Систематический обзор и метаанализ применения шовного материала с покрытием из триклозана для предотвращения инфекций области хирургического вмешательства (ИОХВ)
Автор: Wang Z.X., Jiang C.P., Cao Y., Ding Y.T.
Журнал: Хирургическая практика @spractice
Рубрика: Современное состояние проблемы: обзоры, лекции
Статья в выпуске: 2, 2013 года.
Бесплатный доступ
История вопроса. Инфекции области хирургического вмешательства (ИОХВ) вызывают увеличение заболеваемости и смертности пациентов хирургического профиля и представляют собой серьезное экономическое бремя для системы здравоохранения. Проведенные эксперименты показали, что шовный материал с покрытием из триклозана (ШМТ) способствует предотвращению ИОХВ, однако результаты отдельных рандомизированных контролируемых исследований (РКИ) являются неубедительными. Для оценки эффективности ШМТ при профилактике ИОХВ был проведен метаанализ опубликованных РКИ. Методы. До июня 2012 г. в базах данных медицинских и биологических публикаций PubMed, Embase, MEDLINE, Web of Science*, Cochrane Central Register of Controlled Trials, а также в электронных архивах клинических исследований проводился систематический поиск публикаций РКИ, в которых сравнивалось влияние ШМТ и традиционного шовного материала без покрытия на развитие ИОХВ. Первичной конечной точкой исследования была частота возникновения ИОХВ. Для расчета обобщенных относительных рисков с 95% доверительным интервалом (ДИ) использовалось программное обеспечение RevMan 5.1.6. Результаты. В анализ были включены 17 РКИ, в которых участвовало 3720 пациентов. При сравнении исследований неоднородности статистической достоверности не отмечалось. Использование ШМТ имело существенное преимущество и вызывало снижение частоты возникновения ИОХВ на 30% (относительный риск 0,70, 95% ДИ от 0,57 до 0,85; p Background. Surgical site infections (SSIs) cause an increase in morbidity and mortality in surgical patients and represent a significant economic burden on the healthcare system. The experiments showed that the suture coated with triclosan (CMT) will help prevent wound infection, but the results of individual randomized controlled trials (RCTs) are inconclusive. To evaluate the efficiency of CMT in the prevention of SSIs was conducted a meta-analysis of published RCTs. Methods. Prior to June 2012 in the databases of medical and biological publications PubMed, Embase, MEDLINE, Web of Science *, Cochrane Central Register of Controlled Trials, as well as in the electronic archives of clinical research in systematic search for publications of randomized trials that compared the effects of CMT and traditional suture without covering the development of SSIs. The primary endpoint was the incidence of SSIs. To calculate the distributions of relative risks with 95% confidence intervals (CI) software was used RevMan 5.1.6. Results. In the analysis included 17 studies, involving 3720 patients. In comparison studies inhomogeneity statistical significance was observed. The use of CMT had a significant advantage and caused a decrease in the incidence of wound infection by 30% (relative risk 0.70, 95% CI 0.57 to 0,85; p
Хирургические инфекции, шовный материал, триклоза
Короткий адрес: https://sciup.org/142211507
IDR: 142211507 | УДК: 615.46:617.5
Текст научной статьи Систематический обзор и метаанализ применения шовного материала с покрытием из триклозана для предотвращения инфекций области хирургического вмешательства (ИОХВ)
Опубликована онлайн в «Электронной библиотеке Уайли » (Wiley Online Library) (. DOI (идентификатор документа): 10.1002/bjs.9062
Инфекции области хирургического вмешательства (ИОХВ) остаются одной из самых распространенных проблем современной хирургии. По данным Центров контроля и профилактики заболеваемости США (ЦКЗ), общая частота возникновения ИОХВ в США составляет 2,8% [1], что эквивалентно 756 000 пациентов в год. Согласно отчетности, в Европейских странах этот показатель колеблется в диапазоне от 1,5 до 20%, отражая присущие исследованиям противоречия; однако истинная заболеваемость ИОХВ считается заниженной, из чего можно сделать вывод, что ИОХВ представляют собой серьезную проблему и в Европе [2]. Учитывая широкую распространенность, ИОХВ является тяжелым бременем как для пациентов, так и для системы здравоохранения. ИОХВ не только вызывают существенный рост заболеваемости, повторных госпитализаций, поступлений в отделения интенсивной тера-

пии, а также длительно существующие осложнения в послеоперационной ране, но и приводят к увеличению риска смерти пациентов, подвергающихся хирургическим процедурам [3]. Более того, ИОХВ увеличивают нагрузку на систему здравоохранения, требуя привлечения дополнительного койко-фонда, роста стоимости ресурсов и потери рабочего времени [2, 4, 5].
По оценкам специалистов, 40–60% ИОХВ можно предотвратить [6.] Несмотря на сложную этиологию ИОХВ, хорошо известно, что бактериальное обсеменение шовных материалов является существенным фактором риска развития ИОХВ [7, 8]. В связи с этим была предложена профилактика ИОХВ с помощью шовного материала, обладающего антимикробными свойствами. Для создания такого антибактериального шовного материала применялся триклозан – антисептик широкого спектра действия. В продажу поступило несколько соответствующих продуктов, включая антибактериальный шовный материал на основе покрытого триклозаном полиглактина 910 (Vicryl Plus®; производитель: Ethicon, Johnson & Johnson, Somerville, New Jersey, USA), антибактериальный шовный материал на основе покрытого триклозаном полиглекапрона 25 (Monocryl Plus®; производитель: Ethicon, Johnson & Johnson) и антибактериальный шовный материал на основе покрытого триклозаном полидиоксанона (PDS Plus®;
