Siliceous rocks as modifiers of structure of photocatalytic self-cleaning concrete. Assessment of the effect on the phase composition of cement stone
Автор: Balykov A.S., Nizina T.A., Kyashkin V.M., Chugunov D.B.
Журнал: Nanotechnologies in Construction: A Scientific Internet-Journal @nanobuild-en
Рубрика: The results of the specialists’ and scientists’ researches
Статья в выпуске: 2 Vol.16, 2024 года.
Бесплатный доступ
Introduction. Currently one of the focus areas for the development of construction material science is the creation of self-cleaning concretes characterized by polydisperse multicomponent composition with the presence of nanoscale photocatalytic additives, primarily based on TiO2. These photoactive modifiers give the material a number of positive properties, including the ability to decompose atmospheric pollutants, to self-clean the surface, etc. The promising method for improving the functional characteristics of titanium oxide photocatalysts is the creation of nanostructured systems with ‘core (substrate) – shell (photocatalyst)’ architecture. Previous research results show that the final efficiency of the synthesized composite photocatalytic modifiers largely depends on the level of substrate reactivity in the cement system. The purpose of this study is to investigate the impact of three types of siliceous rocks (diatomite, trepel, and opoka) on cement stone formation processes and to identify the most effective raw materials for use as photocatalytic carriers in self-cleaning concrete compositions. Methods and materials. The methods of Kozeny-Karman, laser diffraction and X-ray fluorescence spectrometry were used to determine the specific surface area and parameters of granulometric and chemical compositions of silicite samples. The phase composition of siliceous rocks and modified cement systems was studied by X-ray powder diffractometry. Results and discussion. The main parameters of granulometric composition of diatomite, trepel and opoka were determined. The predominance of reactive modifications of free silica (47.6–78.0 wt. %), represented by amorphous opal-A or cryptocrystalline OCT-phase (opal-CT), were revealed in the structure of silicites. It was found that increasing the dosages of silica-containing additives from 0 to 10% resulted in decreased by 10–27% in the quantity of portlandite in the phase composition of cement stone aged 28 days, while the content of high-strength low-basic calcium hydrosilicates (C–S–H (I)) increased by 11–27%. Conclusion. The chemical and mineralogical composition peculiarities of silicites, as well as the nature of the impact of silica-containing modifiers on the structure formation processes of cement systems, determine the prospects of using opal-cristobalite rocks as dispersed photocatalyst carriers for self-cleaning concrete.
Self-cleaning concrete, composite photocatalyst, siliceous rock, silica, pozzolanic reactivity, Portland cement, supplementary cementitious material, hydration, cement stone, microstructure, phase composition, X-ray diffraction
Короткий адрес: https://sciup.org/142240856
IDR: 142240856 | DOI: 10.15828/2075-8545-2024-16-2-158-169
Текст научной статьи Siliceous rocks as modifiers of structure of photocatalytic self-cleaning concrete. Assessment of the effect on the phase composition of cement stone
Original article
Today one of the focus areas for the development of building materials science is the creation of high performance cement concretes characterized by polydisperse multicomponent composition with the presence of nano-
and micro-sized modifiers of various types (chemical, mineral, organo-mineral), giving the opportunity to directly influence the structure formation processes and properties of the material [1–10].
The nomenclature of modern modified concretes is quite diverse, including reactive powder concrete [11, 12],
THE RESULTS OF THE SPECIALISTS’ AND SCIENTISTS’ RESEARCHES high-strength concrete [13–15], fiber reinforced concrete [16, 17], self-compacting concrete [18, 19], self-prestressing concrete [18, 20, 21], etc. A separate group contains photocatalytically active cement systems, which acquire a number of positive properties during light irradiation, including the ability to decompose atmospheric pollutants, to self-clean the material surface, etc. [22–27].
The studies [22, 23, 27–33] revealed that the use of photocatalytic additives based on anatase TiO2, characterized by non-toxicity, chemical stability and photoactivity under conditions of exposure to ultraviolet radiation, allowed to achieve the required level of special properties of self-cleaning concrete. The research results [34, 35] showed that the efficiency of photocatalytic process when using titanium dioxide in the composition of cement material could be reduced due to:
-
• reducing the amount of absorbed light and free surface for photochemical reactions because of the high content of micro- and macro-sized components in the concrete;
-
• narrowed spectral action range of TiO2, characterized by band gap of the rutile and anatase forms equal to 3.0 and 3.2 eV, respectively, which requires solving the problem of its sensitization to visible light;
-
• difficulties in ensuring uniform distribution of the photocatalytic nanomodifier in the cement material volume when its dosage is low, etc.
The promising method for improving the functional characteristics of titanium oxide photocatalysts is the creation of nanostructured photocatalytic systems with ‘core – shell’ architecture, the synthesis of which is by precipitation of TiO2 on dispersed carriers (mineral substrates). Using this technology allows to increase the active surface area of titanium dioxide, and also promotes its more more equal distribution in the structure of complex modifier, thereby increasing the photoactivity of ‘carrierphotocatalyst’ compositions in the cement material [35, 36].
The study results by domestic and foreign authors [35–38] indicated that the final characteristics of the synthesized photocatalytic systems with ‘core – shell’ architecture significantly depended on the type of mineral substrate used.
It is known that siliceous raw materials of natural and man-made origin, in particular opal-cristobalite rocks (diatomite, opoka, trepel), are among the most efficient substrates upon obtaining the composite/hybrid photo-catalytic modifiers for cement materials [35, 38–40]. The prospect of using silicites in the composition of self-cleaning concretes lies in their widespread occurrence, high affinity to crystalline hydrates of cement stone, as well as increased pozzolanic activity, owing to the predominance of amorphized varieties of silica in the structure [41, 42].
Notably, the effectiveness of using various opal-cristobalite rocks as photocatalyst carrier for self-cleaning
concrete can vary substantially depending on the composition and properties of silicites, in particular on the level of their reactivity in cement systems.
