Синтез и строение новых дигалогендицианоауратных комплексов
Автор: Шевченко Дмитрий Павлович, Хабина Анастасия Евгеньевна, Сенчурин Владислав Станиславович
Журнал: Вестник Южно-Уральского государственного университета. Серия: Химия @vestnik-susu-chemistry
Рубрика: Химия элементоорганических соединений
Статья в выпуске: 1 т.12, 2020 года.
Бесплатный доступ
Взаимодействием галогенидов тетраорганилфосфония с дихлоро-, дибромо- и дииододицианоауратом калия в воде с последующей перекристаллизацией из ацетонитрила синтезированы ионные комплексы золота(III) [Me4P][Au(CN)2Cl2] (1), [Ph3PR][Au(CN)2Hal2] (Hal = Cl, R = (CH2)6Me (2), (CH2)2C(O)OH (3); Hal = Br, R = CH2CN (4); Hal = I, R = CH2CN (5)) и [Ph3PCH=CHPPh3][Au(CN)2Cl2]2 (6). Аналогичным путем взаимодействием хлорида тетрафенилстибония с дихлородицианоауратом калия получен комплекс [Ph4Sb][Au(CN)2Cl2] (7). Строение комплексов 3, 5-7 установлено методом рентгеноструктурного анализа (РСА). По данным РСА, атомы фосфора и сурьмы в 3, 5-7 имеют искаженную тетраэдрическую координацию (углы СPС 107,5(2)-111,8(3)° (3), 106,0(3)-111,5(3)° (5), 106,7(4)-111,8(4)° (6), CSbC 100,5(7)-114,6(5)° (7); длины связей P-С 1,788(5)-1,807(5) Å (3), 1,765(6)-1,821(6) Å (5), 1,781(8)-1,810(8) Å (6); Sb-C 2,070(11)-2,121(12) Å (7)). Атомы золота в анионах [Au(CN)2Hal2]- имеют малоискаженную плоскоквадратную координацию ( транс -углы HalAuHal и CAuC близки к 180°; цис -углы CAuHal изменяются в интервале 88,05-92,48°), длины связей Au-Hal составляют: Au-Cl 2,328(3) Å (3), 2,393(2), 2,411(2) Å (6), 2,4223(12) Å (7); Au-I 2,609(3), 2,598(3) Å (5), Au-C - 1,981(7) Å (3), 1,996(7), 2,006(8) Å (5), 1,978(12), 2,001(13) Å (6), 2,040(15) (7). Структурная организация в кристаллах 3, 5-7 обусловлена нековалентными взаимодействиями различной природы: С-H∙∙∙N≡C 2,55-2,74 Å (3, 5-7), O-H∙∙∙N≡C 2,03 Å, С-H∙∙∙O=C 2,52 Å, C-H∙∙∙Cl-Au 2,88-2,93 Å (3), Au-I∙∙∙I-Au 3,925(4) Å (5), C-H∙∙∙Cl-Au 2,91 Å (6).
Дигалогендицианоаурат калия, галогениды тетраорганилфосфония, хлорид тетрафенилстибония, синтез, строение, рентгеноструктурный анализ
Короткий адрес: https://sciup.org/147233153
IDR: 147233153 | УДК: 546.593+546.87+546.87+547.53.024+548.312.2 | DOI: 10.14529/chem200103
Текст научной статьи Синтез и строение новых дигалогендицианоауратных комплексов
В последнее время для координационных соединений переходных металлов с цианолигандами открыт ряд практически важных свойств, таких как люминесценция [1–6], магнетизм [6– 13], пористость [14], отрицательный коэффициент термического расширения [15, 16], вапохро-мизм [1, 3, 17–20], двойное лучепреломление [21–28]. Модификация комплексов путем изменения либо внедрения других, отличных от цианидных, лигандов позволяет регулировать их свойства. Так, установлено, что свойство двойного лучепреломления сильнее проявляется в случае дибромдицианоауратных анионов [Au(CN) 2 Br 2 ]– в сравнении с дицианоауратными [Au(CN) 2 ]–, по мнению авторов, из-за межионных галоген-галогенных взаимодействий Br⋯Br и поляризации связей Au–Br [21].
В настоящей работе описан синтез ряда новых дигалогендицианоауратных комплексов с тетраорганилфосфониевыми и тетрафенилстибониевым катионами [Me 4 P][Au(CN) 2 Cl 2 ] ( 1 ), [Ph 3 P(CH 2 ) 6 Me][Au(CN) 2 Cl 2 ] ( 2 ), [Ph 3 P(CH 2 ) 2 C(O)OH][Au(CN) 2 Cl 2 ] ( 3 ), [Ph 3 PCH 2 CN][Au(CN) 2 Br 2 ] ( 4 ), [CH 2 CN][Au(CN) 2 I 2 ] ( 5 ), [Ph 3 PCH=CHPPh 3 ][Au(CN) 2 Cl 2 ] 2 ( 6 ) и [Ph 4 Sb][Au(CN) 2 Cl 2 ] ( 7 ). Для комплексов 3 , 5 – 7 приведены результаты рентгеноструктурного исследования.
Экспериментальная часть
Синтез [Me4P][Au(CN)2Cl2] (1). К раствору 100 мг (0,28 ммоль) дихлородицианоаурата калия в 5 мл воды прибавляли при перемешивании водный раствор 47 мг (0,28 ммоль) бромида тетраметилфосфония. Образовавшийся желтый осадок фильтровали, промывали два раза водой порциями по 5 мл, сушили и навеску массой 50 мг перекристаллизовывали из ацетонитрила. Получили 48 мг (97 %) кристаллов желтого цвета комплекса (1) с т. разл. 142 ° С. ИК-спектр ( v , см - 1): 2999, 2922, 2172, 1427, 1300, 982, 775, 455, 428.