Проведенные на животных эксперименты in vitro и in vivo показали, что шовный материал с покрытием из триклозана (ШМТ) снижает бактериальное обсеменение [9, 10] и угнетает широкий спектр вызывающих ИОХВ патогенных микроорганизмов, не изменяя физических свойств шовного материала, а также не препятствуя процессу заживления раны [7, 18]. Результаты нескольких недавно проведенных клинических исследований свидетельствуют о положительном влиянии ШМТ на предотвращение ИОХВ [19–24]. Тем не менее эффективность ШМТ остается недоказанной и вызывает противоречия, так как некоторые исследования [25–29], включая метаанализ [30], продемонстрировали отсутствие существенной разницы между шовным материалом с покрытием из триклозана и шовным материалом без покрытия с точки зрения возникновения ИОХВ. Однако после проведения вышеупомянутого метаанализа были опубликованы результаты нескольких рандомизированных контролируемых исследований (РКИ) [22–24, 27–29, 31–36]. Целью настоящего систематического обзора являлся анализ имеющихся на сегодняшний день РКИ, в которых сравнивается воздействие ШМТ и традиционного шов-
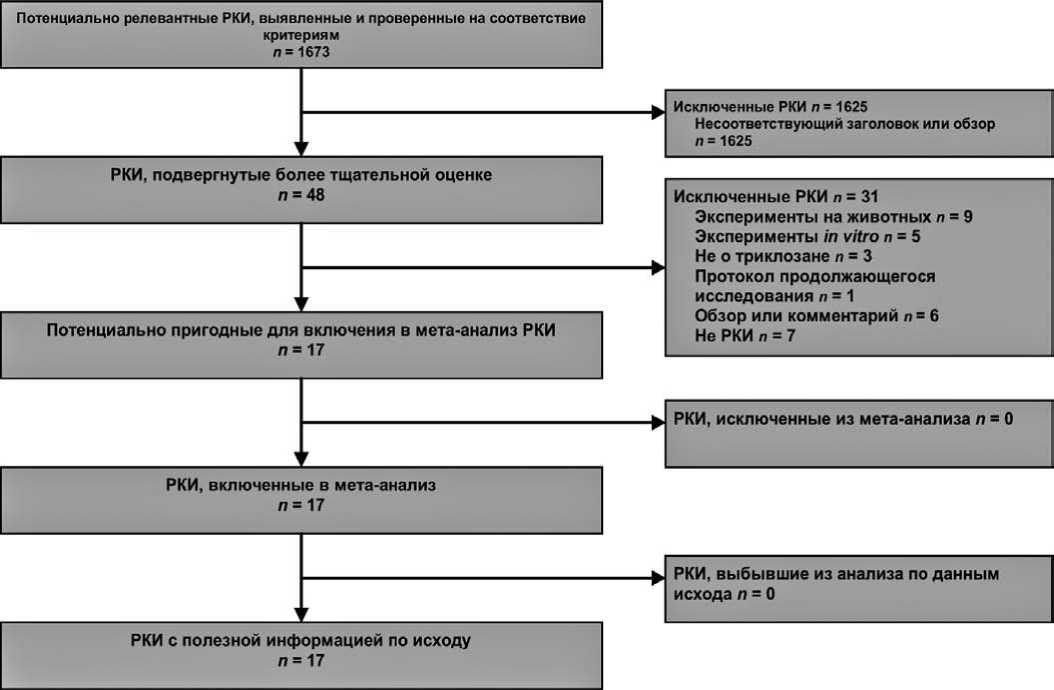
Рис. 1. Блок-схема PRISMA для данного исследования. РКИ – рандомизированное контролируемое исследование

Таблица 1
Характеристики рандомизированных контролируемых исследований шовного материала с покрытием из триклозана
|
Ссылка |
Год |
Объем выборки |
Модель исследования |
Маскировка данных |
Вмешательства |
Длительность периода наблюдения |
|
|
ШМТ |
Контроль |
||||||
|
Baracs et al. [24] |
2011 |
188 |
197 |
Многоцентровое РКИ |
Двойное слепое |
PP в сравн. с P |
30 дней |
|
DeFazio et al. [25] |
2005 |
43 |
50 |
Одноцентровое РКИ |
Двойное слепое |
VP в сравн. с V |
6 недель |
|
Deliaert et al. [47] |
2009 |
26 |
26 |
Одноцентровое РКИ |
Двойное слепое |
VP в сравн. с V |
4 недели |
|
Ford et al. [48] |
2005 |
98 |
49 |
Одноцентровое РКИ |
Открытое |
VP в сравн. с V |
80 ± 5 дней |
|
Galal and El-Hindawy [22] |
2011 |
230 |
220 |
Многоцентровое РКИ |
Двойное слепое |
VP в сравн. с V |
30 дней* |
|
Isikef et al. [36] |
2012 |
170 |
340 |
Одноцентровое РКИ |
Двойное слепое |
VP в сравн. с V |
1 месяц |
|
Khachatryan et al. [33] |
2011 |
65 |
68 |
Одноцентровое РКИ |
Открытое |
VP в сравн. с шовным материалом без покрытия |
НД |
|
Mattavelli et al. [34] |
2011 |
108 |
109 |
Многоцентровое РКИ |
Односторонне слепое |
VP в сравн. с V |
30 дней |
|
Mingmalairak et al. [26] |
2009 |
50 |
50 |
Одноцентровое РКИ |
Двойное слепое |
VP в сравн. с V |
1 год |
|
Rasic et al. [23] |
2011 |
91 |
93 |
Одноцентровое РКИ |
Двойное слепое |
VP в сравн. с V |
НД |
|
Rozzelle et al. [21] |
2008 |
46 |
38 |
Одноцентровое РКИ |
Двойное слепое |
VP в сравн. с V |
6 месяцев |
|
Seim et al. [28] |
2012 |
160 |
163 |
Одноцентровое РКИ |
Односторонне слепое |
VP в сравн. с V |
4 недели |
|
Singh et al. [32] |
2010 |
50 |
50 |
РКИ |
Неизвестно |
VP в сравн. с шовным материалом без покрытия |
30 дней |
|
Turtiainen et al. [29] |
2012 |
139 |
137 |
Многоцентровое РКИ |
Двойное слепое |
VP/MP в сравнении с V/M |
30 дней |
|
Williams et al. [35] |
2011 |
66 |
61 |
Одноцентровое РКИ |
Двойное слепое |
VP/MP в сравнении с V/M |
6 недель |
|
Zhang et al. [27] |
2011 |
46 |
43 |
Многоцентровое РКИ |
Открытое |
MP в сравнении с шелком |
30 дней |
|
Zhuang et al. [31] |
2009 |
150 |
300 |
Одноцентровое РКИ |
Неизвестно |
MP в сравнении с P/шелком |
12–24 месяца |
ного материала без покрытия на частоту возникновения ИОХВ после проведения хирургических процедур.
Методы. Стратегия поиска
Методология настоящего исследования соответствует положениям инструкции «Предпочтительные параметры отчетности для систематических обзоров и мета-анализа (PRISMA)» [37] (рис. 1). При поиске статей в базах данных медицинских и биологических публикаций PubMed, Embase, MEDLINE, Web of Science®, Cochrane Central Register of Controlled Trials (CENTRAL) использовались следующие ключевые слова: «триклозан», «антибактериальный», «антисептический», «Vicryl Plus», «Monocryl Plus», «PDS Plus» и «шовный материал». Поиск выполнялся двумя независимыми исследователями и в последний раз обновлялся 20 июня 2012 г.; ограничения по дате и языку публикаций отсутствовали. Списки литературы проверялись вручную, а электронные архивы клинических исследований [38, 39] использовались для поиска дополнительных исследований по данной теме.
Отбор исследований
Исследования, включенные в настоящий метаанализ, должны были удовлетворять всем нижеследующим критериям: РКИ с оценкой эффективности ШМТ у людей; если обнаруживалась серия из нескольких исследований в одной популяции больных из одной и той же группы, в метаанализ включался только последний отчет. Два ученых проводили поиск исследований для
метаанализа независимо друг от друга. Если возникали какие-либо разногласия, то для обсуждения возникших вопросов и достижения консенсуса приглашался главный исследователь.