The aim of this study was to install the laws of the impact of three types of opal-cristobalite rocks (diatomite, trepel and opoka) from deposits of the Republic of Mordovia on the structure formation processes of cement stone and to find the most effective siliceous raw materials for use as photocatalyst carriers in the composition of self-cleaning concrete.
The following tasks were solved to achieve the aim of this study:
-
1) the chemical and mineralogical composition of opal-cristobalite rocks was established;
-
2) the main parameters of granulometric composition of silicites (specific surface area, particle size distribution) were determined;
-
3) the influence of siliceous rocks on the cement stone phase composition at the project age (28 days) was installed;
-
4) the most efficient types of silicites were identified for use as carriers of photocatalytic agents in the composition of self-cleaning concrete.
METHODS AND MATERIALS
Materials
Portland cement CEM I 42.5R (PC) that meets the requirements of the Russian State Standard GOST 31108-2020 was used as the main component of binder in the composition of cement materials. The researched siliceous rocks were diatomite (DMT), trepel (TPL) and opoka (OPK) from the Atemarsky, Dubensky and Alekseevsky field of the Republic of Mordovia, respectively.
The effect of mineral additives of silicites (MA) on the phase composition of cement stone at the project age (28 days) was studied in the process of experimental study. The variable prescription factors were:
-
• water-binder ratio ( x 1 = W/(PC+MA));
-
• type and content of mineral additives based on opalcristobalite rocks: w 1 (DMT), w 2 (TPL), w 3 (OPK).
The variation levels of the studied prescription factors are presented in Table 1.
Methods
The methods of study silicites and cement systems are given in Table 2.
The crystallinity degree of structure of opal-cristobalite rocks was calculated based on the analysis of diffraction patterns of samples as the ratio of area of all crystalline peaks to the total area of crystalline peaks and amorphous halo.
THE RESULTS OF THE SPECIALISTS’ AND SCIENTISTS’ RESEARCHES
When studying the phase composition of cement stone with additives of silicites, the main controlled parameters were the relative amounts of portlandite, low-basic calcium hydrosilicates (C–S–H(I)) and high-basic calcium hydrosilicates (C–S–H(II)). These parameters were determined as the ratio of intensities of the main reflexes Ca(OH)2 ( d = 4.92–4.96 Å and 2.63–2.65 Å), α-СаО•SiO2 ( d = 3.23–3.25 Å) / β-СаО•SiO2 ( d = 2.97–3.00 Å) and β-2СаО•SiO2 ( d = 2.78–2.79 Å) for samples of modified and control (additive-free) compositions, respectively. At the same time, the intensities of α-CS, β-CS and β-C2S peaks were determined according to the diffraction patterns of cement stone powders calcined at 980–1000оC.
RESULTS AND DISCUSSION
Granulometric composition of silicites
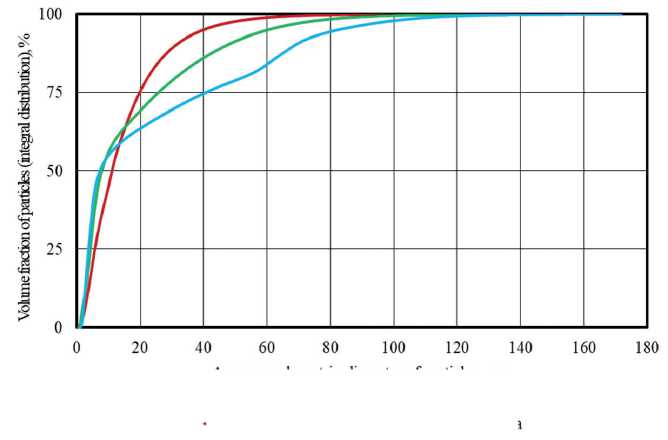
The main parameters of the granulometric composition of opal-cristobalite rock samples were determined based on the study results using the laser diffraction method (Figure 1, Table 3). In particular, the particle size
range of diatomite, trepel and opoka was 0.3–123.4 µm, 0.6–153.8 µm and 0.8–171.7 µm; the median particle size distribution ( d 50%) by volume was 11.1 µm, 7.9 µm and 7.3 µm; the mean particle diameter was 10.3 µm, 9.6 µm and 10.9 µm, respectively.
It was found that the specific surface area of siliceous rock powders increased in the row of trepel → opoka → diatomite with values of 1.2 m2/g, 1.3 m2/g and 2.0 m2/g, respectively. With comparable average particle size of si-licites, the increase by 1.5–1.7 times in the specific surface area of diatomite was due to the significantly higher porosity of its particles compared to opoka and trepel.
Chemical composition of silicites
Table 4 shows the chemical composition of the studied siliceous rocks.
The analysis of experimental data obtained by X-ray fluorescence spectrometry revealed the predominance of silicon, aluminum and iron oxides in the chemical composition of silicites. The content of SiO2, Al2O3 and Fe2O3 was 75.98–82.13 wt.%, 5.34–9.62 wt.% and 3.11–4.11 wt.%, respectively (Table 4). It is worth noting
Table 1
Variation levels of the prescription factors
|
Factors |
Variation levels |
||||
|
–1 |
0 |
+1 |
|||
|
Water-binder ratio, rel. units |
x 1 |
W/(PC+MA) |
0.33 |
0.35 |
0.37 |
|
Type and content of mineral additives based on silicites, % by weight of binder (PC+MA) |
w 1 |
DMT |
0 |
5 |
10 |
|
w 2 |
TPL |
0 |
5 |
10 |
|
|
w 3 |
OPK |
0 |
5 |
10 |
|
Note. The plan for experimental research was compiled taking into account fulfillment of the following conditions: x 1 = –1, 0, +1; wi = –1, 0, +1 ( i = 1, 2, 3).