По аналогичной методике с использованием соответствующих дигалогендицианоауратов калия и галогенидов трифенилорганилфосфония получали комплексы 2–6 , а также взаимодействием хлорида тетрафенилстибония с дихлородицианоауратом калия комплекс 7 .
[Ph3P(CH2)6Me][Au(CN)2Cl2] (2) - неокрашенные кристаллы, выход 91 % , т. пл. 102 ° С. ИК-спектр ( v , см - 1): 3063, 2930, 2868, 2849, 2359, 1825, 1587, 1483, 1460, 1439, 1339, 1317, 1192, 1169, 1113, 995, 930, 750, 721, 691, 532, 511, 494, 451, 430.
[Ph3PCH2CH2C(O)OH][Au(CN)2Cl2]∙2H2O (3) – кристаллы светло-желтого цвета, выход 52 %, т. пл. 156 ° С. ИК-спектр ( v , см - 1): 3094, 3059, 2961, 2916, 1742, 1587, 1485, 1439, 1418, 1396, 1339, 1315, 1236, 1171, 1113, 1042, 997, 895, 799, 746, 725, 689, 527, 505, 430.
[Ph3PCH2СN][Au(CN)2Br2] (4) - кристаллы желтого цвета, выход 72 %, т. пл. 116 ° С. ИК-спектр ( v , см - 1): 3059, 2920, 2878, 2251, 2145, 1585, 1481, 1439, 1385, 1337, 1315, 1240, 1182, 1163, 1113, 1070, 995, 829, 745, 721, 687, 548, 498, 447, 424.
[Ph3PCH2СN][Au(CN)2l2] (5) – кристаллы красно-коричневого цвета, выход 53 %, т. пл. 142 ° С. ИК-спектр ( v , см - 1): 3061, 2914, 2866, 2156, 1585, 1483, 1437, 1385, 1319, 1248, 1186, 1163, 1111, 997, 842, 741, 719, 687, 548, 501, 496, 444.
[Ph3PCH = СHPPh3][Au(CN)2Cl2] (6) - кристаллы желтого цвета, выход 84 %, т. разл. 191 ° С. ИК-спектр ( v , см - 1): 3053, 3017, 2361, 2344, 2332, 1587, 1576, 1481, 1437, 1333, 1314, 1190, 1109, 997, 959, 932, 775, 741, 725, 685, 525, 486, 449, 428.
[Ph4Sb][Au(CN)2Cl2] (7) - кристаллы желтого цвета, выход 91 %, т. пл. 116 ° С. ИК-спектр ( v , см - 1): 3055, 2359, 2332, 2147, 1479, 1435, 1335, 1069, 1020, 995, 731, 687, 457, 444, 419.
ИК-спектры соединений 1–7 записывали на ИК-Фурье спектрометре Shimadzu IRAffinity-1S в таблетке KBr в области 4000 - 400 см - 1.
Рентгеноструктурный анализ ( РСА ) проводили на автоматическом четырехкружном дифрактометре D8 QUEST фирмы Broker (Mo К а -излучение, X = 0,71073 А, графитовый монохроматор). Сбор, редактирование данных и уточнение параметров элементарной ячейки, а также учет поглощения проведены с помощью программ SMART и SAINT-Plus [29]. Все расчеты по определению и уточнению структур выполнены с помощью программ SHELXL/PC [30] и OLEX2 [31]. Структуры определены прямым методом и уточнены методом наименьших квадратов в анизотропном приближении для неводородных атомов. Положение атомов водорода уточняли по модели наездника ( U изо (H) = 1,2 U экв (C)). Кристаллографические данные и результаты уточнения структуры приведены в табл. 1, длины связей и валентные углы – в табл. 2.