Риск системной ошибки
Риск системной ошибки и качество методологии включенных в анализ исследований оценивались с помощью разработанного организацией «Кокрановское Сотрудничество» инструмента оценки риска системной ошибки на основе принципов Кокрановского руководства по систематическим обзорам медицинских вмешательств [40]. Общий риск системной ошибки определялся по следующим критериям: генерация случайной последовательности, сокрытие распределения, маскировка данных об используемых материалах для пациента и медицинского персонала, маскировка оценки исхода и неполные данные об исходе. Исследования, в которых риск по всем пяти критериям оставался низким, были классифицированы как исследования с низким риском системной ошибки. Исследования, в которых риск по какому-либо критерию был неясным или высоким, классифицировались как исследования с неясным или высоким риском системной ошибки. С целью обобщения результатов оценки была составлена таблица показателей риска системной ошибки.
Абстрактное представление данных
Два ученых извлекали данные исследований независимым друг от друга образом. В пригодных для анализа исследованиях выполнялся сбор следующих характеристик: дата публикации, статус публикации, демографические характеристики участников, применявшиеся вмешательства, объем выборки в группах лечения, модель исследования, тип хирургической процедуры, тип разреза по традиционной классификации и длительность периода наблюдения. Данные об исходах были представлены как количество событий на общее число пациентов в группе риска в экспериментальной и контрольной группах. Оба исследователя провели перекрестную проверку результатов абстрагирования и достигли согласия по всем полученным данным. Если исследователи получали разные результаты, то проверяли данные и обсуждали имеющиеся вопросы, пока не приходили к единому мнению. В случае, если разногласия разрешить не удавалось, приглашался главный исследователь. Для получения отсутствующих данных исследователи связывались с соответствующим автором или адаптировали сведения из предыдущего системного обзора [30].
Первичная конечная точка метаанализа и анализа данных в подгруппах
Первичной конечной точкой исследования была частота возникновения ИОХВ. Чтобы получить общую оценку влияния ШМТ на частоту возникновения ИОХВ по сравнению с шовным материалом без покрытия, все исследования с удовлетворительными данными были объединены.
Чтобы дополнительно проверить результат общей оценки в отдельных популяциях больных с относительно однородными характеристиками, был проведен анализ данных по подгруппам, стратифицированный по параметрам участников и типу вмешательства, а именно: возрасту испытуемых, характеру заражения раны согласно традиционной классификации разрезов и виду хирургической процедуры. Также были проанализированы данные сравнения шовных материалов Vicryl Plus® и Vicryl®. Целью такого анализа было определить, усиливал ли триклозан антибактериальные свойства шовного материала Vicryl®, широко применяемого во всем мире.
В соответствии с определением Центра контроля и профилактики заболеваемости США, ИОХВ – это инфекции, возникающие в течение 30 дней после хирургической процедуры (или в течение 1 года, если речь идет об имплантате, который остается в тканях после завершения вмешательства) [41]. Таким образом, для того, чтобы выяснить, влияла ли длительность периода наблюдения в отдельных исследованиях на оценку ИОХВ, исследования были дополнительно стратифицированы по периоду наблюдения (до 1 месяца и более 1 месяца). Кроме того, чтобы оценить надежность и устойчивость результатов мета-анализа, дополнительно был проведен анализ данных в подгруппах по риску системной ошибки и статусу публикации.
Системная ошибка публикации
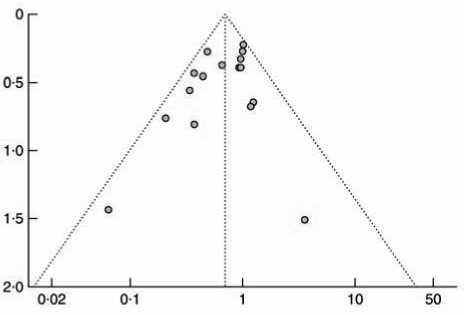
Системные ошибки публикаций оценивались с помощью воронкообразного графика. Асимметричность графика свидетельствовала о системной ошибке публикации.
Статистический анализ
Для синтеза количественных данных использовалось программное обеспечение RevMan 5.1.642. Проверка статистической неоднородности исследований осуществлялась с помощью Q-теста на основе χ2. P > 0,100 в Q -тесте указывало на отсутствие существенной неоднородности исследований [43]. Если существенной неоднородности не обнаруживалось, то обобщенные относительные риски (ОР) с соответствующим 95% доверительным интервалом (ДИ) рассчитывались с помощью модели постоянных эффектов (метод Мантеля–Хэнзеля) [44]. В противном случае для вычисления обобщенных ОР использовалась модель случайных эффектов (метод ДерСимониана и Ларда) [45]. Степень неоднородности измерялась при помощи статистического [46] индекса I2. Значение I2 меньше 25% соответствовало низкому уровню неоднородности, I2 в диапазоне от 25 до 50% считалось показателем умеренной неоднородности, а I2 больше 50% отражало высокую неоднородность. Достоверность обобщенных ОР определялась в соответствии с Z-тестом; при этом Р < 0,050 считалось статистически достоверным. Для обобщения результатов отдельных составляющих метаанализа был построен график «форест-плот».
Для определения чувствительности анализа при каждом цикле исключалось по одному исследованию, что позволяло оценить влияние отдельных наборов данных на обобщенные ОР.
Результаты
Из 1673 источников, найденных в базах данных и других ресурсах, в метаанализ были включены 17 подходящих исследо-
ваний, в которых принимало участие 3720 испытуемых. Блок-схема поиска исследований показана на рис. 1.
Объем выборки каждого из включенных РКИ варьировался от 52 до 510 испытуемых; случайным образом 1726 участников были распределены в группу, где применялся ШМТ и 1994 – в группу, где использовался шовный материал без покрытия; при этом период наблюдения колебался в диапазоне от 4 недель до 24 месяцев. Исследуемый ШМТ: Vicryl Plus®, Monocryl Plus® и PDS Plus®. Подробные характеристики включенных исследований представлены в таблице 1.
Качественные показатели и риски системных ошибок включенных исследований отражены в таблице 2. Три исследования были отнесены к группе «с высоким качеством и низким риском системной ошибки», шесть исследований – к группе с «низким качеством и высоким риском системной ошибки». Вследствие недостаточной информации о модели исследования и отсутствия доказательств, говорящих о наличии риска системной ошибки, оставшиеся восемь РКИ были классифицированы как «исследования со средним качеством и неясным риском системной ошибки». В целом качественные характери- стики включенных в метаанализ исследований были приемлемыми; риск системной ошибки оставался умеренным.
Влияние шовного материала с покрытием из триклозана на инфекции послеоперационной раны по сравнению с шовным материалом без покрытия
В семнадцати исследованиях содержались данные о частоте возникновения ИОХВ в группах больных, где использовался ШМТ, и в контрольных группах [21–29, 31–36, 47, 48]. Метаанализ этих РКИ показал, что применение ШМТ имело существенные преимущества; обобщенный ОР составил 0,70 (95% ДИ 0,57 – 0,85; P < 0,001) при отсутствии статистической неоднородности (P в Q-тесте = 0,129, I2 = 29%). Это указывает на то, что использование ШМТ привело к значительному снижению частоты возникновения ИОХВ (рис. 2). Результаты анализа чувствительности свидетельствуют о том, что ни один из наборов данных не вызывал существенного изменения неоднородности или обобщенных ОР ИОХВ. Данный факт указывает на устойчивость результатов метаанализа.