Table 2
Methods for studying silicites and cement systems
|
Studied parameter |
Used research method |
|
Parameters of granulometric composition and specific surface area of siliceous rock powders |
Laser diffraction and Kozeny-Karman methods using the Shimadzu Sald-3101 analyzer and the PSKh-12 device. |
|
Chemical composition of silicites |
X-ray fluorescence spectrometry using the ARL Perform’X 4200 spectrometer (Rh Kα radiation). |
|
Phase composition of opal-cristobalite rocks and cement stone |
X-ray powder diffraction on the PANalytical Empyrean multipurpose diffractometer with the Bragg-Brentano focusing geometry in the θ–2θ scanning mode. The survey was performed using CuKα radiation characterized by weighted average wavelength λ = 1.54 Å. Phase identification was carried out on the basis of the Powder Diffraction File (PDF-2) from the International Center for Diffraction Data (ICDD). |
THE RESULTS OF THE SPECIALISTS’ AND SCIENTISTS’ RESEARCHES
Average vokunetric diameter of paiticles, pm Type of siliceous rock:
—Diatomite — Trepel ---Opoka
Fig. 1. Integral curves of the volumetric particle size distribution for silicite samples (according to laser diffraction)
Table 3
Main parameters of the granulometric composition of silicite powders
|
Parameter of granulometric composition |
Type of siliceous rock |
|||
|
Diatomite |
Trepel |
Opoka |
||
|
Specific surface area, m2/g |
2.0 |
1.2 |
1.3 |
|
|
Particle size range, µm |
0.3–123.4 |
0.6–153.8 |
0.8–171.7 |
|
|
Particle size, µm |
d 25 % |
5.6 |
4.2 |
3.6 |
|
d 50 % |
11.1 |
7.9 |
7.3 |
|
|
d 75 % |
19.8 |
25.6 |
40.5 |
|
|
median |
11.1 |
7.9 |
7.3 |
|
|
mean |
10.3 |
9.6 |
10.9 |
|
Table 4
Chemical composition of siliceous rocks (in recalculation on oxides)
|
Chemical composition |
Siliceous rock |
||
|
Diatomite |
Trepel |
Opoka |
|
|
Content, wt.% |
|||
|
SiO2 |
81.47 |
75.98 |
82.13 |
|
Al 2 O 3 |
5.34 |
9.62 |
6.05 |
|
Fe 2 O 3 |
3.11 |
4.11 |
3.48 |
|
CaO |
0.91 |
1.59 |
1.05 |
|
MgO |
0.67 |
1.01 |
0.84 |
|
K2O |
0.97 |
1.49 |
1.16 |
|
Na2O |
0.43 |
0.50 |
0.48 |
|
TiO2 |
0.37 |
0.29 |
0.32 |
|
SO3 |
0.12 |
0.31 |
0.30 |
|
other (loss on ignition) |
6.61 |
5.10 |
4.19 |
THE RESULTS OF THE SPECIALISTS’ AND SCIENTISTS’ RESEARCHES that the higher concentration of alumina recorded for the trepel sample (9.62%) were indicative of the increased content of clay minerals in it compared to diatomite and opoka. At the same time, increased weight losses during calcination of diatomite (6.61%) were associated with the growth of opal content in its structure, the amount of water in which can reach 10–20 wt.%.
Phase composition of silicites
Figure 2 and Table 5 show the results of qualitative and quantitative X-ray diffraction analysis of siliceous rock samples.
The phase composition of silicites consisted (Table 5) mainly of various polymorphic modifications of free silica (66.0–84.5 wt.%), in particular, amorphous opal (opal-A) and crystalline quartz for diatomite; crystalline quartz and quasi-crystalline (structurally disordered) opal-cristobalite-tridymite (OCT) phase for trepel and opoka. The OCT-phase (opal-CT) is the crystallization product of opal-A, and is the single mineral formation in the form of complex of amorphized/cryptocrystalline and crystalline varieties of silica (opal, cristobalite and tridymite).
It was found that despite significant differences in the opal-CT content of trepel and opoka (47.6 wt.% and 69.0 wt.%, respectively), the OCT-phase has a close ratio of components, in particular, opal/cristobalite/tridy-mite = 68–69/17/14–15 (Table 5). It is worth noting that according to [43], the fixed ratio of the OCT-phase components with almost equal concentrations of cristo-
balite and tridymite is specific to siliceous rocks located in platform areas.
It was revealed (Table 5) that the content of amorphous phase (opal) increased in the row of trepel → opoka → diatomite (33.0 wt.%, 47.0 wt.% and 78.0 wt.%), while the crystallinity degree of the studied siliceous rocks correspondingly decreased (67.0%, 53.0% and 22.0%). At the same time, decrease of 1.7 and 2.4 times in the amount of X-ray amorphous opal for opoka and trepel compared to diatomite was associated with increase in the content of quartz in their phase composition by 2.1 and 2.8 times (from 6.5 wt. % to 13.7 wt.% and 18.4 wt.%, respectively).
It was established that clay minerals in the amount of 11.6–17.7 wt.% (Table 5), represented mainly by micas and hydromicas from subgroups of muscovite and illite, were the second structural component of the studied silic-ites after free silica in terms of content. The insignificant amounts of smectites from the montmorillonite subgroup were also present in diatomite and trepel, as evidenced by the presence of typical reflex with d = 15.6–15.0 Å and 2θ = 5.7–5.9о (Figure 2).
X-ray diffraction analysis showed the presence in the siliceous rock structure of feldspars in the amount of 1.9–5.7 wt.%. These phases are group of framework aluminosilicates of potassium (microcline, orthoclase), sodium (albite) and calcium (anorthite). In addition, the mineralogical composition of trepel contained 12.3 wt.% of zeolites from the subgroups of clinoptilolite and heulandite (Table 5).
