Somatic embryogenesis, plantlet regeneration and in vitro flowering from cotyledon culture in a legume oil crop, peanut (Arachis hypogaea L.) under NaCl-stress conditions
Автор: Abirami K., Vikrant
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 4 т.20, 2024 года.
Бесплатный доступ
Present study aims to establish somatic embryogenesis and plantlet regeneration from cotyledon culture in peanut ( Arachis hypogaea L. cv. TMV13) under NaCl-stress conditions. Cotyledon tissue was found to be efficient for somatic embryogenesis in MS medium fortified with 10mg/L of 2,4-dichlorophenoxyacetic acid (2,4-D). Moreover, in order to achieve salt-tolerant regenerants in peanut, cotyledons were inoculated on MS-medium supplemented with various concentrations of NaCl-salt in presence of 2,4-D (10mg/L). Results indicate that with the increase in NaCl-concentrations in medium, frequency of embryogenic callus formation gradually declines and obtained as minimum (17.1±0.16%) with high concentration (150mM) of NaCl-salt while NaCl (200mM) was proved to be lethal. Further, salt-tolerant embryogenic callus was transferred to medium added with (0.5mg/L, 1.0mg/L, 1.5mg/L, and 2.0mg/L) of BAP in combination with (1.5mg/L) of IAA and NaCl (100mM) for the germination of somatic embryos followed by shoot regeneration. Significantly, the maximum frequency (70.2±0.59%) of shoot regeneration was obtained on medium containing BAP (1.5mg/L), IAA (1.5mg/L) and NaCl (100mM). Moreover, salt-tolerant regenerated shoots were further transferred to medium containing kinetin (1.0mg/L, 2.0mg/L, 3.0mg/L, and 5.0mg/L) along with NAA (1.5mg/L) and NaCl (100mM) for root initiation. The high frequency (51±0.7%) of root regeneration was observed on ½ MS medium containing kinetin (2.0mg/L), NAA (1.5mg/L), and NaCl (100mM). Interestingly, rooting medium fortified with NaCl (100mM) was also proved to be effective for precocious induction of in vitro flowering (26.66±0.12%) in the regenerated plantlets. The regenerated plantlets were further transferred to plastic cup soil and acclimatized under greenhouse conditions. The result indicated, salt-tolerant peanut cotyledon culture also shows In Vitro plantlet regeneration and flower formation.
Cotyledon, in vitro, flowering, regeneration, salinity, somatic embryos
Короткий адрес: https://sciup.org/143183440
IDR: 143183440
Текст научной статьи Somatic embryogenesis, plantlet regeneration and in vitro flowering from cotyledon culture in a legume oil crop, peanut (Arachis hypogaea L.) under NaCl-stress conditions
Abbreviations: 2,4-D-2,4-Dichlorophenoxyacetic acid; BAP-6-Benzylaminopurine; IAA-
Indole-3-acetic acid; NAA-1-Naphthaleneacetic acid; NaCl-Sodium Chloride.
Arachis hypogaea L. (peanut or groundnut) is the most important oilseed legume crop, native to South America. It contains oil (44-56%), protein (22-30%) and also is a source of P, Ca, Mg, Vitamin E, Vitamin , and Vitamin B2. It is consumed as oilseed crop in the region of warmed condition and also used for industrial purpose (Cheng et al., 1992). Peanut oil is also consumed for digestible purpose because it contains high quantity of certain biological active compounds such as tocopherols, flavonoids, and phytosterols (Tuberoso et al ., 2007).
During recent past, irreversible increase in salt concentrations in soil or irrigation water has proved as a major threat to agricultural production in arid and semi-arid regions. Higher concentrations of salts in soil cause water deficit conditions leading to accumulation of Na+ and Cl- ions which have detrimental impacts on food crops including peanuts. Moreover, high salt content in soil or irrigating water causes a drastic reduction in crop yield by inhibiting seed germination, seedling growth, flowering, and fruit set (Sairam and Tyagi, 2004). Remarkably, peanut crop is known as susceptible crop to salinity, and therefore, productivity is severely affected (Meena et al ., 2012).