Таблица 1
Кристаллографические данные, параметры эксперимента и уточнения структур 3, 5 – 7
|
Параметр |
3 |
5 |
6 |
7 |
|
Формула |
C 22 H 24 NO 4 PClAu |
C 22 H 17 N 3 PI 2 Au |
C 21 H 16 N 2 PCl 2 Au |
C 26 H 20 N 2 Cl 2 SbAu |
|
М |
629,81 |
805,12 |
595,19 |
750,06 |
|
Т , К |
293,15 |
293,15 |
293,15 |
293,15 |
|
Сингония |
Моноклинная |
Триклинная |
Моноклинная |
Моноклинная |
|
Пр. группа |
C 2/ c |
P –1 |
P 2 1 / n |
С 2 /с |
|
a , Å |
14,731(7) |
8,638(9) |
11,435(5) |
17,853(7) |
|
b, Å |
19,543(9) |
12,416(13) |
14,593(12) |
8,170(4) |
|
c, Å |
17,162(12) |
12,869(14) |
13,619(6) |
18,548(9) |
|
α, град. |
90,00 |
66,75(4) |
90,00 |
90,00 |
|
β, град. |
94,96(3) |
83,74(6) |
107,459(14) |
94,471(18) |
|
γ, град. |
90,00 |
70,69(5) |
90,00 |
90,00 |
|
V , Å3 |
4922(5) |
1196(2) |
2168(2) |
2697(2) |
|
Z |
8 |
2 |
4 |
4 |
|
р (выч.), г/см3 |
1,700 |
2,235 |
1,824 |
1,847 |
|
ц , мм-1 |
6,177 |
8,808 |
7,115 |
6,648 |
|
F (000) |
2448,0 |
740,0 |
1136,0 |
1416,0 |
|
Форма кристалла (размер, мм) |
обломок (0,52 × 0,3 × 0,19) |
обломок (0,63 × 0,46 × 0,19) |
обломок (0,65 × 0,31 × 0,21) |
обломок (0,34 × 0,29 × 0,12) |
Окончание табл. 1
|
Параметр |
3 |
5 |
6 |
7 |
|
Область сбора данных по 9 , град. |
6,08–59,14 |
6–82,72 |
6,22–72,76 |
5,98–70,18 |
|
Интервалы индексов отражений |
–20 ≤ h ≤ 20, –27 ≤ k ≤ 27, –23 ≤ l ≤ 23 |
–15 ≤ h ≤ 15, –22 ≤ k ≤ 22, –23 ≤ l ≤ 23 |
–19 ≤ h ≤ 18, –24 ≤ k ≤ 24, –22 ≤ l ≤ 22 |
–28 ≤ h ≤ 28, –13 ≤ k ≤ 13, –30 ≤ l ≤ 29 |
|
Измерено отражений |
69321 |
91207 |
74481 |
49825 |
|
Независимых отражений |
6870 |
14470 |
10486 |
5927 |
|
R int |
0,0471 |
0,0641 |
0,0689 |
0,0517 |
|
Переменных уточнения |
274 |
265 |
244 |
147 |
|
GOOF |
1,075 |
1,084 |
1,032 |
1,326 |
|
R -факторы по F2 > 2 g (F2) |
R 1 = 0,0447, wR 2 = 0,1217 |
R 1 = 0,0824, wR 2 = 0,1341 |
R 1 = 0,0966, wR 2 = 0,2886 |
R 1 = 0,1079, wR 2 = 0,3248 |
|
R -факторы по всем отражениям |
R 1 = 0,0546, wR 2 = 0,1304 |
R 1 = 0,1492, wR 2 = 0,1589 |
R 1 = 0,1372, wR 2 = 0,3243 |
R 1 = 0,1562, wR 2 = 0,3593 |
|
Остаточная электронная плотность (min/max), e/A3 |
–2,32/1,95 |
–2,86/2,53 |
–6,23/8,30 |
–3,09/9,42 |
Таблица 2
|
Связь d , Å |
Угол го, ° |
||
|
3 |
|||
|
Au(1)Cl(1) |
2,328(3) |
С(10)Au(1)Cl(1) |
90,3(3) |
|
Au(1)C(10) |
1,981(7) |
C(1)P(1)C(11) |
107,5(2) |
|
P(1)C(1) |
1,788(5) |
C(1)P(1)C(7) |
108,0 |
|
P(1)C(11) |
1,794(6) |
C(1)P(1)C(21) |
111,5(3) |
|
P(1)C(7) |
1,807(5) |
C(11)P(1)C(7) |
111,8(3) |
|
P(1)C(21) |
1,795(6) |
C(11)P(1)C(21) |
109,0(3) |
|
C(21)P(1)C(7) |
109,0(3) |
||
|
5 |
|||
|
Au(1)–I(1a) |
2,609(3) |
I(1)Au(1)I(1a) |
180,0 |
|
Au(1)–I(1) |
2,609(3) |
С(9а)Au(1)I(1) |
89,9(2) |
|
Au(1)–C(9a) |
1,996(7) |
C(9)Au(1)I(1) |
90,1(2) |
|
Au(1)–C(9) |
1,996(7) |
C(9a)Au(1)I(1a) |
90,1(2) |
|
Au(2)–I(2) |
2,598(3) |
C(9)Au(1)I(1a) |
89,9(2) |
|
Au(2)–I(2b) |
2,598(3) |
C(9a)Au(1)C(9) |
180,0 |
|
Au(2)–C(10) |
2,006(8) |
I(2b)Au(2)I(2) |
180,000(1) |
|
Au(2)–C(10a) |
2,006(8) |
C(10)Au(2)I(2) |
89,1(2) |
|
P(1)–C(21) |
1,780(6) |
C(10)Au(2)I(2b) |
90,9(2) |
|
P(1)–C(1) |
1,765(6) |
C(10b)Au(2)I(2) |
90,9(2) |
|
P(1)–C(11) |
1,776(6) |
C(10b)Au(2)I(2b) |
89,1(2) |
|
P(1)–C(7) |
1,821(6) |
C(10)Au(2)C(10b) |
179,999(1) |
|
N(3)–C(8) |
1,131(9) |
C(21)P(1)C(7) |
110,3(3) |
|
N(1)–C(9) |
1,131(9) |