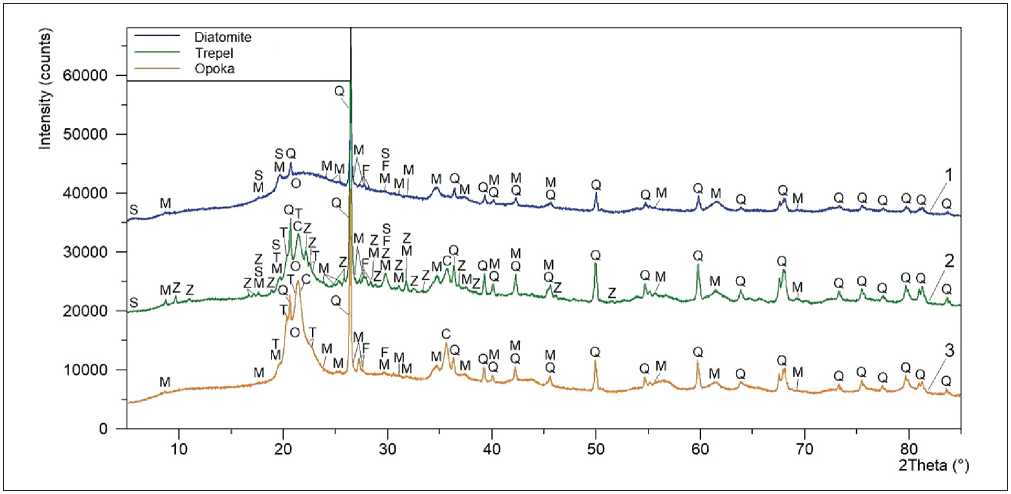
Fig. 2. Results of qualitative X-ray diffraction analysis of samples of diatomite (1), trepel (2) and opoka (3):
opal (O); cristobalite (C); tridymite (T); quartz (Q); micas and hydromicas (M) in the form of muscovite and illite; smectites (S) in the form of montmorillonite; feldspars (F); zeolites (Z) in the form of clinoptilolite and heulandite
THE RESULTS OF THE SPECIALISTS’ AND SCIENTISTS’ RESEARCHES
Thus, despite the close total SiO2 content (75.98– 82.13 wt.% (Table 4)), the studied silicites had different ratios of structure-forming phases, primarily polymorphic modifications of free silica (opal, OCT-phase, quartz) and clay minerals. The phase composition variation predetermined the complex of the main functional properties of siliceous rocks, including their chemical activity in cement systems.
Influence of silicites on the phase composition of cement stone
It is known that calcium silicate hydrates (C–S–H) of different basicity and portlandite (Ca(OH)2) are the main hydrate phases of cement stone due to the predominance
of alite (3CaO•SiO2) and belite (β-2CaO•SiO2) in the mineralogical composition of Portland cement clinker.
During the hardening of cement systems, the resulting calcium silicate hydrates have different composition and degree of crystallization (amorphization), which vary in wide ranges. Taking into account the chemical and mineralogical features, calcium silicate hydrates are conditionally divided into two main groups [44–46]:
-
1) high-strength low-basic calcium silicate hydrates (C–S–H (I)) with CaO/SiO2 molar ratio less than 1.5, characterized by crystalline hydrates of colloidal size (less than 100 nm);
-
2) high-basic calcium silicate hydrates (C–S–H (II)) that are larger and less durable than C–S–H(I) and that have CaO/SiO2 molar ratio at least 1.5.
Table 5
Results of quantitative X-ray diffraction analysis of siliceous rock samples
|
Description of phases |
Position of the main / typical reflexes d , Å (2θ, °) |
Siliceous rock |
|||||
|
Type |
Group |
Subgroup, name |
Diatomite |
Trepel |
Opoka |
||
|
Phase composition, wt. % |
|||||||
|
3 и g d |
Opal |
halo corresponding to 4,9–3,6 Å (18–25°) maximum in the area of 4,1 Å (21,6°) |
78.0 |
33.0 |
47.0 |
||
|
Cristobalite |
4.13; 2.51 Å (21.5; 35.7°) |
– |
8.0 |
11.8 |
|||
|
Tridymite |
4.36; 4.13; 3.94–3.88 Å (20.4; 21.5; 22.6–22.9°) |
– |
6.6 |
10.2 |
|||
|
Quartz |
4.28; 3.36; 1.82 Å (20.7; 26.5; 50.0°) |
6.5 |
18.4 |
13.7 |
|||
|
Clay minerals |
Micas and hydromicas (muscovite, illite) |
10.10; 5.02; 4.50; 2.58 Å (8.7; 17.6; 19.7; 34.7°) |
13.6 |
17.7 |
11.6 |
||
|
Smectites (montmorillonite) |
15.6–15.0; 5.02; 4.50 Å (5.7–5.9; 17.6; 19.7°) |
||||||
|
Zeolites |
Clinoptilolite, heulandite |
9.10; 8.02; 5.16; 4.00 Å (9.7; 11.0; 17.2; 22.2°) |
– |
12.3 |
– |
||
|
Alkaline feldspars (microcline, orthoclase) |
3.26; 3.22–3.18 Å (27.3; 27.7–28.0°) |
1.9 |
4.0 |
5.7 |
|||
|
Plagioclases (albite, anorthite) |
3.26; 3.22–3.18; 3.16; 3.14 Å (27.3; 27.7–28.0; 28.2; 28.4°) |
||||||
|
Characteristics of the opal-cristobalite-tridymite (OCT) phase |
|||||||
|
Content, wt.% |
– |
47.6 |
69.0 |
||||
|
Ratio of opal/cristobalite/tridymite in the OCT-phase, % |
– (opal is 100 %) |
69/17/14 |
68/17/15 |
||||
THE RESULTS OF THE SPECIALISTS’ AND SCIENTISTS’ RESEARCHES
Typical representatives of low-basic calcium silicate hydrates of C–S–H (I) type are:
-
• tobermorite group with the CaO/SiO2 molar ratio from 0.8 to 1.0, in particular, 9.3 Å tobermorite (riv-ersideite, 5CaO•6SiO2•3H2O), 11.3 Å tobermorite (5CaO•6SiO2•5H2O) and 14 Å tobermorite (plom-bierite, 5CaO•6SiO2•10.5H2O);
-
• compounds with the CaO/SiO2 molar ratio from 0.5 to 1.3 that are similar to the wollastonite structure, in particular, nekoite (3CaO•6SiO2•8H2O), okenite (CaO•2SiO2•2H2O), xonotlite (6CaO•6SiO2•H2O), foshagite (4CaO•3SiO2•1.5H2O);
-
• gyrolite group with the CaO/SiO2 molar ratio from 0.5 to 0.7, in particular, gyrolite (2CaO•3SiO2•2.5H2O), truscottite (CaO•2SiO2•0.67H2O).