Although, it has been possible to develop salt resistance against stress via breeding of salt-tolerant plants (Witcombe et al ., 2008). However, plants tolerant to salinity can be also obtained through cell and tissue culture techniques by two approaches either through selection of mutant cell lines or screening of germplasm of the plant for salt tolerance under in vitro conditions .
Significantly, susceptible plants are known to confine the uptake of salts in plant and adjust their cellular osmotic pressures by the synthesis of compatible solutes or signalling molecules such as proline, glycine betaine, and sugars whereas the tolerant plants have been identified to accumulate salts in cell vacuoles for regulating salt concentrations in the cytosol as well as ensuing maintenance of high cytosolic +/Na+ ratio in plant cell ( rishna et al ., 2015).
The development of suitable protocols for plant regeneration has been suggested as one of the main prerequisites for the genetic improvement of crop plants using biotechnological methods. In order to achieve stable genetic transformation in plants, plants regenerated through somatic embryogenesis has been considered as more useful than plants obtained through organogenesis (Bhanumati et al., 2005).
However, literature also reveals plantlet regeneration via somatic embryogenesis from immature cotyledon explants (Baker and Wetzstein, 1994; im et al. , 2004), cotyledon (Maina et al., 2010), cotyledonary nodes (Limbua et al ., 2019; Swathy Anuradha et al ., 2006), de– embryonated cotyledon (Hoa et al ., 2021), immature zygotic embryos (Jayabalan et al ., 2004), immature embryonic axes (Eapen and George, 1993a), mature embryo axes (Baker et al ., 1995), leaflets (Chengalrayan et al ., 2001), epicotyls (Little et al ., 2000) and embryo axes, mature whole seed (Radhakrishnan et al ., 2000) could be possible in peanut tissue culture.
Additionally, various growth regulators have been also used for somatic embryogenesis such as 2,4-D (Eapen and George, 1993; Baker et al ., 1995; Roja Rani and Padmaja, 2005) and picloram (Little et al ., 2000). Significantly, present study is aimed for establishing a stable and efficient protocol for differentiation of somatic embryos from the peanut cotyledon explant tissues through callus formation that could be useful for handling gene transfer experiments in peanut and also from one species to others.
Although peanut has been studied extensively for regeneration under in vitro conditions, however, none of the existing regeneration protocols is established under salinity stress conditions. Moreover, the productivity and nutritional value of this crop can be improved by developing cultivars tolerant to salinity stresses in order to grow in saline soil/water conditions and also to maintain protein and oil quality/quantity in peanut. Therefore, present study was undertaken to establish somatic embryogenesis followed by plantlet regeneration and precocious in vitro flowering under NaCl-salinity stress conditions to obtain salinity tolerant peanut plants.
MATERIALS AND METHODS
Seed Collection and Sterilization Procedure: Mature and dry peanut seeds (cv. TMV13) were collected from Tamil Nadu Agriculture University (TNAU) Coimbatore, India. To begin with, healthy peanut seeds were initially surface sterilized with detergent (Tween-20) for 5-7 mins. Properly washed peanut seeds were further treated with ethanol (70%) for 45-60 seconds followed by HgCl2 (0.1%) treatment for 7-10mins. Finally, treated peanut seeds were washed with sterile distilled water (SDW) for 4-5 times.
Nutrient Medium Formulation : MS (Murashige and Skoog, 1962) medium supplemented with sucrose (3%) as carbon source and further agar (0.8%) was used to solidify the medium. Different growth hormones (auxin/cytokinin) were used either alone or in combinations for achieving somatic embryogenesis and plantlets regeneration. Moreover, pH of the nutrient medium was also adjusted to 5.5-5.8 and the nutrient medium was finally sterilized under 121ºC at 15 psi for 15 minutes.