Риски системных ошибок
Таблица 2
|
Ссылка |
Генерация случайной последовательности (системная ошибка отбора) |
Сокрытие распределения (системная ошибка отбора) |
Маскировка данных для участников исследования и персонала (системная ошибка, связанная с ходом исследования) |
Маскировка оценки исхода (системная ошибка, связанная с выявлением исхода) |
Неполные данные об исходе (системная ошибка, связанная с выбыванием участников) |
Избират. предост. данных в отчетах |
Другие систем. ошибки |
|
Baracs et al. [24] |
+ |
? |
? |
? |
– |
+ |
+ |
|
DeFazio et al. [25] |
? |
+ |
+ |
+ |
? |
+ |
– |
|
Deliaert et al. [47] |
? |
+ |
+ |
+ |
+ |
– |
+ |
|
Ford et al. [48] |
? |
? |
– |
– |
– |
+ |
+ |
|
Galal and El-Hindawy [22] |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
|
Isikef et al. [36] |
? |
? |
? |
? |
+ |
? |
+ |
|
Khachatryan et al. [33] |
? |
? |
– |
– |
? |
? |
? |
|
Mattavelli et al. [34] |
? |
? |
– |
– |
? |
? |
? |
|
Mingmalairak et al. [26] |
+ |
+ |
+ |
+ |
+ |
? |
+ |
|
Rasic et al. [23] |
+ |
+ |
? |
? |
+ |
? |
? |
|
Rozzelle et al. [21] |
? |
+ |
+ |
+ |
+ |
+ |
+ |
|
Seim et al. [28] |
? |
+ |
– |
– |
+ |
+ |
+ |
|
Singh et al. [32] |
? |
? |
? |
? |
? |
? |
? |
|
Turtiainen et al. [29] |
? |
+ |
+ |
+ |
+ |
+ |
+ |
|
Williams et al. [35] |
+ |
+ |
+ |
+ |
+ |
– |
+ |
|
Zhang et al. [27] |
+ |
+ |
– |
– |
+ |
+ |
– |
|
Zhuang et al. [31] |
? |
? |
? |
+ |
+ |
? |
? |
«+» — низкий риск; «?» — неясный риск; «-» — высокий риск.
|
Ссылка |
ИОХВ |
Вес (%) |
Относительный риск |
Относительный риск |
||
|
Триклозан |
Контроль |
|||||
|
Baracs et al. [24] |
23 из 188 |
24 из 197 |
10,8 |
1,00 (0,59 1,72) |
||
|
DeFazio et al. [25] |
4 из 43 |
4 из 50 |
1,7 |
1,16 (0,31, 4,37) |
||
|
Deliaert et al. [47] |
0 из 26 |
0 из 26 |
Невозможно оценить |
|||
|
Ford et al. [48] |
3 из 98 |
0 из 49 |
0,3 |
3,54 (0,19, 67,12) |
||
|
Galal and El-Hindawy [22] |
17 из 230 |
33 из 220 |
15,5 |
0,49 (0,28, 0,86) |
||
|
Isikef et al. [36] |
9 из 170 |
19 из 340 |
5,8 |
0,95 (0,44, 2,05) |
||
|
Khachatryan et al. [33] |
6 из 65 |
14 из 68 |
6,3 |
0,45 (0,18, 1,10) |
||
|
Mattavelli et al. [34] |
11 из 108 |
12 из 109 |
5,5 |
0,93 (0,43, 2,01) |
||
|
Mingmalairak et al. [26] |
5 из 50 |
4 из 50 |
1,8 |
1,25 (0,36, 4,38) |
||
|
Rasic et al. [23] |
4 из 91 |
12 из 93 |
5,5 |
0,34 (0,11, 1,02) |
||
|
Rozzelle et al. [21] |
2 из 46 |
8 из 38 |
4,0 |
0,21 (0,05, 0,92) |
_l________1________________________1________L_ 002 0-1 1 10 50 |
|
|
Seim et al. [28] |
16 из 160 |
17 из 163 |
7,7 |
0,96 (0,50, 1,83) |
||
|
Singh et al. [32] |
6 из 50 |
16 из 50 |
7,4 |
0,38 (0,16, 0,88) |
||
|
Turtiainen et al. [29] |
31 из 139 |
30 из 137 |
13,9 |
1,02 (0,65, 1,59) |
||
|
Williams et al. [35] |
10 из 66 |
14 из 61 |
6,7 |
0,66 (0,32, 1,37) |
||
|
Zhang et al. [27] |
2 из 46 |
5 из 43 |
2,4 |
0,37 (0,08, 1,83) |
||
|
Zhuang et al. [31] |
0 из 150 |
15 из 300 |
4,8 |
0,06 (0,00, 1,07) |
||
|
Всего |
149 из 1726 |
227 из 1994 |
100 |
0,70 (0,57, 0,85) |
||
|
Неоднородность: χ2 = 21,26, 15 d.f., P = 0,129; I2 = 29% Кри терий общего эффекта: Z = 3,61, P < 0, 001 |
В пользу триклозана |
В пользу контроля |
||||
Рис. 2. График «форест-плот», построенный по данным метаанализа для сравнения частоты возникновения инфекций послеоперационной раны (ИОХВ) в группах пациентов, где применялся шовный материал с покрытием из триклозана и шовный материал без покрытия (контрольная группа). Для проведения метаанализа использовалась модель постоянных эффектов Мантеля–Хэнзеля. Значения относительного риска представлены с 95% доверительным интервалом
Анализ данных в подгруппах
При анализе данных в подгруппах, стратифицированном по характеристикам испытуемых и применяемым вмешательствам, благотворное влияние ШМТ на предотвращение ИОХВ носило постоянный и достоверный характер в группе взрослых пациентов, в группе больных с операциями на брюшной полости, а также в группах чистых и условно-чистых хирургических ран. Однако в группе пациентов детского возраста, пациентов с зараженными или грязными ранами, ранами после хирургического вмешательства на молочной железе и сердце такого положительного влияния не наблюдалось.
Что касается РКИ, в которых изучалась только эффективность шовного материала Vicryl Plus® по сравнению с Vicryl®, обобщенная оценка говорит в пользу Vicryl Plus® (ОР 0,70, 95% ДИ от 0,53 до 0,94; Р = 0,016). Более того, преимущество применения ШМТ по сравнению с традиционным шовным материалом оставалось устойчивым независимо от длительности периода наблюдения.