The most common high-basic calcium silicate hydrates of C–S–H (II) type are afwillite (3CaO•2SiO2•3H2O), hillebrandite (2CaO•SiO2•1.17H2O), α-hydrate C2S (2CaO•SiO2•H2O), dellaite (6CaO•3SiO2•H2O), calcium chondrodite (5CaO•2SiO2•H2O).
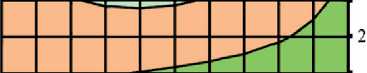
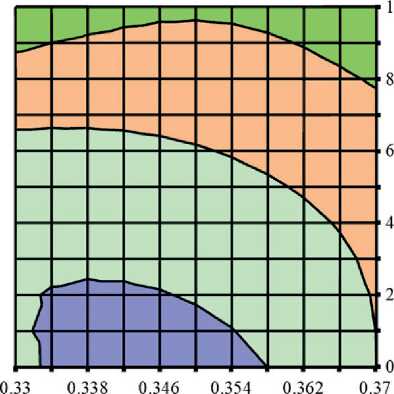
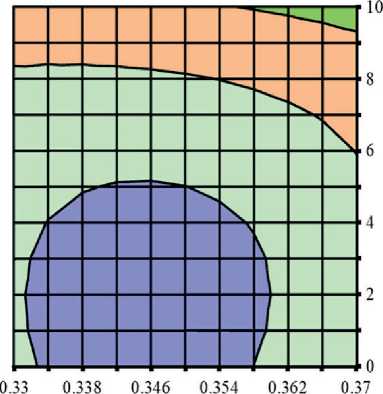
Figures 3 and 4 show the study results of the effect of silicite and water dosages on the content of portlandite and low-basic calcium silicate hydrates of C–S–H (I) type in the structure of cement stone relative to the con-
а
b
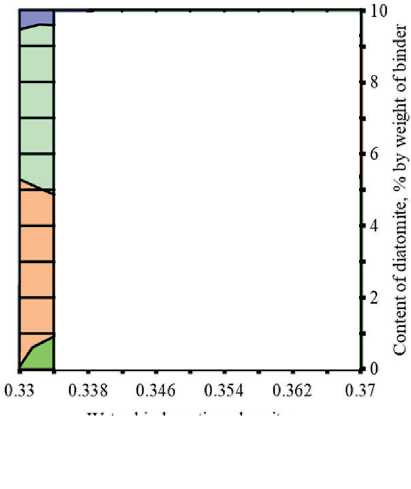
Water-binder ratio, rel. units Relative content of Ca(OH)2, rel. units:
□0.6-0.7 Q0.7-0.8 00.8-0.9 □ 0.9-1
Water-binder ratio, rel. luiits
Relative content of Ca(OH)2, rel. units:
□ 0.6-0.7 D0.7-0.8 口 0.8-0.9 DO.9-1
c



















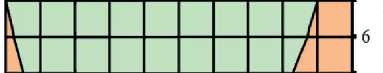

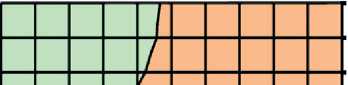
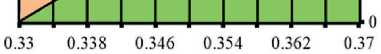
Fig. 3. Isolines of changes in the relative content of portlandite (Ca(OH)2) in the structure of cement stone at the age of 28 days (relative to the additive-free composition with W/(PC+MA) = 0.37) depending on the water-binder ratio of the composition, type and dosage of silica-containing additive (Table 1): diatomite (a); trepel (b); opoka (c)

033 0.338 0.346 0.354 0.362 0.37
A^ter-binder ratio, reL units
Relative content of Ca(OH)2? rel units:
□ 0.6-0.7 00.7-0.8 CI0.8-0.9 DO.9-1
І ә р-aq go 一иІӘЛЛ Kq %«Nodo jo =ІӘ=Ю。
THE RESULTS OF THE SPECIALISTS’ AND SCIENTISTS’ RESEARCHES
а
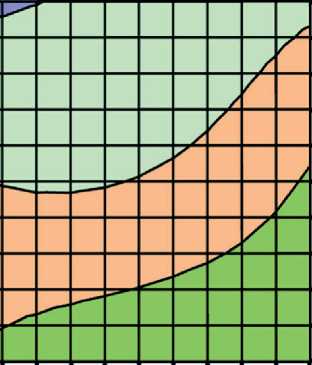
.IluPIqq jo =『 'SA\ Aq % ^^so^re^^o B^§^
о
Water-binder ratio, rel. miits
Relative content of C-S-H (I)} rel. imits: □0.6-0.8 QO.8-1 01-1.2 OL2-L4
b
.І ә р-Sq jo Jqs-SAV Aq % Tudau jo HIQHIOU
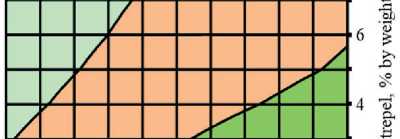
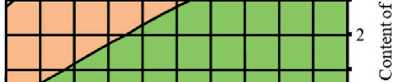
Fig. 4. Isolines of changes in the relative content of low-basic calcium silicate hydrates (C–S–H (I)) in the structure of cement stone at the age of 28 days (relative to the additive-free composition with W/(PC+MA) = 0.37) depending on the water-binder ratio of the composition, type and dosage of silica-containing additive (Table 1): diatomite (a); trepel (b); opoka (c)
Water-bindei ratio, rel. units
Relative content of C-S-H (I), rel. units: □0.6-0.8 DO.8-1 □ 1-1.2 □!.2-1.4
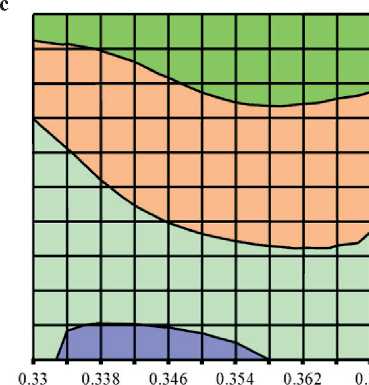
Water-binder ratio, rel. units Relative content of C-S-H (I), rel. units: □0.6-0,8 00.8-1 Ql-1.2 01.2-1.4

trol additive-free composition with water-binder ratio of 0.37.
