Solitary metastasis of gastric carcinoma in the right rectus abdominis muscle 15 years after total gastrectomy
Автор: Bayramov R.B., Huseynova S.E., Maharramov Z.A., Bayramli F.R.
Журнал: Сибирский онкологический журнал @siboncoj
Рубрика: Случай из клинической практики
Статья в выпуске: 6 т.24, 2025 года.
Бесплатный доступ
Background. Recurrence of gastric carcinoma most often occurs within 5 years after radical surgery, with 60–70 % of recurrence detected within first two years and 4–5 % after 5 years. The liver and peritoneum are the most common sites of gastric cancer recurrence, but it can also spread to other distant sites. Metastases to skeletal muscle from any primary tumor are rare, with a reported incidence ranging from 0.03 to 0.16 %. Isolated hematogenous metastasis bypassing the portal and pulmonary circulation from gastric primary is a rare event. Here we report a case of a patient who developed isolated solitary metastasis in the right rectus abdominis muscle 15 years after total gastrectomy (without perioperative or adjuvant chemotherapy) for gastric carcinoma. Case presentation. A 64-year-old patient who had undergone gastrectomy for gastric cancer 15 years earlier presented with a tumor in the right rectus abdominis muscle. Histopathological examination of the ultrasound-guided Tru-Cut biopsy confirmed the presence of adenocarcinoma. A CT scan of the chest, abdomen, and pelvis, as well as a colonoscopy, revealed no synchronous primary tumor. Wide excision of the tumor was performed, including full-thickness abdominal wall resection, with reconstruction of the defect using polypropylene mesh. Conclusion. Our case report demonstrates that gastric cancer metastases to skeletal muscle can occur even 15 years after a curative gastrectomy. In other words, the detection of soft tissue tumors developing in patients even 15 years after gastrectomy for gastric cancer does not rule out the possibility of metachronous metastasis from the primary gastric tumor.
Gastric carcinoma, gastrectomy, solitary metastasis from gastric carcinoma into skeletal muscles metastasis, abdominal wall resection
Короткий адрес: https://sciup.org/140313338
IDR: 140313338 | УДК: 616.33-006.66-089.87-033.2:611.95 | DOI: 10.21294/1814-4861-2025-24-6-192-198
Текст научной статьи Solitary metastasis of gastric carcinoma in the right rectus abdominis muscle 15 years after total gastrectomy
As the fifth most common cancer and the third leading cause of cancer-related death worldwide, gastric carcinoma remains a major public health issue in most countries [1]. Curative surgery including systemic lymph node dissection is the only treatment modality offering chance for cure in patients with gastric carcinoma. Despite the fact that perioperative (or adjuvant) chemotherapy can present additional effect in cases of advanced-stage gastric carcinoma, a significant portion of patients develop recurrence during follow-up and substantial part of patients with a history of gastrectomy for advanced-stage gastric carcinoma die from recurrent disease [2, 3].
Recurrence of gastric carcinoma is strongly dependent on the extent of the disease, as indicated by the TNM stage. The TNM classification system accurately predicts the overall survival, but is unable to provide data in terms of overall recurrence, time-specific recurrence, and site-specific first recurrence [2]. Thus, the patients who have undergone gastrectomy for gastric carcinoma are routinely searched for intra-abdominal (including hepatic) recurrence during the follow-up period with checking other sites if clinically indicated.
The definition of early and late recurrence varies across studies. Mostly, late recurrence is defined as recurrence ≥2 years after radical-intent surgery [4]. Most follow-up programs end 5 years after primary treatment [2]. Nevertheless, recurrence at >5 years after radical gastrectomy and adjuvant chemotherapy is observed frequently. Recurrence can also be found in atypical distant sites. Skeletal muscle metastases from any primary tumor are rare occurrences with a reported incidence of 0.03 to 0.16 % [5]. In the literature, very few cases of solitary muscle metastasis from a gastric primary have been reported.
Here we report a patient who developed a recurrence in the right rectus abdominis muscle 15 years after total gastrectomy (without adjuvant chemotherapy) for gastric carcinoma. The purpose of this case report is to enrich the relevant cases described in the literature, which emphasizes the possibility of distant hematogenous metastasis in non-typical sites after long time from primary treatment.