C(1)P(1)C(21) |
109,3(3) |
|
N(2)–C(10) |
1,120(10) |
C(1)P(1)C(11) |
109,7(3) |
|
Преобразования симметрии: a)1–X, –Y,1–Z; b)1–X,2–Y, –Z |
|||
|
6 |
|||
|
Au(1)–Cl(1) |
2,393(2) |
Cl(1)Au(1)Cl(2) |
179,36(6) |
|
Au(1)–Cl(2) |
2,411(2) |
C(8)Au(1)Cl(1) |
89,8(3) |
|
Au(1)–C(8) |
2,001(13) |
C(8)Au(1)Cl(2) |
90,2(3) |
|
Au(1)–C(9) |
1,978(12) |
C(9)Au(1)Cl(1) |
92,0(4) |
Окончание табл. 2
|
Связь d , Å |
Угол ю, ° |
||
|
P(1)–C(7) |
1,810(8) |
C(9)Au(1)Cl(2) |
88,1(4) |
|
P(1)–C(11) |
1,806(10) |
C(9)Au(1)C(8) |
176,3(5) |
|
P(1)–C(1) |
1,790(9) |
C(11)P(1)C(7) |
106,7(4) |
|
P(1)–C(21) |
1,781(8) |
C(1)P(1)C(7) |
110,9(4) |
|
C(7)–C(7a) |
1,342(17) |
C(1)P(1)C(11) |
111,8(4) |
|
N(1)–C(8) |
1,135(16) |
C(21)P(1)C(7) |
110,1(4) |
|
N(2)–C(9) |
1,186(16) |
C(21)P(1)C(11) |
107,6(5) |
|
C(21)P(1)C(1) |
109,5(4) |
||
|
Преобразования симметрии: а) 1–X,1–Y,1–Z |
|||
|
7 |
|||
|
Au(1)–Cl(1) |
2,4223(18) |
Сl(1a)Au(1)Cl(1) |
180,0 |
|
Au(1)–Cl(1a) |
2,4223(18) |
C(7)Au(1)Cl(1) |
90,0(4) |
|
Au(1)–C(7a) |
2,040(15) |
C(7a)Au(1)Cl(1a) |
90,0(4) |
|
Au(1)–C(7) |
2,040(15) |
C(7)Au(1)Cl(1a) |
90,0(4) |
|
Sb(1)–C(11b) |
2,121(12) |
C(7a)Au(1)Cl(1) |
90,0(4) |
|
Sb(1)–C(11) |
2,121(11) |
C(7)Au(1)C(7a) |
179,999(2) |
|
Sb(1)–C(1b) |
2,070(11) |
C(11b)Sb(1)Cl(1) |
100,5(7) |
|
Sb(1)–C(1) |
2,070(11) |
C(1b)Sb(1)C(11) |
108,2(4) |
|
N(1)–C(7) |
1,07(2) |
C(1b)Sb(1)C(11b) |
114,6(5) |
|
C(1)Sb(1)C(11b) |
108,2(4) |
||
|
C(1)Sb(1)C(11) |
114,6(5) |
||
|
C(1b)Sb(1)C(1) |
110,5(6) |
||
|
Преобразования симметрии: а) 1/2–X,5/2–Y,1–Z; b) 1–X,+Y,3/2–Z |
|||
Полные таблицы координат атомов, длин связей и валентных углов депонированы в Кембриджском банке структурных данных (№ 1963507 (3), 1957186 (5), 1957184 (6), 1912896 (7); ; .
Обсуждение результатов
В продолжение исследования синтеза комплексов золота [32–34], в том числе с дигалогендициа-ноауратными анионами [15, 16, 20, 21, 34 - 39], получено семь неизвестных ранее дигалогенодициа-ноауратных ионных комплекса с тетраорганилфосфониевыми и тетрафенилстибониевым катионами: [Me 4 P][Au(CN) 2 Cl 2 ] ( 1 ), [Ph 3 P(CH 2 ) 6 Me][Au(CN) 2 Cl 2 ] ( 2 ), [Ph 3 P(CH 2 ) 2 C(O)OH][Au(CN) 2 Cl 2 ] ( 3 ), [Ph 3 PCH 2 CN][Au(CN) 2 Br 2 ] ( 4 ), [Ph 3 PCH 2 CN][Au(CN) 2 I 2 ] ( 5 ), [Ph 3 PCH=CHPPh 3 ][Au(CN) 2 Cl 2 ] 2 ( 6 ) и [Ph4Sb][Au(CN)2Cl2] ( 7 ). Комплексы 1 – 6 синтезировали взаимодействием водных растворов дихлоро-, дибромо- или дииододицианоаурата калия с водными растворами галогенидов тетраорга-нилфосфония с последующей перекристаллизацией высушенного осадка из ацетонитрила:
[Me 4 P]Br + K[Au(CN) 2 Cl 2 ] [Me 4 P][Au(CN) 2 Cl 2 ] + KBr
[Ph 3 PR]Hal' + K[Au(CN) 2 Hal 2 ] [Ph 3 PR][Au(CN) 2 Hal 2 ] + KHal'
Hal = Cl, Hal' = Cl, R = (CH 2 ) 2 C(O)OH ( 3 ); Hal' = Br, R = (CH 2 ) 6 Me ( 2 );
Hal = Br, R = CH 2 CN ( 4 );
Hal = I, R = CH 2 CN ( 5 ).
[Ph 3 PCH=CHPPh 3 ]Br 2 + 2K[Au(CN) 2 Cl 2 ] [Ph 3 PCH=CHPPh 3 ][Au(CN) 2 Cl 2 ] 2 + 2KBr
Комплекс 7 получен по реакции дихлородицианоаурата калия с хлоридом тетрафенилстибония:
[Ph 4 Sb]Cl + K[Au(CN) 2 Cl 2 ] [Ph 4 Sb][Au(CN) 2 Cl 2 ] + KBr
По данным РСА, комплексы 3 , 5 – 7 состоят из тетраэдрических трифенилорганилфосфоние-вых или тетрафенилстибониевых катионов и плоскоквадратных моноядерных дигалогенодициа-ноаурат-анионов (рис. 1–4).