There was direct relationship between the water content of composition and the concentration of portlandite in the structure of the cement stone at the project age (28 days). It was established that reduction in the water-binder ratio from 0.37 to 0.33 rel. units led to decrease in the relative amount of Ca(OH)2 in the phase composition of cement stone: by 10% in the control additive-free compositions (without silicites); by 12%, 16% and 3% in the modified compositions with dosage of diatomite, trepel and opoka equal to 10%
by weight of the binder, respectively (Figure 3). The increase in the content of portlandite with the growth of the water-binder ratio of cement systems in the accepted range of variation (0.33–0.37 relative units) was largely due to increase in the degree of Portland cement hydration in compositions with higher water content, as evidenced by the results of numerous studies, in particular [44, 45].
It was found that increase in the dosages of siliceous rocks in the modified cement systems from 0 to 10% by weight of the binder led to the growth in the relative content of low-basic calcium silicate hydrates of C–S–H (I)
THE RESULTS OF THE SPECIALISTS’ AND SCIENTISTS’ RESEARCHES type by 1.22–1.35 times, 1.11–1.26 times and 1.26– 1.40 times in the compositions with diatomite (DMT), trepel (TPL) and opoka (OPK), respectively (Figure 4). At the same time, replacing 10 % of Portland cement with DMT, TPL and OPK helped reduce the relative amount of portlandite in the phase composition of cement stone by 22–27%, 10–19% and 13–21%, respectively (Figure 3). Thus, the study results of the Ca(OH)2 content in the structure of cement systems indicated an increase in the pozzolanic activity of opal-cristobalite rocks in the row of trepel → opoka → diatomite.
To summarize, the use of siliceous rocks made it possible to significantly change the quantitative relationship between the main hydrate phases of cement stone such as portlandite and calcium silicate hydrates of different basicity. The established shift in the balance towards an increase in the volume of high-strength low-basic calcium silicate hydrates of C–S–H (I) type (so-called secondary phases with CaO/SiO2 molar ratio less than 1.5) instead of primary crystalline hydrates of Ca(OH)2 and high-basic calcium silicate hydrates of C–S–H (II) type, characterized by lower dispersion, strength and corrosion resistance, was the manifestation of the chemical effect [46, 47] in the action mechanism of silica-containing modifiers. This chemical effect was associated with the pozzolanic activity of silicites and was due to the predominance of reactive varieties of silica in their mineralogical composition, in particular, amorphous opal-A for diatomite and quasi-crystalline (structurally disordered) OCT-phase (opal-CT) for tre-pel and opoka.
CONCLUSION
The following scientific results were obtained in the paper:
-
1. The chemical and mineralogical composition of opal-cristobalite rocks was established.
-
2. The main parameters of granulometric composition of silicites (specific surface area, particle size distribution) were determined.
-
3. The influence of siliceous rocks on the phase composition of cement stone at the project age (28 days) was installed.
-
4. The most effective types of silicites were identified for use as carriers of photocatalytic agents in the composition of self-cleaning concrete.
The use of opal-cristobalite rocks made it possible to purposefully change the phase composition of cement stone by:
-
• decreasing the number of the Ca(OH)2 crystals, characterized by low strength and reduced corrosion resistance;
-
• increasing the density and strength of calcium silicate hydrates by shifting the balance towards increasing the content of highly dispersed low-basic phases of the C– S–H (I) type instead of larger high-basic compounds of C–S–H (II) type.
The established nature of the influence of silicites on the phase composition of cement systems indicates their increased chemical activity due to the predominance in the structure of reactive modifications of silica (opal-A, opal-CT). It determines the prospects of using siliceous rocks as dispersed carriers of photocatalytic agents for self-cleaning concrete.
Список литературы Siliceous rocks as modifiers of structure of photocatalytic self-cleaning concrete. Assessment of the effect on the phase composition of cement stone
- Khan K., Johari M.A.M., Amin M.N., Nasir M. Development and evaluation of basaltic volcanic ash based high performance concrete incorporating metakaolin, micro and nano-silica. Developments in the Built Environment. 2024; 17: 100330. https://doi.org/10.1016/j.dibe.2024.100330
- Jiang J., Qin J., Chu H. Improving mechanical properties and microstructure of ultra-high-performance lightweight concrete via graphene oxide. Journal of Building Engineering. 2023; 80: 108038. https://doi.org/10.1016/j.jobe.2023.108038
- Tayeh B.A., Akeed M.H., Qaidi S., Bakar B.H.A. Ultra-high-performance concrete: Impacts of steel fibre shape and content on flowability, compressive strength and modulus of rupture. Case Studies in Construction Materials. 2022; 17: e01615. https://doi.org/10.1016/j.cscm.2022.e01615
- O’Hegarty R., Kinnane O., Newell J., West R. High performance, low carbon concrete for building cladding applications. Journal of Building Engineering. 2021; 43: 102566. https://doi.org/10.1016/j.jobe.2021.102566
- Shin H.O., Yoo D.Y., Lee J.H., Lee S.H., Yoon Y.S. Optimized mix design for 180 MPa ultra-high-strength concrete. Journal of Materials Research and Technology. 2019; 8: 4182–4197. https://doi.org/10.1016/j.jmrt.2019.07.027
- Strokova V.V., Markova I.Yu., Markov A.Yu., Stepanenko M.A., Nerovnaya S.V., Bondarenko D.O., Botsman L.N. Properties of a composite cement binder using fuel ashes. Key Engineering Materials. 2022; 909: 184–190. https://doi.org/10.4028/p-tm4y4j
- Kalashnikov V.I. Evolution of Development of Concretes Compositions and Change in Concrete Strength. Concretes of Present and Future. Part 1. Change in Compositions and Strength of Concretes. Construction Materials. 2016; 1-2: 96–103.