Explant preparation and culture : The sterilized peanut seeds were placed on a sterile filter paper kept in sterile petridish. The seed coats and the embryos from sterilized peanut seeds were also removed without damaging the cotyledons. The cotyledon explants were then inoculated on the MS-medium for embryogenic callus induction. The cultures were incubated at 16h light and 8h dark.
Embryogenesis induction medium : Nutrient medium was containing MS-salts with sucrose (3%), agar (0.8%) and various concentrations (5mg/L, 10mg/L, 15mg/L, 20mg/L, 25mg/L, 30mg/L, and 40mg/L) of 2,4-Dichlorophenoxyacetic acid (2,4-D) for induction of callus and somatic embryogenesis.
Somatic embryo germination or shoot regeneration medium : Induced embryogenic callus was sub-cultured on MS-medium supplemented with various concentrations (0.5mg/L, 1.0mg/L, 1.5mg/L, and 2.0mg/L) of 6-benzylaminopurine (BAP) in combination with (1.5mg/L) of indole-3-acetic acid (IAA) for somatic embryo germination leading to shoot-regeneration.
Root induction medium: Regenerated shoots from the germinated somatic embryos were then transferred on ½-MS-medium with various concentrations of kinetin (1.0mg/L, 2.0mg/L, 3.0mg/L, and 5.0mg/L) in combination with (1.5mg/L) of α-naphthaleneacetic acid (NAA) for root induction and complete plantlets formation.
Salinity Stress Treatments : During this study, MS-nutrient medium was supplemented with various concentrations of NaCl (10mM, 25mM, 50mM, 75mM, 100mM, 150mM, and 200mM) salt along with 2,4-D (10mg/L) for induction of embryogenic callus and differentiation of somatic embryos. Further, for the germination of somatic embryo and shoot regeneration, embryogenic calli were transferred to MS-medium supplemented with BAP (0.5mg/L, 1.0mg/L, 1.5mg/L, and 2.0mg/L), IAA (1.5mg/L) and NaCl (100mM)-salt.
Furthermore, for root regeneration, salt-tolerant regenerated shoots were further transferred to ½-MS-medium supplemented with kinetin (1.0mg/L, 2.0mg/L, 3.0mg/L, and 5.0mg/L) in combination with NAA (1.5mg/L) and NaCl (100mM)-salt.
In Vitro Flowering : Regenerated salt-tolerant shoots were transferred to ½-MS-rooting medium supplemented with kinetin (1.0mg/L, 2.0mg/L, 3.0mg/L, and 5.0mg/L) in combination with (1.5mg/L) of NAA and NaCl (100mM)-salt.
Transplantation to Soil : The regenerated plantlets with complete root and shoot raised during cotyledon cultures were transferred and gradually acclimatized in plastic cups containing sterilized mixture of red soil (20%), vermiculite (40%), and compost (40%) for hardening under greenhouse conditions.
Statistical Analysis
A minimum of 25-30 explants were used for each treatment and each experiment was repeated at three times. The mean frequency of explants showing somatic embryogenesis, somatic embryos showing shoot regeneration, regenerated shoots showing root formation, and frequency of plantlets exhibit precocious flowering was recorded and calculated. The data were analyzed based on SPSS software.
RESULTS
Induction of Callus and Somatic embryogenesis
Significantly, frequency of embryogenic callus induction was recorded as the low (35.0±0.13%) in cotyledon explants that were treated with the very low concentration (5mg/L) of 2,4-D while further increased 2,4-D concentration (10mg/L) was proved to enhance frequency of somatic embryogenesis and it was obtained as the maximum (60.0±2.11%) and also the maximum number (5.84±0.04) of somatic embryos per embryogenic callus ( Table-1 ). However, very high concentration (40mg/L) of 2,4-D proves to be strongly inhibitory and frequency of somatic embryogenesis (20.3±0.11%) and also number of somatic embryos per embryogenic callus was found to be the minimum (1.09±0.005).