При анализе, стратифицированном по риску системной ошибки, в подгруппе исследований с неясным риском была выявлена существенная статистическая неоднородность. В исследованиях с низким риском преимущество ШМТ было достоверным (ОР 0,60, от 0,39 до 0,90; Р = 0,015), тогда как в исследованиях с неясным риском наблюдалась тенденция к снижению частоты ИОХВ в группе пациентов, где применялся ШМТ, при пограничном значении Р, равном 0,051 (ОР 0,57, от 0,32 до 1,00). При оценке исследований с высоким риском системной ошибки невозможно было выявить преимущества ШМТ над шовным материалом без покрытия (ОР 0,85, от 0,62 до 1,18; Р = 0,332). Более того, анализ данных в подгруппах, который проводился в отношении исследований, опубликованных в виде полных статей или обзоров конференций, соответствовал результатам общей оценки.
Системная ошибка публикации
Распределение исследований на воронкообразном графике было симметричным. Свидетельств существенных ошибок публикаций в данном метаанализе выявлено не было (рис. 3).
Комментарии
Данный систематический обзор и метаанализ доказывают, что использование ШМТ вызывало 30% снижение риска ИОХВ, особенно в группе взрослых пациентов, больных с вмешательствами на брюшной полости, а также в группах чистых
Таблица 3
|
Количество исследований |
Количество участников |
Инфекция послеоперационной раны |
Относительный риск |
P* |
P в Q-тесте † |
I2 (%) |
||
|
TCSs |
Контроль |
|||||||
|
Всего |
17 |
3720 |
149 из 1726 |
227 из 1994 |
0,70 (0,57, 0,85) |
< 0,001 |
0,129 |
29 |
|
Возрастная группа |
||||||||
|
Взрослые |
15 |
3489 |
144 из 1582 |
219 из 1907 |
0,71 (0,58, 0,87) |
< 0,001 |
0,185 |
25 |
|
Дети |
2 |
231 |
5 из 144 |
8 из 87 |
0,64 (0,04, 1,01) |
0,749 |
0,087 |
66 |
|
Заражение раны |
||||||||
|
Чистые |
9 |
1797 |
80 из 820 |
117 из 977 |
0,73 (0,56, 0,95) |
0,021 |
0,219 |
26 |
|
Условно-чистые |
6 |
1146 |
53 из 566 |
79 из 580 |
0,69 (0,50, 0,96) |
0,026 |
0,349 |
10 |
|
Зараженные или грязные |
2 |
87 |
8 из 42 |
12 из 45 |
1,10 (0,14, 8,43) |
0,928 |
0,065 |
71 |
|
Тип хирургической процедуры |
||||||||
|
Вмешательство на брюшной полости |
7 |
1562 |
53 из 695 |
85 из 867 |
0,69 (0,50, 0,97) |
0,030 |
0,169 |
34 |
|
Вмешательство на молочной железе |
3 |
268 |
12 из 138 |
19 из 130 |
0,59 (0,30, 1,14) |
0,114 |
0,522 |
0 |
|
Вмешательство на сердце |
3 |
933 |
31 из 380 |
52 из 553 |
0,75 (0,49, 1,14) |
0,180 |
0,178 |
42 |
|
Период наблюдения (месяцев) |
||||||||
|
1 |
9 |
2402 |
115 из 1117 |
156 из 1285 |
0,79 (0,63, 0,99) |
0,037 |
0,230 |
25 |
|
> 1 |
6 |
1001 |
24 из 453 |
45 из 548 |
0,56 (0,35, 0,92) |
0,021 |
0,136 |
40 |
|
Риск системной ошибки |
||||||||
|
Низкий |
3 |
677 |
32 из 346 |
51 из 331 |
0,60 (0,39, 0,90) |
0,015 |
0,395 |
0 |
|
Неясный |
8 |
1749 |
56 из 715 |
104 из 1034 |
0,57 (0,32, 1,00) |
0,051 |
0,034 |
56 |
|
Высокий |
6 |
1294 |
61 из 665 |
72 из 629 |
0,85 (0,62, 1,18) |
0,332 |
0,487 |
0 |
|
Статус публикации |
||||||||
|
Полный объем |
13 |
3177 |
122 из 1460 |
181 из 1717 |
0,72 (0,58, 0,90) |
0,003 |
0,116 |
34 |
|
Обзор |
4 |
543 |
27 из 266 |
46 из 277 |
0,61 (0,39, 0,94) |
0,026 |
0,292 |
20 |
|
Vicryl Plus® в сравнении с Vicryl® |
10 |
2160 |
71 из 1022 |
109 из 1138 |
0,70 (0,53, 0,94) |
0,016 |
0,243 |
22 |
Значения в скобках отражают 95% доверительные интервалы. ШМТ – шовный материал с покрытием из триклозана. *Z-тест; †χ2 тест
Обзор анализа данных в подгруппах
и условно-чистых ран. Применение ШМТ может оказаться полезным в клинической практике для снижения частоты возникновения ИОХВ и уменьшения дополнительных затрат, связанных с такими инфекциями.
Шовный материал играет существенную роль в развитии ИОХВ, являясь поверхностью, к которой могут прикрепляться микроорганизмы [49]. При обсеменении шовного материала патогенными микроорганизмами в дальнейшем может формироваться биопленка, которая позволяет инфекционным агентам оставаться в тканях и усиливает их устойчивость к воздействию иммунной системы и антибактериальному лечению, что способствует развитию инфекции в ране [50–52]. Со- ответственно, возможность применения шовного материала с покрытием из противомикробных агентов, таких как серебро или антибиотики, рассматривалась с 1950-х годов [53–55]. Триклозан – антисептик широкого спектра действия с хорошо изученным профилем безопасности, широко используется в фармацевтических и гигиенических изделиях для человека уже более 30 лет [56, 57]. Недавно для борьбы с ИОХВ был разработан шовный материал с покрытием из триклозана, обладающий противомикробной активностью.
Подтверждая многообещающие результаты, полученные в экспериментах in vitro и in vivo , разнообразные клинические исследования продемонстрировали преимущество ШМТ над
STATUS Р^ттЕПЕТТ*^
O.
О га io га" ч га
О
Log (ОР)
Рис. 3. Воронкообразный график оценки системной ошибки публикаций. ОР – относительный риск. Симметричный воронкообразный график свидетельствует об отсутствии явных системных ошибок публикации традиционным шовным материалом без покрытия при профилактике ИОХВ. Однако результаты отдельных РКИ были неубедительными и противоречивыми, указывая на то, что ограниченный объем выборки некоторых исследований имел недостаточную статистическую мощность для определения истинного эффекта ШМТ. В соответствии с расчетами, для демонстрации статистически достоверной разницы в частоте возникновения ИОХВ между группой, где применялся ШМТ, и контрольной группой через 2 и 6 недель после хирургического вмешательства на молочной железе, потребовалось бы примерно в 13 и 3 раза больше участников соответственно, чем количество испытуемых, задействованных в данном исследовании [35]. В подобных обстоятельствах метаанализ может быть действительно полезным, поскольку он включает в себя количественный синтез данных многих РКИ, способствуя более всесторонней оценке с высокой статистической мощностью [58]. Объединив данные о 3720 хирургических пациентах, данный систематический обзор подтвердил благотворное воздействие ШМТ на предотвращение ИОХВ.