- Kaprielov S.S., Sheinfeld A.V., Kardumyan G.S., Chilin I.A. About selection of compositions of high-quality concretes with organic-mineral modifiers. Construction Materials. 2017; 12: 58–63.
- Zagorodnuk L.Kh., Elistratkin M.Yu., Podgornyi D.S., Al Mamuri S. Сomposite binders for 3d additive technologies. The Russian Automobile and Highway Industry Journal. 2021; 18(4): 428–439. https://doi.org/10.26518/2071-7296-2021-18-4-428-439
- Balykov A.S., Nizina T.A., Volodin S.V. Optimization of technological parameters for obtaining mineral additives based on calcined clays and carbonate rocks for cement systems. Nanotechnologies in Construction. 2022; 14(2): 145–155. https://doi.org/10.15828/2075-8545-2022-14-2-145-155
- Ge L., Zhang Y., Sayed U., Li H. Study on properties of basalt fiber reinforcing reactive powder concrete under different curing conditions. Journal of Materials Research and Technology. 2023; 27: 5739–5751. https://doi.org/10.1016/j.jmrt.2023.10.289
- Tolstoy A.D., Lesovik V.S., Zagorodnyuk L.Kh., Kovaleva I.A. Powder concretes with technogenic materials. Proceedings of Moscow State University of Civil Engineering. 2015; 11: 101–109.
- Sifan M., Nagaratnam B., Thamboo J., Poologanathan K., Corradi M. Development and prospectives of lightweight high strength concrete using lightweight aggregates. Construction and Building Materials. 2023; 362: 129628. https://doi.org/10.1016/j.conbuildmat.2022.129628
- Rassokhin A.S., Ponomarev A.N., Figovsky O.L. Silica fumes of different types for high-performance finegrained concrete. Magazine of Civil Engineering. 2018; 78: 151–160. https://doi.org/10.18720/MCE.78.12
- Balykov A.S., Nizina T.A., Makarova L.V. Criteria of Efficiency of Cement Concretes and Their Use for Analyzing Compositions of High-Strength Composites. Construction Materials. 2017; 6: 69–75.
- Chen L., Chen Z., Xie Z., Wei L., Hua J., Huang L., Yap P.-S. Recent developments on natural fiber concrete: A review of properties, sustainability, applications, barriers, and opportunities. Developments in the Built Environment. 2023; 16: 100255. https://doi.org/10.1016/j.dibe.2023.100255
- Nizina T.A., Balykov A.S., Korovkin D.I., Volodin V.V. Physical and mechanical properties of modified finegrained fibre-reinforced concretes containing carbon nanostructures. International Journal of Nanotechnology. 2019; 16: 496–509. https://doi.org/10.1504/IJNT.2019.106621
- Carballosa P., García Calvo J.L., Revuelta D., Sánchez J.J., Gutiérrez J.P. Influence of cement and expansive additive types in the performance of self-stressing and self-compacting concretes for structural elements. Construction and Building Materials. 2015; 93: 223–229. https://doi.org/10.1016/j.conbuildmat.2015.05.113
- Nizina T.A., Balykov A.S., Korovkin D.I., Volodin V.V. Modified fine-grained concretes based on highly filled self-compacting mixtures. IOP Conference Series: Materials Science and Engineering. 2019; 481: 012048. https://doi.org/10.1088/1757-899X/481/1/012048
- Wang W., Wu J., Yang W., Wang S., Wu H., Zhu Z., Wang L., Ding Q., Wang H., Zhou X. Towards lowtemperature shrinkable synthetic fibers for internally self-prestressing concrete. Journal of Building Engineering. 2023; 73: 106769. https://doi.org/10.1016/j.jobe.2023.106769
- Dhahir M.K., Marx S. Development of expansive concrete for chemical prestressing applications. Case Studies in Construction Materials. 2023; 19: e02611. https://doi.org/10.1016/j.cscm.2023.e02611
- Amor F., Baudys M., Racova Z., Scheinherrová L., Ingrisova L., Hajek P. Contribution of TiO2 and ZnO nanoparticles to the hydration of Portland cement and photocatalytic properties of High Performance Concrete. Case Studies in Construction Materials. 2022; 16: e00965. https://doi.org/10.1016/j.cscm.2022.e00965
- Janczarek M., Klapiszewski Ł., Jędrzejczak P., Klapiszewska I., Ślosarczyk A., Jesionowski T. Progress of functionalized TiO2-based nanomaterials in the construction industry: A comprehensive review. Chemical Engineering Journal. 2022; 430(3): 132062. https://doi.org/10.1016/j.cej.2021.132062
- Yang L., Hakki A., Wang F., Macphee D.E. Photocatalyst efficiencies in concrete technology: The effect of photocatalyst placement. Applied Catalysis B: Environmental. 2018; 222: 200–208. https://doi.org/10.1016/j.apcatb.2017.10.013
- George C., Beeldens A., Barmpas F., Doussin J.F., Manganelli G., Herrmann H., Kleffmann J., Mellouki A. Impact of photocatalytic remediation of pollutants on urban air quality. Frontiers of Environmental Science & Engineering. 2016; 10(5): 2. https://doi.org/10.1007/s11783-016-0834-1
- Gallus M., Akylas V., Barmpas F. et al. Photocatalytic de-pollution in the Leopold II tunnel in Brussels: NOx abatement results. Building and Environment. 2015; 84: 125–133. https://doi.org/10.1016/j.buildenv.2014.10.032