Effects of NaCl-Salt Stress on Somatic Embryogenesis
In comparison to control-treatment as MS-medium with 2,4-D (10mg/L) and without NaCl-salt, nutrient medium with NaCl-salt was found to be influencing factor during induction and development of somatic embryo from cotyledon culture. Moreover, with the increase in NaCl-salt concentrations in MS-medium in presence of 2,4-D (10mg/L), the frequency of somatic embryogenesis and number of somatic embryos/explants was found to gradually decline.
After 3-weeks of culture initiation in cotyledon explants that were growing in MS-medium supplemented with various concentrations (10mM, 25mM, 50mM, 75mM, 100mM, 150mM, and 200mM) of
NaCl with 2,4-D (10mg/L), induction and development of somatic embryos could be seen in various shape, morphology, and texture ( FIG. 1E-I ) indicating the morpho-regulatory response of salinity stress on somatic embryogenesis. However, lower concentrations of NaCl (10mM, 25mM, and 50mM), differentiated somatic embryos were found to be normal and with well-defined typical structures ( FIG. 1E, F, & G ) respectively.
Significantly, cotyledons that were treated with 50mM of NaCl along with 2,4-D (10mg/L), differentiated somatic embryos were found to be normal in growth and morphological appearance ( FIG. 1G ). However, cotyledon explants growing with higher concentrations of NaCl (100mM), number of differentiated somatic embryos were limited and also appeared to be abnormal in shape and suppressed in growth ( FIG. 1H ) at the end of 3rd week of culture initiation. Interestingly, cotyledon explants that were treated with very high concentration 150mM of NaCl along with 2,4-D (10mg/L), establishment of somatic embryogenesis was remarkably inhibited and somatic embryos were observed to be morphologically altered ( FIG. 1I ).
Moreover, the least frequency of somatic embryogenesis (17.1±1.03%) and minimum number of somatic embryos/embryogenic callus (2.6±0.5) was recorded in case of cotyledon explants that were treated with the 150mM of NaCl salt-added nutrient medium while the low dose of NaCl (10mM) treatment was turned out to be poorly inhibitory (75.0±2.69%/17.5±1.2) in terms of both frequency of somatic embryogenesis and number of somatic embryos/explants respectively. Further, very high concentration of NaCl (200mM) was proved as completely toxic and lethal for somatic embryogenesis ( Table-1 ).
Germination of Somatic Embryos and Shoot Regeneration
Embryogenic callus induced on 2,4-D supplemented nutrient medium was further transferred to MS-basal medium and also on MS- medium containing various concentrations (0.5mg/L, 1.0mg/L, 1.5mg/L, and 2.0mg/L) of BAP along with IAA (1.5mg/L) in order to induce the germination of somatic embryos followed by shoot regeneration. Significantly, embryogenic callus transferred on BAP (1.5mg/L) along with IAA (1.5mg/L) medium was turned out to be the best nutrient medium for germination of somatic embryos and shoot regeneration (FIG. 2A).
Moreover, percentage of embryogenic callus showing somatic embryo germination or shoot regeneration and also number of regenerated shoots per embryogenic callus was recorded as maximum (84±1.7%) and (3.98±0.2) respectively. However, further higher concentration of BAP (2.0mg/L) along with IAA (1.5mg/L) was proved relatively inhibitory for both somatic embryo germination and shoot regeneration.
Therefore, percentage of embryogenic callus with regenerated shoots and also number of regenerated shoots per embryogenic callus was recorded as maximum (66±0.2%) and (2.60±0.03) respectively. Moreover, other lower concentrations of BAP (0.5mg/L and 1.0mg/L) with IAA (1.5mg/L) were proved to be equally good for somatic embryo germination and shoot regeneration from embryogenic callus ( Table-2 ).