Case presentation
A 49-year-old male patient with gastric carcinoma was admitted to Thoracoabdomunal Unit of Oncologic Clinic, Azerbaijan Medical University in April, 2008. On the bases of preoperative clinic-radiological evaluation, the patient was diagnosed with gastric adenocarcinoma involved the antrum and corpus of the stomach (poor-differentiated adenocarcinoma, cT3NXM0) and complicated with decompensated pyloric stenosis. Total gastrectomy was planned for radical-intent treatment. Roux-en-Y total gastrectomy, D2 extended lymph node dissection were performed. Pathological report of the resected specimen and pathological staging: mucinous carcinoma, metastases in 2 regional lymph nodes (in stations 3 and 5); pT3N1M0. Patient did not receive adjuvant chemotherapy and never applied for follow-up examination.
Fifteen years after surgery, the patient returned with a tumor in the anterior abdominal wall. On inspection, a tumor with a long diameter of approximately 10.0 cm was seen in the right half of the anterior abdominal wall, which was mobile on palpation without clinical signs of fixation. On abdominal CT scan, a hypodense tumor measuring 7.0×5.0×4.0 cm was detected in the right rectus sheath without signs of adjacent organ invasion (Fig. 1). Tru-Cut biopsy under US-guidance was conducted. Histopathological examination of biopsy specimen revealed poor-differentiated adenocarcinoma. Taking into the account the tumor histology, all possible primary tumor foci were searched by thoracic and pelvic CT scan, total colonoscopy. No any primary tumor was detected. The tumor was considered to be late metachronous metastasis from gastric carcinoma removed 15 years ago. Wide excision of the tumor with reconstruction of abdominal wall with prolene mesh was planned according to the multidisciplinary team decision.
Through the right transrectal incision, the abdominal wall skin was cut in an ellipsoid form, and the
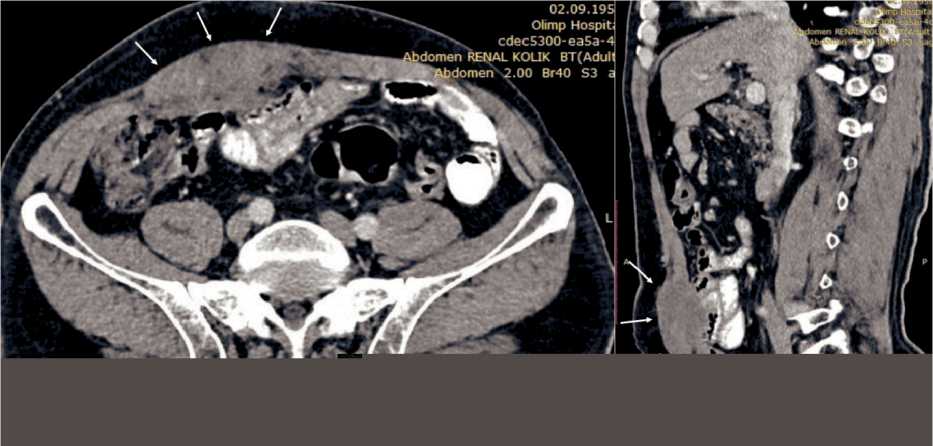
Fig. 1. CT scan of the tumor in the right rectus abdominis muscle (arrows). A – axial slice; B – sagittal slice. Note: created by the authors Рис. 1. КТ опухоли в правой прямой мышце живота (указана стрелками). А – аксиальный срез; Б – сагиттальный срез. Примечание: рисунок выполнен авторами
borders of the tumor were determined by palpation. Visually, the tumor did not invade the anterior layer of the right rectus sheath and did not invade into the linea alba. The tumor was completely excised with a >1.0-cm resection margin via full-thickness resection of the abdominal wall. Macroscopically, the tumor invaded the parietal peritoneum and no visible seedlings were found on the parietal and visceral peritoneal surface. The abdominal wall defect was reconstructed with prolene mesh.