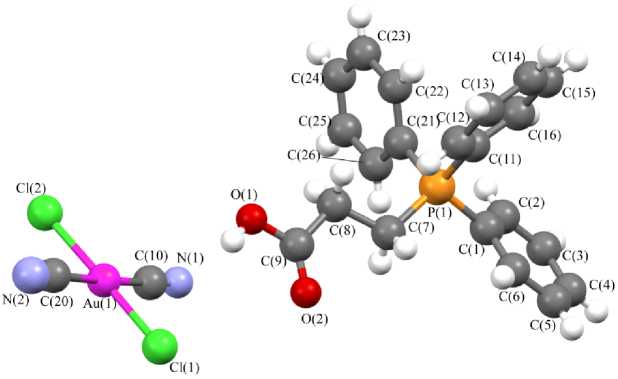
Рис. 1. Строение комплекса 3
В катионах 3 , 5 и 6 углы CPC изменяются в интервале 107,5(2) - 111,8(3) ° , 106,0(3) - 111,5(3) ° и 106,7(4) - 111,8(4) ° соответственно; длины связей P-C (1,788(5) - 1,807(5) А ( 3 ), 1,765(6) 1,821(6) А ( 5 ) и 1,781(8) - 1,810(8) А ( 6 )) меньше суммы ковалентных радиусов атомов фосфора и углерода (1,88 А [40]). Углы CSbC в катионе 7 изменяются в интервале 100,5(7) - 114,6(5) ° ; расстояния Sb-C (2,070(11) - 2,121(12) А) практически совпадают с суммой ковалентных радиусов сурьмы и углерода (2,12 Å [40]).
В центросимметричных плоскоквадратных анионах [Au(CN)2Hal2] - транс -углы HalAuHal и CAuC близки к 180 ° ; цис -углы CAuHal изменяются в интервале 88,05-92,48 ° . В кристалле комплекса 5 присутствует два типа кристаллографически независимых анионов [Au(CN)2I2] - с близкими геометрическими параметрами. Длины связей Au–Cl (2,328(3) Å ( 3 ), 2,393(2), 2,411(2) Å ( 6 ), 2,4223(12) Å ( 7 ) и Au–I 2,609(3), 2,598(3) Å ( 5 )) близки или меньше сумм ковалентных радиусов атомов золота и соответствующего галогена (Au–Cl 2,38 Å, Au–I 2,75 Å [40]). Длины связей Au–C (1,981(7) Å ( 3 ), 1,996(7), 2,006(8) Å ( 5 ), 1,978(12), 2,001(13) Å ( 6 ), 2,040(15) ( 7 )) меньше суммы ковалентных радиусов атомов золота и углерода (2,05 Å [40]).
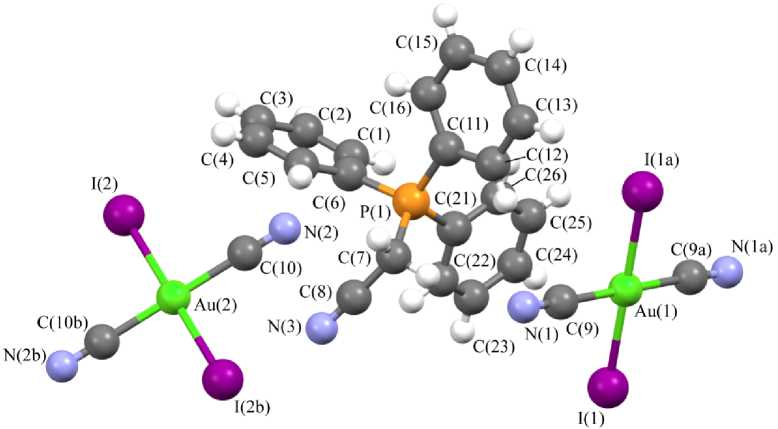
Рис. 2. Строение комплекса 5
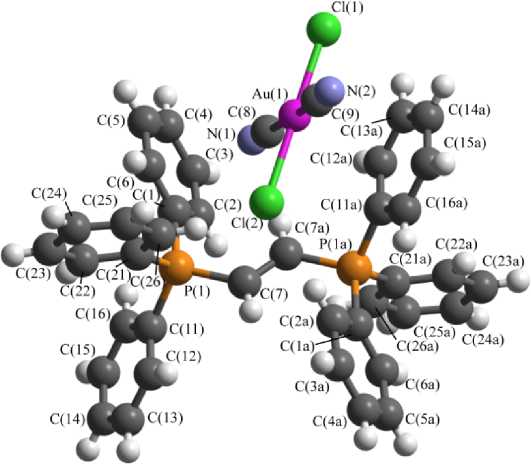
Рис. 3. Строение комплекса 6
Cl(la)
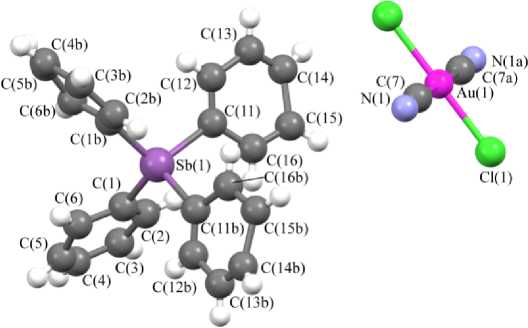
Рис. 4. Строение комплекса 7
В кристаллах соединений 3 , 5 – 7 присутствуют межионные контакты С–H∙∙∙N≡C (2,55–2,74 Å), близкие к сумме ван-дер-ваальсовых радиусов атомов водорода и азота (2,65 Å [41]). В кристалле 3 также наблюдаются контакты O–H∙∙∙N≡C (2,03 Å) и С–H∙∙∙O=C (2,52 Å). Кроме того, в кристаллах некоторых комплексов присутствуют контакты C–H∙∙∙Cl–Au (2,88–2,93 Å ( 3 ), 2,91 Å ( 6 )), близкие к суммам ван-дер-ваальсовых радиусов атомов водорода и хлора (H∙∙∙Cl 2,85 Å [41]). В кристалле же комплекса 5 обнаружены галоген-галогенные Au–I∙∙∙I–Au контакты первого типа [42] (расстояния I∙∙∙I 3,925(4) Å не превышают суммы ван-дер-ваальсовых радиусов атомов иода 4,05 Å [41]).