Применение ШМТ может оказать существенное влияние на современную клиническую практику, способствуя не только снижению заболеваемости и риска смерти хирургических пациентов, но и уменьшению общих непрямых затрат на лечение [59, 60]. Предшествующие отчеты о затратах здравоохранения свидетельствуют о значительных экономических убытках, связанных с ИОХВ [60, 61]. За счет небольших дополнительных расходов ШМТ может существенно снизить риск повторных госпитализаций и длительность пребывания в стационаре, а значит, и лишние затраты на медицинское обслуживание [19, 61]. Результаты данного систематического обзора свидетельствуют в пользу стандартного применения ШМТ, особенно в группе взрослых пациентов, больных с вмешательствами на брюшной полости, а также в группах чистых и условно-чистых ран.
Заключение настоящего метаанализа отличается от результатов предыдущего метаанализа по данной теме [30]. Предше- ствующий метаанализ включал всего семь РКИ и не выявил преимущества в использовании ШМТ. Однако большинство проанализированных исследований характеризовались низким качеством и высокой неоднородностью. Также ограниченное число исследований не позволило авторам изучить потенциальный эффект ШМТ в отдельных популяциях больных. В отличие от вышеупомянутого обзора, данный систематический обзор и метаанализ представляют собой дополненную всестороннюю оценку эффективности ШМТ на основе последних данных, полученных в ходе нескольких РКИ. Десять новых опубликованных исследований [22, 23, 28, 29, 31–36] и обновленные данные двух других исследований [24, 27] позволили увеличить общий объем выборки с 836 до 3720, что значительно повысило статистическую мощность данного метаанализа. Качество исследований также улучшилось: если в предыдущий метаанализ было включено одно исследование с низким риском, три – с неясным риском и три – с высоким риском, то в настоящем обзоре использовались три исследования с низким риском, восемь – с неясным риском и шесть – с высоким риском. Анализ данных в подгруппах, проведенный в рамках настоящего метаанализа, говорит о тенденции к увеличению статистической достоверности в соответствии с улучшением качества исследований. Это может, по крайней мере, частично объяснить противоречие с результатом предыдущего исследования [30] и еще раз подчеркивает важность качества методологии РКИ. Более того, 17 исследований, включенных в данный метаанализ, можно было разделить на несколько категорий в зависимости от сходных клинических моделей, что позволило авторам решить проблему неоднородности путем анализа данных в подгруппах. Результаты, подтвержденные анализом данных в подгруппах, являются более надежными и информативными, так как описывают эффективность ШМТ в аналогичных клинических ситуациях, характеризующихся более единообразными исходными характеристиками и меньшей клинической неоднородностью. Особенно важно то, что в настоящем систематическом обзоре был проведен анализ данных в нескольких подгруппах в отношении качества модели исследований и потенциального риска системной ошибки. Согласующиеся между собой выводы еще раз доказывают надежность и устойчивость результатов метаанализа.
Следует отметить, что интерпретация результатов требует определенной осторожности, учитывая некоторые ограничения данного систематического обзора и метаанализа. Во-первых, качество исследований по-прежнему нельзя назвать полностью удовлетворительным. Поскольку надежность метаанализа определяется главным образом качеством включенных в него исследований, результаты настоящего метаанализа должны интерпретироваться с осторожностью. Необходимы дальнейшие РКИ с хорошо продуманной моделью и высоким качеством методологии. Во-вторых, установление клинического диагноза ИОХВ сильно зависит от мнения специалистов. Только в пяти исследованиях [22, 27, 29, 35, 36] были четко определены диагностические критерии ИОХВ, разработанные
Центром контроля и профилактики заболеваемости США [62], тогда как авторы остальных исследований не придерживались данных критериев. Это может служить причиной клинической неоднородности, поэтому нельзя исключить потенциальную системную ошибку. Более того, хотя во всех включенных исследованиях конечной точкой считалась частота возникновения ИОХВ после проведения хирургических процедур, в трех из них ИОХВ не были отмечены как первичные исходы [27, 47, 48]. Неоднородность отчетности об исходах могла стать причиной потенциальной системной ошибки и исказить данные, полученные в указанных исследованиях. В-третьих, исследования, включенные в настоящий метаанализ, проводились на испытуемых с разными состояниями и после разных хирургических процедур. Результаты необходимо интерпретировать осторожно. Кроме того, следует рассматривать определенные клинические сценарии. Наконец, недостаточность данных о пациентах помешала авторам провести метаанализ, основанный на подробной персональной информации. Стратификация по факторам риска ИОХВ, таким как диабет, употребление стероидов и курение, также оказалась невозможной вследствие недостаточной информации. Эти факторы могли исказить эффект ШМТ и, следовательно, повлиять на оценку.
Z.X. Wang и C.P. Jiang внесли одинаковый вклад в эту работу. Данное исследование проводилось при поддержке программы «Грантов для главных клинических центров институтов» (№ ZX201105) и «Научного фонда министерства здравоохранения Китая» (№ LW201008). Декларация финансовой незаинтересованности: авторы заявляют об отсутствии конфликта интересов.
Список литературы Систематический обзор и метаанализ применения шовного материала с покрытием из триклозана для предотвращения инфекций области хирургического вмешательства (ИОХВ)
- Barie PS. Surgical site infections: epidemiology and prevention//Surg. Infect. (Larchmt). 2002. Vol. 3 (Suppl. 1). S. 9-21.
- Leaper D.J., van Goor H., Reilly J., Petrosillo N., Geiss H.K., Torres A. J. et al. Surgical site infection -a European perspective of incidence and economic burden//Int. Wound J. 2004. Vol. 1. P. 247-273.
- Hawn M, Vick C.C., Richman J., Holman W., Deierhoi R.J., Graham L.A. et al. Surgical site infection prevention: time to move beyond the surgical care improvement program//Ann. Surg. 2011. Vol. 254. P. 494-499.
- Patkar A.D., Magee G., Vaughn B., Edmiston C.E., Vardireddy N. The economic burden of surgical site infection using therapeutic antibiotic utilization measure -comparison of two time periods//Value in Health. 2010. Vol. 13. A432.
- Alexander J.W., Solomkin J.S., Edwards M.J. Updated recommendations for control of surgical site infections//Ann. Surg. 2011. Vol. 253. P. 10821093.
- Odom-Forren J. Preventing surgical site infections//Nursing. 2006. Vol. 36. P. 58-63.
- Alexander J.W., Kaplan J.Z., Altemeier W.A. Role of suture materials in the development of wound infection.//Ann. Surg. 1967. Vol. 165. P. 192-199.
- Katz S., Izhar M., Mirelman D. Bacterial adherence to surgical sutures. A possible factor in suture induced infection.//Ann. Surg. 1981. Vol. 194. P. 35-41.