Root Induction on Regenerated Shoots
Regenerated shoots from embryogenic callus growing on various concentrations of BAP (0.5mg/L, 1.0mg/L, 1.5mg/L, and 2.0mg/L) supplemented regeneration nutrient media were transferred to root induction ½-MS-medium containing various concentrations of kinetin (1.0mg/L, 2.0mg/L, 3.0mg/L, and 5.0mg/L) along with NAA (1.5mg/L). Significantly, kinetin (2.0mg/L) along with NAA (1.5mg/L) was emerged as the best root induction medium and exhibited the maximum response.
Furthermore, an increase of BAP (1.0mg/L) in regeneration medium added with IAA (1.5mg/L) and NaCl (100mM), germination of somatic embryos and shoot regeneration was more apparent on regeneration medium ( FIG. 2C ) and frequency of embryogenic callus showing shoot regeneration and number of regenerated shoots/embryogenic callus (58±0.1%/2.69±0.07) respectively ( Table-2 .).
Significantly, further higher concentration of BAP (1.5mg/L) with IAA (1.5mg/L) and NaCl (100mM) was proved to be the best and optimal nutrient medium for somatic embryo germination and shoot regeneration
( FIG. 2D & E ) and hence, maximum frequency of shoot regeneration (70.2±0.59%) and also number of regenerated shoots (2.76±0.5) per embryogenic callus was obtained with this nutrient combination.
Moreover, during present study, very high concentration of BAP (2.0mg/L), frequency (55.6±1.5%) of rooting in regenerated shoots was observed ( Table-3 ). While other concentrations of kinetin (1.0mg/L, 3.0mg/L and 5.0mg/L) along with NAA (1.5mg/L) were proved to be less efficient for root induction in regenerated shoots. Moreover, in all tested concentrations of kinetin in present study, kinetin (5.0mg/L) along with NAA (1.5mg/L) was turned out to be the least effective (37.2±0.05%) for root initiation in regenerated shoots ( Table-3 ).
Effects of Salinity Stress on Somatic embryo germination/Shoot regeneration
Once somatic embryos were differentiated on various concentrations of NaCl (10mM, 25mM, 50mM, 75mM, 100mM, and 150mM) supplemented nutrient media, embryogenic callus was transferred to the germination and shoot regeneration MS-medium added with various concentrations (0.5mg/L, 1.0mg/L, 1.5mg/L, and 2.0mg/L) of BAP in combination with 1.5 mg/L of IAA and 100mM of NaCl-salt.
Somatic embryos differentiated in the embryogenic callus could show immediate germination after transferring on regeneration medium containing very low concentration of BAP (0.5mg/L) along with IAA (1.5mg/L) and NaCl (100mM). Moreover, various stages of developing somatic embryos were apparent after one-week of transfer on regeneration medium ( FIG. 2B ). However, frequency of embryogenic callus showing shoot regeneration was recorded as the minimum (44±0.1%) with 0.5mg/L of BAP treatments and number of regenerated shoots per embryogenic callus was obtained as (2.24±0.01).
Moreover, BAP (2.0mg/L) along with IAA (1.5mg/L) and NaCl (100mM) was turned out to be relatively inhibitory for somatic embryo germination and shoot regeneration. Germinated somatic embryos were apparent morphologically aberrant and inhibited (FIG. 2F). This could be due to either presence of high BAP or salinity stress caused by NaCl present in the regeneration medium.
However, frequency of regenerated shoots per embryogenic callus was found to be high (60.3±0.15%) but interestingly, the number of regenerated shoots/embryogenic callus was obtained as the minimum (2.09±0.01) ( Table-2 ). Results indicate that high concentration of BAP (2.0mg/L) proves to be slightly inhibitory for somatic embryo germination and shoot regeneration from embryogenic callus.
Effects of Salinity Stress on Root Development in Regenerated Shoot
During evaluation of NaCl- salinity stress effects on root induction in regenerated shoots grown on various concentrations of BAP (0.5mg/L, 1.0mg/L, 1.5mg/L, and 2.0mg/L), regenerated shoots were transferred to root induction ½-MS-medium containing various concentrations of kinetin (1.0mg/L, 2.0mg/L, 3.0mg/L, and 5.0mg/L) along with NAA (1.5mg/L) and NaCl (100mM).