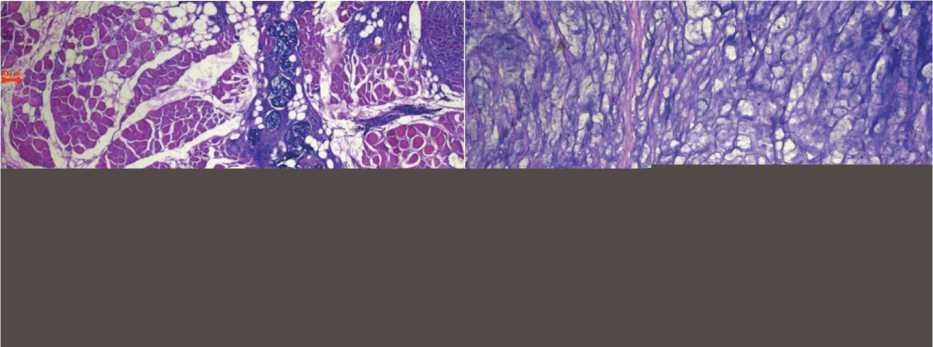
No complications were observed in the postoperative period. The pathological examination of the excised tumor revealed the tumor measuring 72×51×41 mm, not extending beyond the anterior and medial wall of the sheath of the right rectus abdominis muscle; mucinous cancer infiltrating the striated muscles with invasion into the parietal peritoneum; negative resection margins. Histopathological specimens of the primary tumor were retrieved from the archives and sent, along with specimens of the metastatic tumor, to another oncology pathologist. The final consensual histopathological diagnosis was mucinous carcinoma in both cases (Fig. 2). Metastatic tissue was stained for CDX2 and CK20 on paraffin-embedded tissue. Nuclear expression of CDX2 and cytoplasmic staining for CK20 were observed (Fig. 3). The patient underwent eight cycles of adjuvant chemotherapy. A six-month follow-up examination revealed no signs of local recurrence or metastases.
Discussion
Recurrence of gastric carcinoma typically occurs within 5 years after radical surgery, with peritoneum and liver being the most frequent sites of metastasis, and late recurrences are extremely rare [4]. Limited reports suggest that <10 % of patients with gastric cancer who undergo radical surgery experience recurrence at 5 years, and <2 % at 10 years [6].
C.H. Shin et al. reported on 266 (266/1299) patients with recurrence after curative gastrectomy. The early
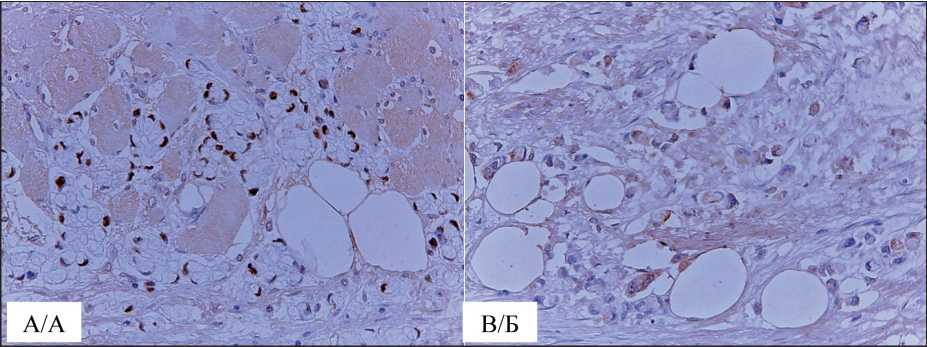
Fig. 3. Microphoto. Immunohistochemical staining of metastatic tumors for CDX2 and CK20. A – nuclear expression of CDX2; B – cytoplasmic staining for CK20, ×200. Note: created by the authors
Рис. 3. Микрофото. Иммуногистохимическое окрашивание метастатической опухоли на CDX2 и CK20. A – нуклеарная экспрессия CDX2; Б – цитоплазматическое окрашивания CK20, ×200. Примечание: рисунок выполнен авторами
(<2 years) recurrence rate was 68.4 %, the intermediate (2 to 5 years) rate was 22.9 %, and the late (>5 years) rate was 8.6 % [2]. According to the authors, no clear definition has been established for late recurrence of gastric carcinoma. Most reports have described cases recurring over 5 years after surgery.
Extra-abdominal solitary recurrences after radical gastrectomy for gastric cancer are very rare, but can be explained by the mechanisms of metastasis. Skeletal muscle metastasis from carcinoma is very rare, and the most common primary sites are lung (25 %), followed by genitourinary (22.3 %), gastrointestinal (21 %), and breast (8.2 %) [5].