- Storch M.L., Rothenburger S.J., Jacinto G. Experimental efficacy study of coated VICRYL Plus antibacterial suture in guinea pigs challenged with Staphylococcus aureus//Surg. Infect. (Larchmt). 2004. Vol. 5. P. 281-288.
- Edmiston C.E., Seabrook G.R., Goheen M.P., Krepel C.J., Johnson C.P., Lewis B.D. et al. Bacterial adherence to surgical sutures: can antibacterial-coated sutures reduce the risk of microbial contamination?//J. Am. Coll. Surg. 2006. Vol. 203. P. 481-489.
- Rothenburger S., Spangler D., Bhende S., Burkley D. In vitro antimicrobial evaluation of coated VICRYL* Plus antibacterial suture (coated polyglactin 910 with triclosan) using zone of inhibition assays//Surg. Infect. (Larchmt). 2002. Vol. 3 (Suppl. 1). S. 79-87.
- MingX., Rothenburger S., Yang D. In vitro antibacterial efficacy of MONOCRYL Plus antibacterial suture (poliglecaprone 25 with triclosan)//Surg. Infect. (Larchmt). 2007. Vol. 8. P. 201-208.
- Bojar W., Kazmierska K., Szalwinski M., Zareba T. Triclosan-coated sutures in oral surgery//Advances in Clinical and Experimental Medicine. 2009. Vol. 18. P. 401-405.
- Marco F., Vallez R., Gonzalez P., Ortega L., De La Lama J., Lopez-Duran L. Study of the efficacy of coated Vicryl Plus* antibacterial suture in an animal model of orthopedic surgery//Surg. Infect. (Larchmt). 2007. Vol. 8. P. 359-365.
- MingX., Nichols M., Rothenburger S. In vivo antibacterial efficacy of MONOCRYL Plus antibacterial suture (poliglecaprone 25 with triclosan)//Surg. Infect. (Larchmt). 2007. Vol. 8. P. 209-214.
- Ming X., Rothenburger S., Nichols M.M. In vivo and in vitro antibacterial efficacy of PDS Plus (polidioxanone with triclosan) suture//Surg. Infect. (Larchmt). 2008. Vol. 9. P. 451-457.
- Storch M., Perry L.C., Davidson J.M., Ward J.J. A28-day study of the effect of coated VICRYL* Plus antibacterial suture (coated polyglactin 910 suture with triclosan) on wound healing in guinea pig linear incisional skin wounds//Surg. Infect. (Larchmt). 2002. Vol. 3 (Suppl. 1). S. 89-98.
- Storch M., Scalzo H., Van Lue S., Jacinto G. Physical and functional comparison of coated VICRYL* Plus antibacterial suture (coated polyglactin 910 suture with triclosan) with coated VICRYL* suture (coated polyglactin 910 suture)//Surg. Infect. (Larchmt). 2002. Vol. 3 (Suppl. 1). S. 65-77.
- Fleck T., Moidl R., Blacky A., Fleck M., Wolner E., Grabenwoger M. et al. Triclosan-coated sutures for the reduction of sternal wound infections: economic considerations//Ann. Thorac. Surg. 2007. Vol. 84. P. 232-236.
- Justinger C., Schuld J., Sperling J., Kollmar O., Richter S., Schilling M.K. Triclosan-coated sutures reduce wound infections after hepatobiliary surgery -a prospective non-randomized clinical pathway driven study//Langenbecks Arch. Surg. 2011. Vol. 396. P. 845-850.
- Rozzelle C.J., Leonardo J., Li V. Antimicrobial suture wound closure for cerebrospinal fluid shunt surgery: a prospective, doubleblinded, randomized controlled trial//J. Neurosurg. Pediatr. 2008. Vol. 2. P. 111 -117.
- Galal I., El-Hindawy K. Impact of using triclosan-antibacterial sutures on incidence of surgical site infection//Am. J. Surg. 2011. Vol. 202. P. 133-138.
- Rasic Z., Schwarz D., Adam V.N., Sever M., Lojo N., Rasic D. et al. Efficacy of antimicrobial triclosan-coated polyglactin 910 (Vicryl* Plus) suture for closure of the abdominal wall after colorectal surgery//Coll. Antropol. 2011. Vol. 35. P. 439-443.
- Baracs J., Husza'r O., Sajjadi S.G., Horva'th O.P. Surgical site infections after abdominal closure in colorectal surgery using triclosan-coated absorbable suture (PDS Plus) vs. uncoated sutures (PDS II): a randomized multicenter study//Surg. Infect. (Larchmt). 2011. Vol. 12. P. 483-489.
- DeFazio A., Datta M.S., Nezhat C. Does the use of Vicryl Plus antibacterial suture decrease the incidence of umbilical infection when compared to Vicryl suture?//Fertil. Steril. 2005. Vol. 84 (Suppl. 1). S. 161.
- Mingmalairak C., Ungbhakorn P., Paocharoen V. Efficacy of antimicrobial coating suture coated polyglactin 910 with triclosan (Vicryl Plus) compared with polyglactin 910 (Vicryl) in reduced surgical site infection of appendicitis, double blind randomized control trial, preliminary safety report//J. Med. Assoc. Thai. 2009. Vol. 92. P. 770-775.
- Zhang Z.T., Zhang H.W., Fang X.D., Wang L.M., Li X.X., Li Y.F. et al. Cosmetic outcome and surgical site infection rates of antibacterial absorbable (polyglactin 910) suture compared to Chinese silk suture in breast cancer surgery: a randomized pilot research//Chin. Med. J. (Engl.). 2011. Vol. 124. P. 719-724.
- Seim B.E., Tennessen T., Woldbaek P.R. Triclosan-coated sutures do not reduce leg wound infections after coronary artery bypass grafting//Interact. Cardiovasc. Thorac. Surg. 2012. Vol. 15. P. 411-415.
- Turtiainen J., Saimanen E.I., Ma'kinen K.T., Nyka'nen A.I., Venermo M.A., Uurto I.T. et al. Effect of triclosan-coated sutures on the incidence of surgical wound infection after lower limb revascularization surgery: a randomized controlled trial//World J. Surg. 2012. Vol. 36. P. 2528-2534.
- Chang W.K., Srinivasa S., Morton R., Hill A.G. Triclosan-impregnated sutures to decrease surgical site infections: systematic review and meta-analysis of randomized trials//Ann. Surg. 2012. Vol. 255. P. 854-859.
- Zhuang C.P., Cai G.Y., Wang Y.Q. Comparison of two absorbable sutures in abdominal wall incision//Journal of Clinical Rehabilitative Tissue Engineering Research. 2009. Vol. 13. P. 4045-4048.
- Singh H., Emmert M.Y., Sakaguchi H., Neng Lee C., Kofidis T. Antibacterial suture reduces surgical site infections in coronary artery bypass grafting//Heart Surgery Forum. 2010. Vol. 13. S. 85.
