinum crispum (Mill) Leaves Grown in Saudi Arabia. Molecules. 2022, 27, 934.
- Yeh, Y.C.; et al. Identification of NSP3 (SH2D3C) as a Prognostic Biomarker of Tumor Progression and Immune Evasion for Lung Cancer and Evaluation of Organosulfur Compounds from Allium sativum L. as Therapeutic Candidates. Biomedicines. 2021, 9, 1582.
- Alehaideb, Z.I.; et al. Bursatella leachii Purple Ink Secretion Concentrate Exerts Cytotoxic Properties against Human Hepatocarcinoma Cell Line (HepG2): In Vitro and In Silico Studies. Molecules. 2022, 27, 826.
- Nasrin, S.; et al. Chemical profiles and pharmacological insights of Anisomeles indica Kuntze: An experimental chemico-biological interaction. Biomed. Pharmacother. 2022, 149, 112842.
- Mácsai, L.; et al. Biological Activities of Four Adaptogenic Plant Extracts and Their Active Substances on a Rotifer Model. Evid. Based Complement. Alternat. Med. 2018, 2018.
- Amujuri, D.; et al. Synthesis and biological evaluation of Schizandrin derivatives as potential anti-cancer agents. European Journal of Medicinal Chemistry. 2018, 149, 182-192.
- Yoganathan, S.; et al. Ellagic Acid and Schisandrins: Natural Biaryl Polyphenols with Therapeutic Potential to Overcome Multidrug Resistance in Cancer. Cells 2021, 10, 458. DOI: 10.3390/cells10020458
- Dileep Kumar, G.; et al. Synthesis and biological evaluation of Schizandrin derivatives as tubulin polymerization inhibitors. Bioorg Med Chem Lett. 2020, 30, 127354.
- Xu, G.; et al. Molecular Mechanism of the Regulatory Effect of Schisandrol A on the Immune Function of Mice Based on a Transcription Factor Regulatory Network. Frontiers in Pharmacology. 2021, 12.
- Lin, X.; et al. Regulation of cell signaling pathways by Schisandrin in different cancers: Opting for “Swiss Army Knife” instead of “Blunderbuss.” Cellular and Molecular Biology. 2021, 67, 25-32.
- Zhu, P.; et al. Schisandra fruits for the management of drug-induced liver injury in China: A review. Phytomedicine. 2019, 59, 152760.
- Xin, Y.; Yang, Y.; Yu, K.; Wang, H. Filtration of Active Components with Antioxidant Activity Based on the Differing Antioxidant Abilities of Schisandrae Sphenantherae Fructus and Schisandrae Chinensis Fructus through UPLC/MS Coupling with Network Pharmacology. Evidence-Based Complementary and Alternative Medicine 2021, 2021, 1-13.
- Wang, Z.; et al. Schisantherin A induces cell apoptosis through ROS/JNK signaling pathway in human gastric cancer cells. Biochemical Pharmacology. 2020, 173, 113673.
- Zhang, L.; et al. Schisantherin A protects against 6-OHDA-induced dopaminergic neuron damage in zebrafish and cytotoxicity in SH-SY5Y cells through the ROS/NO and AKT/GSK3β pathways. Journal of Ethnopharmacology. 2015, 170, 8-15.
- Panossian, A., et al. The adaptogens Rhodiola and Schizandra modify the response to immobilization stress in rabbits by suppressing the increase of phosphorylated stress‐activated protein kinase, nitric oxide and cortisol. Drug Targets Insights. 2007, 2, 39‐54
- Inglis, J.E.; Lin, P.J.; Kerns, S.L. Nutritional interventions for treating cancer‐related fatigue: a qualitative review. Nutr Cancer. 2019, 71, 21‐40.