- Emeline A.V., Rudakova A.V., Sakai M., Murakami T., Fujishima A. Factors affecting UV-induced superhydrophilic conversion of TiO2 surface. The Journal of Physical Chemistry C. 2013; 117(23): 12086–12092. https://doi.org/10.1021/jp400421v
- Li Y.-N., Chen Z.-Y., Bao S.-J., Wang M.-Q., Song C.-L., Pu S., Long D. Ultrafine TiO2 encapsulated in nitrogen-doped porous carbon framework for photocatalytic degradation of ammonia gas. Chemical Engineering Journal. 2018; 331: 383–388. https://doi.org/10.1016/j.cej.2017.08.119
- Henderson M.A. A surface science perspective on TiO2 photocatalysis. Surface Science Reports. 2011; 66: 185–297. https://doi.org/10.1016/j.surfrep.2011.01.001
- Nakata K., Fujishima A. TiO2 photocatalysis: Design and applications. Journal of Photochemistry and Photobiology C: Photochemistry Reviews. 2012; 13(3): 169–189. https://doi.org/10.1016/j.jphotochemrev.2012.06.001
- Tyukavkina V.V., Tsyryatyeva A.V. The structure of the cement stone modified by nanodispersed titaniumbearing additive. Proceedings of the Fersman Scientific Session of the State Institute of the CSC RAS. 2019; 16: 597–601. https://doi.org/10.31241/FNS.2019.16.122
- Lukuttsova N.P., Pykin A.A., Postnikova O.A., Golovin S.N., Borovik E.G. The structure of cement stone with dispersed titanium dioxide in daily age. Bulletin of Belgorod State Technological University named after V.G. Shukhov. 2016; 11: 13–17. https://doi.org/10.12737/22432
- Timokhin D.K., Geranina Yu.S. Titanium Dioxide as a Photocatalyst in Cement Concrete. Scientific Review. 2015; 8: 46–50.
- Hela R., Bodnarova L. Research of Possibilities of Testing Effectiveness of Photoactive TiO2 in Conrete. Construction Materials. 2015; 2: 77–81.
- Strokova V.V., Gubareva E.N., Ogurtsova Yu.N. Evaluation of the properties of the silica raw materials as a substrate as component of composite photocatalytic material. Bulletin of Belgorod State Technological University named after V.G. Shukhov. 2017; 2: 6–12. https://doi.org/10.12737/23819
- Falikman V.R., Vainer A.Y. New high performance nanoadditives for photocatalytic concrete: synthesis and study. Nanotechnologies in Construction. 2015; 7(1): 18–28. https://doi.org/10.15828/2075-8545-2015-7-1-18-28
- Wang D., Hou P., Stephan D., Huang S., Zhang L., Yang P., Cheng X. SiO2/TiO2 composite powders deposited on cement-based materials: Rhodamine B removal and the bonding mechanism. Construction and Building Materials. 2020; 241: 118124. https://doi.org/10.1016/j.conbuildmat.2020.118124
- Balykov A.S., Nizina T.A., Kyashkin V.M., Volodin S.V. Evaluation of the effectiveness of mineral additives in cement systems in the development of “core – shell” photocatalytic compositions. Nanotechnologies in Construction. 2022; 14(5): 405–418. https://doi.org/10.15828/2075-8545-2022-14-5-405-418
- Fatimah I., Prakoso N.I., Sahroni I., Miqdam Musawwa M., Sim Y.-L., Kooli F., Muraza O. Physicochemical characteristics and photocatalytic performance of TiO2/SiO2 catalyst synthesized using biogenic silica from bamboo leaves. Heliyon. 2019; 5(11): e02766. https://doi.org/10.1016/j.heliyon.2019.e02766
- Pal A., Jana T.K., Chatterjee K. Silica supported TiO2 nanostructures for highly efficient photocatalytic application under visible light irradiation. Materials Research Bulletin. 2016; 76: 353-357. https://doi.org/10.1016/j.materresbull.2015.12.040
- Taoukil D., El meski Y., Lahlaouti M.L., Djedjig R., El bouardi A. Effect of the use of diatomite as partial replacement of sand on thermal and mechanical properties of mortars. Journal of Building Engineering. 2021; 42: 103038. https://doi.org/10.1016/j.jobe.2021.103038
- Balykov A.S., Nizina T.A., Kyashkin V.M., Volodin S.V. Prescription and technological efficiency of sedimentary rocks of various composition and genesis in cement systems. Nanotechnologies in Construction. 2022; 14(1): 53–61. https://doi.org/10.15828/2075-8545-2022-14-1-53-61
- Ilicheva O.M., Naumkina N.I., Lygina T.Z. Phase and structural variability in sedimentary siliceous rocks as the basis for evaluation of the quality. Exploration and protection of subsoil. 2012; 5: 50–53.
- Kaprielov S.S., Sheinfeld A.V., Dondukov V.G. Cements and Additives for Producing High-Strength Concretes. Construction Materials. 2017; 11: 4–10.
- Chen J.J., Thomas J.J., Taylor H.F.W., Jennings H.M. Solubility and structure of calcium silicate hydrate. Cement and Concrete Research. 2004; 34(9): 1499–1519. https://doi.org/10.1016/j.cemconres.2004.04.034
- Nizina T.A., Balykov A.S., Volodin V.V., Kyashkin V.M. Structure and properties of cement systems with additives of calcined clay and carbonate rocks. Magazine of Civil Engineering. 2022; 116(8): 11602. https://doi.org/10.34910/MCE.116.2
- Detwiler R.J., Mehta P.K. Chemical and physical effects of silica fume on the mechanical behavior of concrete. ACI Materials Journal. 1989; 86(6): 609–614.