Выводы
Таким образом, взаимодействием галогенидов тетраорганилфосфония и тетрафенилстибония с дихлоро-, дибромо- и дииододицианоауратом калия синтезирован ряд новых дигалогендициа-ноауратных комплексов: [Me 4 P][Au(CN) 2 Cl 2 ] ( 1 ), [Ph 3 P(CH 2 ) 6 Me][Au(CN) 2 Cl 2 ] ( 2 ),
[Ph 3 P(CH 2 ) 2 C(O)OH][Au(CN) 2 Cl 2 ] ( 3 ), [Ph 3 PCH 2 CN][Au(CN) 2 Br 2 ] ( 4 ), [Ph 3 PCH 2 CN][Au-(CN) 2 I 2 ] ( 5 ), [Ph 3 PCH=CHPPh 3 ][Au(CN) 2 Cl 2 ] 2 ( 6 ) и [Ph 4 Sb][Au(CN) 2 Cl 2 ] ( 7 ), из которых комплексы 3 , 5 – 7 были структурно охарактеризованы.
Выражаем признательность проф. В.В. Шарутину за рентгеноструктурный анализ кристаллов соединений 3 , 5 – 7 .
Список литературы Синтез и строение новых дигалогендицианоауратных комплексов
- Polymorphism of Zn[Au(CN)2]2 and Its Luminescent Sensory Response to NH3 Vapor / M.J. Katz, T. Ramnial, H. Yu, D. Leznoff // J. Am. Chem. Soc. - 2008. - V. 130, № 32. - P. 10662-10673. DOI: 10.1021/ja801773p
- Photophysical Investigation of Silver/Gold Dicyanometallates and Tetramethylammonium Networks. An Experimental and Theoretical Investigation / A.D. Nicholas, R.M. Bullard, R.D. Pike et al. // Eur. J. Inorg. Chem. - 2018. - № 7. - P. 956-962. DOI: 10.1002/ejic.201801407
- Ovens, J.S. Designing Tunable White-Light Emission from an Aurophilic CuI/AuI Coordination Polymer with Thioether Ligands / J.S. Ovens, P.R. Christensen, D.B. Leznoff // Chem. - A Eur. J. - 2016. - V. 22, № 24. - P. 8234-8239. DOI: 10.1002/chem.201505075
- Roberts, R.J. Color-Tunable and White-Light Luminescence in Lanthanide-Dicyanoaurate Coordination Polymers / R.J. Roberts, D.Le, D.B. Leznoff // Inorg. Chem. - 2017. - V. 56, № 14. - P. 7948-7959. DOI: 10.1021/acs.inorgchem.7b00735
- Cyanide-Assembled d10 Coordination Polymers and Cycles: Excited State Metallophilic Modulation of Solid-State Luminescence / A. Belyaev, T. Eskelinen, T.M. Dau et al. // Chem. - A Eur. J. - 2017. - V. 24, № 6. - P. 1404-1415. DOI: 10.1002/chem.201704642
- Effect of Noble Metals on Luminescence and Single-Molecule Magnet Behavior in the Cyanido-Bridged Ln-Ag and Ln-Au (Ln = Dy, Yb, Er) Complexes / K. Kumar, O. Stefańczyk, S. Chorazy et al. // Inorg. Chem. - 2019. - V. 58, № 9. - P. 5677-5687.
- DOI: 10.1021/acs.inorgchem.8b03634
- Precise Electrochemical Control of Ferromagnetism in a Cyanide-Bridged Bimetallic Coordination Polymer / Y. Mizuno, M. Okubo, K. Kagesawa et al. // Inorg. Chem. - 2012. - V. 51, № 19. - P. 10311-10316.
- DOI: 10.1021/ic301361h
- Synergistic photomagnetic effects in coordination polymer heterostructure particles of Hofmann-like Fe(4-phenylpyridine)2[Ni(CN)4]·0.5H2O and K0.4Ni[Cr(CN)6]0.8·nH2O / C.R. Gros, M.K. Peprah, A.C. Felts et al. // Dalton Trans. - 2016. - V. 45, № 42. - P. 16624-16634.
- DOI: 10.1039/c6dt02353c
- A New Basic Motif in Cyanometallate Coordination Polymers: Structure and Magnetic Behavior of M(μ-OH2)2[Au(CN)2]2 (M = Cu, Ni) / J. Lefebvre, F. Callaghan, M.J. Katz et al. // Chem. - Eur. J. - 2006. - V. 12, № 26. - P. 6748-6761.
- DOI: 10.1002/chem.200600303
- Magnetic Frustration and Spin Disorder in Isostructural M(μ-OH2)2[Au(CN)2]2 (M = Mn, Fe, Co) Coordination Polymers Containing Double Aqua-Bridged Chains: SQUID and μSR Studies / J. Lefebvre, P. Tyagi et al. // Inorg. Chem. - 2009. - V. 48, № 1. - P. 55-67.