- Khachatryan N., Dibirov M., Omelyanovsky V., Chupalov M, Gasanova G. Prevention of postoperative infections in abdominal surgery using reabsorbable suture with antibacterial activity (Vicryl Plus) versus reabsorbable standard sutures//Surg. Infect. (Larchmt). 2011. Vol. 12. P. 13-14.
- Mattavelli I., Nespoli L., Alfieri S., Cantore F., Sebastian-Douglas S., Cobianchi L. et al. Triclosan-coated suture to reduce surgical site infection after colorectal surgery//Surg. Infect. (Larchmt). 2011. Vol. 12. P. 14-15.
- Williams N., Sweetland H., Goyal S., Ivins N., Leaper D.J. Randomized trial of antimicrobial-coated sutures to prevent surgical site infection after breast cancer surgery//Surg. Infect. (Larchmt). 2011. Vol. 12. P. 469-474.
- Isik I., Selimen D., Senay S., Alhan C. Efficiency of antibacterial suture material in cardiac surgery: a double-blind randomized prospective study//The Heart Surgery Forum. 2012. Vol. 15. P. 40-45.
- MoherD., LiberatiA., TetzlaffJ., Altman D.G. The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement//Open. Med. 2009. Vol. 3. P. 123-130.
- ClinicalTrials.gov. http://www.clinicaltrial.gov [accessed 9 May 2012].
- World Health Organization. International Clinical Trials Registry Platform Search Portal. http://apps.who.int/trialsearch/Default.aspx [accessed 9 May 2012].
- The Cochrane Collaboration. Cochrane Handbook for Systematic Reviews of Interventions. http://www.cochrane-handbook.org [accessed 9 May 2012].
- Mangram A.J., Horan T.C., Pearson M.L., Silver L.C., Jarvis W.R. Guideline for prevention of surgical site infection, 1999. Centers for Disease Control and Prevention (CDC) Hospital Infection Control Practices Advisory Committee//Am.J. Infect. Control. 1999. Vol. 27. P. 97-132.
- The Cochrane Collaboration. Review Manager. Version 5.1.6. The Nordic Cochrane Centre, The Cochrane Collaboration: Copenhagen, 2011.
- Lau J., Ioannidis J.P., Schmid C.H. Quantitative synthesis in systematic reviews//Ann. Intern. Med. 1997. Vol. 127. P. 820-826.
- Mantel N., Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease//J. Natl. Cancer Inst. 1959. Vol. 22. P. 719-748.
- DerSimonian R., Laird N. Meta-analysis in clinical trials//Control Clin. Trials. 1986. Vol. 7. P. 177-188.
- Higgins J.P., Thompson S.G. Quantifying heterogeneity in a metaanalysis//Stat. Med. 2002. Vol. 21. P. 1539-1558.
- Deliaert A.E., Van den Kerckhove E., Tuinder S., Fieuws S., Sawor J.H., Meesters-Caberg M.A. et al. The effect of triclosan-coated sutures in wound healing. A double blind randomised prospective pilot study//J. Plast. Reconstr. Aesthet. Surg. 2009. Vol. 62. P. 771-773.
- Ford H.R., Jones P., Gaines B., Reblock K., Simpkins D.L. Intraoperative handling and wound healing: controlled clinical trial comparing coated VICRYL Plus antibacterial suture (coated polyglactin 910 suture with triclosan) with coated VICRYL suture (coated polyglactin 910 suture)//Surg. Infect. (Larchmt). 2005. Vol. 6. P. 313-321.
- Masini B.D., Stinner D.J., Waterman S.M., Wenke J.C. Bacterial adherence to suture materials//J. Surg. Educ. 2011. Vol. 68. P. 101-104.
- Gristina A.G., Price J.L., Hobgood C.D., Webb L.X., Costerton J.W. Bacterial colonization of percutaneous sutures//Surgery. 1985. Vol. 98. P. 12-19.
- Go'mez-Alonso A., Garc'ta-Criado F.J., Parren~o-Manchado F.C., Garc'ta-Sa'nchez J.E., Garc'ta-Sa'nchezE., Parren~o-Manchado A. et al. Study of the efficacy of coated VICRYL Plus antibacterial suture (coated polyglactin 910 suture with triclosan) in two animal models of general surgery//J. Infect. 2007. Vol. 54. P. 82-88.
- Wolcott R., Cutting K.F., Dowd S.E. Surgical site infections: biofilms, dehiscence and delayed healing//Wounds UK. 2008. Vol. 4. P. 108-113.
- Glassman J.A., Fowler E.F., Novak M.V. An experimental study of sulfonamide impregnated sutures//Surg. Obstet. Gynecol. 1944. Vol. 78. P. 359-363.
- Darouiche R.O., Meade R., Mansouri M., Raad II. In vivo efficacy of antimicrobial-coated fabric from prosthetic heart valve sewing rings//J. Heart Valve Dis. 1998. Vol. 7. P. 639-646.
- Blaker J.J., Nazhat S.N., Boccaccini A.R. Development and characterisation of silver-doped bioactive glass-coated sutures for tissue engineering and wound healing applications//Biomaterials. 2004. Vol. 25. P. 1319-1329.
- Barbolt T.A. Chemistry and safety of triclosan, and its use as an antimicrobial coating on coated VICRYL* Plus antibacterial suture (coated polyglactin 910 suture with triclosan)//Surg. Infect. (Larchmt). 2002. Vol. 3 (Suppl. 1). S. 45-53.
- Leaper D., Assadian O., Hubner N.O., McBain A., Barbolt T., Rothenburger S. et al. Antimicrobial sutures and prevention of surgical site infection: assessment of the safety of the antiseptic triclosan//Int. Wound J. 2011. Vol. 8. P. 556-566.
- Sacks H.S., Berrier J., Reitman D., Ancona-Berk V.A., Chalmers T.C. Meta-analyses of randomized controlled trials//N. Engl. J. Med. 1987. Vol. 316. P. 450-455.
- Gaynes R.P., Culver D.H., Horan T.C., Edwards J.R., Richards C., Tolson J.S. Surgical site infection (SSI) rates in the United States, 19921998: the National Nosocomial Infections Surveillance System basic SSI risk index//Clin. Infect. Dis. 2001. Vol. 33 (Suppl. 2). S. 69-77.
- Fry D.E. The economic costs of surgical site infection//Surg. Infect. (Larchmt). 2002. Vol. 3 (Suppl. 1). S. 37-43.
- Edmiston C.E., Patkar A.D., Magee G., Seabrook G.R., Vaughn B. Use of an observational, nationwide inpatient discharge database to document the economic benefits associated with innovative antimicrobial technology to reduce the risk of surgical site infection (SSI)//Am. J. Infect. Control. 2010. Vol. 38. P. 38.
- Horan T.C., Gaynes R.P., Martone W.J., Jarvis W.R., Emori T.G. CDC definitions of nosocomial surgical site infections, 1992: a modification of CDC definitions of surgical wound infections//Am. J. Infect. Control. 1992. Vol. 20. P. 271-274.