Despite the substantial long time from the removal of the primary tumor and non-specific site of distant metastasis in our patient with a history of gastric carcinoma, the tumor was considered to be late metastasis from the primary tumor on the bases of the same histopathological type of the tumors and absence of any primary carcinomas in other sites. In addition, the metastatic tumor expressed nuclear staining for CDX2 and cytoplasmic staining for CK20, which was consistent with the gastrointestinal origin of the tumor. As mentioned earlier, total colonoscopy detected no lesions suggestive of colorectal carcinoma.
According to studies of Tuoheti et al., only 30 cases of skeletal muscle metastasis from gastric carcinoma had been reported in the literature since the 1960s till 2004 [5]. In most reported cases, skeletal muscle involvement was associated with synchronous hepatic and/or lung metastasis. Our case is similar to that reported by Koga et al. and that by Aguirre et al., in which solitary skeletal muscle metastasis developed in the absence of liver or lung involvement [7, 8].
According to some authors, from a physiological perspective, it is difficult to explain why skeletal muscle is only rarely affected by hematogenous dissemination given its high vascularity and total mass accounting for nearly 50 % of total body weight [8, 9, 10]. The most reasonable mechanism for solitary skeletal muscle metastasis can be liver- and lung- bypass of carcinoma cells, the likelihood of which is extremely rare. Skeletal muscle metastasis associated with involvement of the liver and/or lung can likely develop as a re-metastasis from the secondary foci.
From a scientific and clinical point of view, another interesting aspect of this case is that the metastasis was detected after a very long period of time. In the medical literature such metastases are called “late metastases”, which are detected more than 10 years from the elimination of the primary tumor and without evidences of a local recurrence. This latency explained by the dormancy of the extravasated cancer cells, which has also been termed “a temporary mitotic arrest” [11]. It should be noted that very small number of cancer cells, that successfully extravasate, result in metastases. Most extravasated cancer cells remain as single cells or form small clusters called micrometastases and become dormant [12]. Dormant state is defined as nonproliferating state or cell cycle arrest that results in a prolonged G0 phase [11, 12]. Dormancy results from the absence of growth factor signaling, the actions of suppressor genes, the absence of an activated angiogenesis at the micrometastatic site and the presence of immunological factors [12]. Extravasated cancer cells can escape elimination by the immune system through several mechanisms. Release of cytokines and chem-okines from cancer cells contributes to the creation of immunosuppressive environment. Recruitment of immature dendritic cells and macrophages, which suppress the abilities of mature counterparts to eliminate tumor cells by blocking T-cell activation and phagocytosis, maintains this environment as well. Formation of immunosuppressive environment is also maintained by so-called hybrid E/M (epithelial/mesenchymal) cells, which are formed through the programmed process of epithelial mesenchymal transition (EMT). Activation of EMT causes carcinoma cells to lose their epithelial properties (apical-basal polarity and intercellular junctions) and acquire invasive and migratory capabilities, which are characteristics of mesenchymal cells. Those gained mesenchymal properties are the primary drivers of the metastasis. In other words, through the process of EMT, carcinoma cells acquire stem-like properties and become chemoresistant [13]. It should be noted that when micrometastases escape the aforementioned obstacles, the cancer cells can form macrometastases defined as metastases greater than 2 mm in diameter [12].
Late metastases are of particular concern and become a subject of attention after organ transplantation from donors with a long history of malignancy. Clinical evidence proved that the transplanted organ could inadvertently transmit an undetected population of tumor cells even decades after the removal of the primary tumor in the donor. Approximately 40 % of recipients of organs from donors with a history of cancer may develop related malignancies. The tumor cell population was confirmed to be of donor origin, as shown by karyotyping or other markers [11]. One of the consequences of the skepticism on this issue is that some of these “late” metastases may have been classified as “tumors of unknown primary” [14].
The clinical features of carcinoma metastasis to skeletal muscles closely resemble those of soft tissue sarcomas in many respects. Diagnosis is confirmed on the bases of histopathological examination of biopsy specimens taken by needle. However, prior to pathological diagnosis, radiographic evaluation of the mass often provides valuable information with regard to