- DOI: 10.1021/ic801094m
- Magnetic Properties of Isostructural M(H2O)4[Au(CN)4]2-based Coordination Polymers (M = Mn, Co, Ni, Cu, Zn) by SQUID and μsR Studies / A.R. Geisheimer, W. Huang, V. Pacradouni et al. // Dalton Trans. - 2011. - V. 40, № 29. - P. 7505-7516.
- DOI: 10.1039/c0dt01546f
- Lefebvre, J. Synthesis, Structure and Magnetic Properties of 2-D and 3-D [cation]{M[Au(CN)2]3} (M = Ni, Co) Coordination Polymers / J. Lefebvre, D. Chartrand, D.B. Leznoff // Polyhedron. - 2007. - V. 26, № 9-11. - P. 2189-2199.
- DOI: 10.1016/j.poly.2006.10.045
- Miller, J.S. Organometallic- and Organic-based Magnets: New Chemistry and New Materials for the New Millennium / J.S. Miller // Inorg. Chem. - 2000. - V. 39, № 20. - P. 4392-4408.
- DOI: 10.1021/ic000540x
- Single-crystal X-ray Diffraction Studies on Structural Transformations of Porous Coordination Polymers / J.-P. Zhang, P.-Q. Liao, H.-L Zhou et al. // Chem. Soc. Rev. - 2014. - V. 43, № 16. - P. 5789-5814.
- DOI: 10.1039/c4cs00129j
- Ovens, J.S. Thermal Expansion Behavior of M[AuX2(CN)2]-Based Coordination Polymers (M = Ag, Cu; X = CN, Cl, Br) / J.S. Ovens, D.B. Leznoff // Inorg. Chem. - 2017. - V. 56, № 13. - P. 7332-7343.
- DOI: 10.1021/acs.inorgchem.6b03153
- Ovens, J.S. Probing Halogen⋯Halogen Interactions via Thermal Expansion Analysis / J.S. Ovens, D.B. Leznoff // Cryst. Eng. Comm. - 2018. - V. 20, № 13. - P. 1769-1773.
- DOI: 10.1039/c7ce02167d
- Lefebvre, J. Cu[Au(CN)2]2(DMSO)2: Golden Polymorphs that Exhibit Vapochromic Behavior / J. Lefebvre, R.J. Batchelor, D.B. Leznoff // J. Am. Chem. Soc. - 2004. - V. 126, № 49. - P. 16117-16125.
- DOI: 10.1021/ja049069n
- Vapochromic Behaviour of M[Au(CN)2]2 - Based Coordination Polymers (M = Co, Ni) / J. Lefebvre, J.L. Korčok, M.J. Katz et al. // Sensors. - 2012. - V. 12, № 3. - P. 3669-3692.
- DOI: 10.3390/s120303669
- Varju, B.R. Mixed Cu(I)/Au(I) Coordination Polymers as Reversible Turn-on Vapoluminescent Sensors for Volatile Thioethers / B.R. Varju, J.S. Ovens, D.B. Leznoff // Chem. Comm. - 2017. - V. 53, № 48. - P. 6500-6503.
- DOI: 10.1039/c7cc03428h
- Ovens, J.S. Raman Detected Sensing of Volatile Organic Compounds by Vapochromic Cu[AuX2(CN)2]2 (X = Cl, Br) Coordination Polymer Materials / J.S. Ovens, D.B. Leznoff // Chem. Mater. - 2015. - V. 27, № 5. - P. 1465-1478.
- DOI: 10.1021/cm502998w
- The Use of Polarizable [AuX2(CN)2]- (X = Br, I) Building Blocks Toward the Formation of Birefringent Coordination Polymers / J.S. Ovens, A.R. Geisheimer, A.A. Bokov et al. // Inorg. Chem. - 2010. - V. 49, № 20. - P. 9609-9616.
- DOI: 10.1021/ic101357y
- Guan, D. Emissive and birefringent Hg(CN)2-based coordination polymer materials with very distorted coordination geometries / D. Guan, J.R. Thompson, D.B. Leznoff // Can. J. Chem. - 2018. - V. 96, № 2. - P. 226-234.
- DOI: 10.1139/cjc-2017-0589
- Katz, M.J. Highly Birefringent Cyanoaurate Coordination Polymers: The Effect of Polarizable C-X Bonds (X = Cl, Br) / M.J. Katz, D.B. Leznoff // J. Am. Chem. Soc. - 2009. - V. 131, № 51. - P. 18435-18444.
- DOI: 10.1021/ja907519c
- Thompson, J.R. Birefringent, Emissive Cyanometallate-Based Coordination Polymer Materials Containing Group(II) Metal-Terpyridine Building Blocks / J.R. Thompson, K.A.S. Goodman-Rendall, D.B. Leznoff // Polyhedron. - 2016. - V. 108. - P. 93-99.
- DOI: 10.1016/j.poly.2015.12.026
- Highly Birefringent Materials Designed Using Coordination Polymer Synthetic Methodology / M.J. Katz, H. Kaluarachchi, R.J. Batchelor et al. // Angew. Chem., Int. Ed. - 2007. - V. 46, № 46. - P. 8804-8807.
- DOI: 10.1002/anie.200702885
- Birefringent, Emissive Coordination Polymers Incorporating Bis(benzimidazole)pyridine as an Anisotropic Building Block / J.R. Thompson, R.J. Roberts, V.E. Williams et al. // Cryst. Eng. Comm. - 2013. - V. 15, № 45. - P. 9387-9393.
- DOI: 10.1039/c3ce41556b
- Thompson, J.R. Structural Design Parameters for Highly Birefringent Coordination Polymers / J.R. Thompson, M.J. Katz, V.E. Williams // Inorg. Chem. - 2015. - V. 54, № 13. - P. 6462-6471.
- DOI: 10.1021/acs.inorgchem.5b00749
- Assembly and Dichroism of a Four-Component Halogen-Bonded Metal-Organic Cocrystal Salt Solvate Involving Dicyanoaurate(I) Acceptors / J.-C. Christopherson, K.P. Potts, O.S. Bushuev et al. // Faraday Discuss. - 2017. - № 203. - P. 441-457.
- DOI: 10.1039/c7fd00114b
- Bruker. SMART and SAINT-Plus. Versions 5.0. Data Collection and Processing Software for the SMART System. Bruker AXS Inc., Madison, Wisconsin, USA, 1998.
- Bruker. SHELXTL/PC. Versions 5.10. An Integrated System for Solving, Refining and Displaying Crystal Structures From Diffraction Data. Bruker AXS Inc., Madison, Wisconsin, USA, 1998.
- OLEX2: Complete Structure Solution, Refinement and Analysis Program / O.V. Dolomanov, L.J. Bourhis, R.J. Gildea et al. // J. Appl. Cryst. - 2009. - V. 42. - P. 339-341.
- DOI: 10.1107/S0021889808042726
- Синтез и строение комплексов золота и меди [Ph3PCH2Ph]+[AuCl4]-, [NH(C2H4OH)3]+[AuCl4]- · H2O и [Ph3EtP]+2[Cu2Cl6]2- / В.В. Шарутин, В.С. Сенчурин, А.П. Пакусина и др. // Журн. неорган. химии. - 2010. - Т. 55, № 9. - С. 1499-1505.
- Шарутин, В.В. Синтез и строение комплексов золота [Ph3PCH2CH=CHCH2PPh3]2+[AuCl4]-2 и [Ph3PCH2CH2COOH]+[AuCl4]- / В.В. Шарутин, О.К. Шарутина, В.С. Сенчурин // Журн. неорган. химии. - 2015. - Т. 60, № 8. - С. 1040-1045.
- Сенчурин, В.С. Комплексы золота [Ph4Bi][Au(CN)2Hal2] (Hal = Cl, Br). Синтез и строение / В.С. Сенчурин // Вестник ЮУрГУ. Серия "Химия". - 2019. - Т. 11, № 3. - С. 50-58.
- DOI: 10.14529/chem190306
- Ovens, J.S. Thermally Triggered Reductive Elimination of Bromine from Au(III) as a Path to Au(I)-based Coordination Polymers / J.S. Ovens, D.B. Leznoff // Dalton Trans. - 2011. - V. 40. - P. 4140-4146.
- DOI: 10.1039/C0DT01772H
- Crystal Structures and Properties of [Au(phen){(CN)0.92Br0.08}2]Br and [Au(phen)(CN){(CN)0.82Br0.18}]·0.5trans-[Au(CN)2Br2]·0.5Br·phen (phen = 1,10-phenanthroline) Obtained by Disproportionation of Five-co-ordinate Bromodicyano(1,10-phenanthroline)gold(III). Two Examples of Secondary Co-ordination and CN/Br Disorder in Square-planar Gold(III) Complexes / G. Marangoni, B. Pitteri, V. Bertolasi et al. // J. Chem. Soc., Dalton Trans. - 1987. - P. 2235-2240.
- DOI: 10.1039/DT9870002235
- Ovens, J.S. Targeting [AuCl2(CN)2]- Units as Halophilic Building Blocks in Coordination Polymers / J.S. Ovens, K.N. Truong, D.B. Leznoff // Inorg. Chim. Acta. - 2013. - V. 403. - P. 127-135.
- DOI: 10.1016/j.ica.2013.02.011
- Ovens, J.S. Structural Organization and Dimensionality at the Hands of Weak Intermolecular Au⋯Au, Au⋯X and X⋯X (X = Cl, Br, I) Interactions / J.S. Ovens, K.N. Truong, D.B. Leznoff // Dalton Trans. - 2012. - V. 41. - P. 1345-1351.
- DOI: 10.1039/C1DT11741F
- Pitteri, B. Chelate Polypyridine Ligand Rearrangement in Au(III) / B. Pitteri, M. Bortoluzzi, V. Bertolasi // Complexes Transition Met. Chem. - 2008. - V. 33, № 5. - P. 649-654.
- DOI: 10.1007/s11243-008-9092-9
- Covalent Radii Rrevisited / B. Cordero, V. Gómez, A.E. Platero-Prats et al. // Dalton Trans. - 2008. - Iss. 21. - P. 2832-2838.
- DOI: 10.1039/B801115J
- Consistent Van der Waals Radii for the Whole Main Group / M. Mantina, A.C. Chamberlin, R. Valero et al. // J. Phys. Chem. A. - 2009. - V. 113, iss. 19. - P. 5806-5812.
- DOI: 10.1021/jp8111556
- Sakurai, T. A Nuclear Quadrupole Resonance and X-ray Study of the Crystal Structure of 2,5-Dichloroaniline / T. Sakurai, M. Sundaralingam, G.A. Jeffrey // Acta Crystallogr. - 1963. - V. 16, № 5. - P. 354-363.
- DOI: 10.1107/S0365110X63000979