Significantly, kinetin (2.0mg/L) along with NAA (1.5mg/L) and NaCl (100mM) was proved as the most suitable nutrient medium for root induction efficiently and exhibited the maximum frequency (51±1.07%) of rooting in regenerated shoots while high concentration (5.0mg/L) of kinetin along with NAA (1.5mg/L) and NaCl (100mM) was turned out to be the least effective (29.9±0.1%) for root initiation in regenerated shoots ( Table-3 ).
Results indicate that optimal kinetin concentration (2.0mg/L) along with NAA (1.5mg/L) supports maximum potential of root induction in regenerated shoots even under salinity stress conditions and rood initiation could be observed after 67-days of culture initiation (FIG. 2G). However, same 100mM of NaCl plays an inhibitory role during root initiation in regenerated shoots when high kinetin (5.0mg/L) with NAA (1.5mg/L) is present in ½-MS-nutrient medium.
In Vitro Precocious Flowering
Significantly, root induction ½-MS medium containing kinetin (2.0mg/L) with NAA (1.5mg/L) and NaCl (100mM) could be also effective for precocious flower bud induction followed by development of flower in regenerated shoots and thus, regenerated complete plantlets with well-developed roots and shoots could be seen with fully developed flower ( FIG. 2H ) after 11th week of culture initiation. Furthermore, the maximum frequency (26.66±0.12%) of in vitro flowering was recorded in regenerated shoots with induced roots that were growing in nutrient medium containing kinetin (2.0mg/L), NAA (1.5mg/L) and NaCl (100mM).
However, flowering was completely lacking in regenerated shoots which were growing with higher concentration of kinetin (1.0mg/L and 5.0mg/L) in presence of NaCl (100mM). In contrast, nutrient medium containing lower concentration of kinetin (1.0mg/L) with NAA (1.5mg/L) and NaCl (100mM) was proved as poorly effective (6.7%±0.06) for in vitro flowering ( Table-3 ). Significantly, during present study, regenerated shoots growing on ½-MS-medium fortified with various concentrations of kinetin (1.0mg/L, 2.0mg/L, 3.0mg/L, and 5.0mg/L) along with NAA (1.5mg/L) and without NaCl-salt, flowering symptoms were completely lacking.
Table-1. Peanut (Arachis hypogaea L.); Cotyledon culture- effects of various concentrations of 2,4-D and NaCl on percentage of somatic embryogenesis and number of somatic embryos per embryogenic callus growing on MS-nutrient medium supplemented with or without 2,4-D and NaCl.
|
Concentration of 2,4-D (mg/L) |
% of Embryoge-nic Callus Formation (Mean±SE) |
No. of Somatic Embryos/Embryoge-nic Callus (Mean±SD) |
Concentration of NaCl (mM) with 2,4-D (10mg/L) |
% of Embryogenic Callus Formation (Mean±SE) |
No. of Somatic Embryos/Embryo genic Callus (Mean±SD) |
|
0 |
0 |
0 |
0 |
0 |
0 |
|
5 |
35.0±0.13 |
3.21±0.05 |
10 |
75.0±2.69 |
17.5±1.2 |
|
10 |
60.0±2.11 |
5.84±1.04 |
25 |
63.2±1.6 |
11.3±0.5 |
|
15 |
56.1±1.62 |
4.30±0.15 |
50 |
47.4±1.12 |
9.1±1.11 |
|
20 |
53.3±1.44 |
3.69±0.10 |
75 |
40.7±1.14 |
7.2±0.2 |
|
25 |
36.7±0.68 |
2.19±0.03 |
100 |
35.1±0.23 |
3.2±0.1 |
|
30 |
31.5± 0.26 |
2.11±0.01 |
150 |
17.1±0.16 |
2.6±0.5 |
|
40 |
20.3±0.11 |
1.09±0.005 |
200 |
0 |
0 |
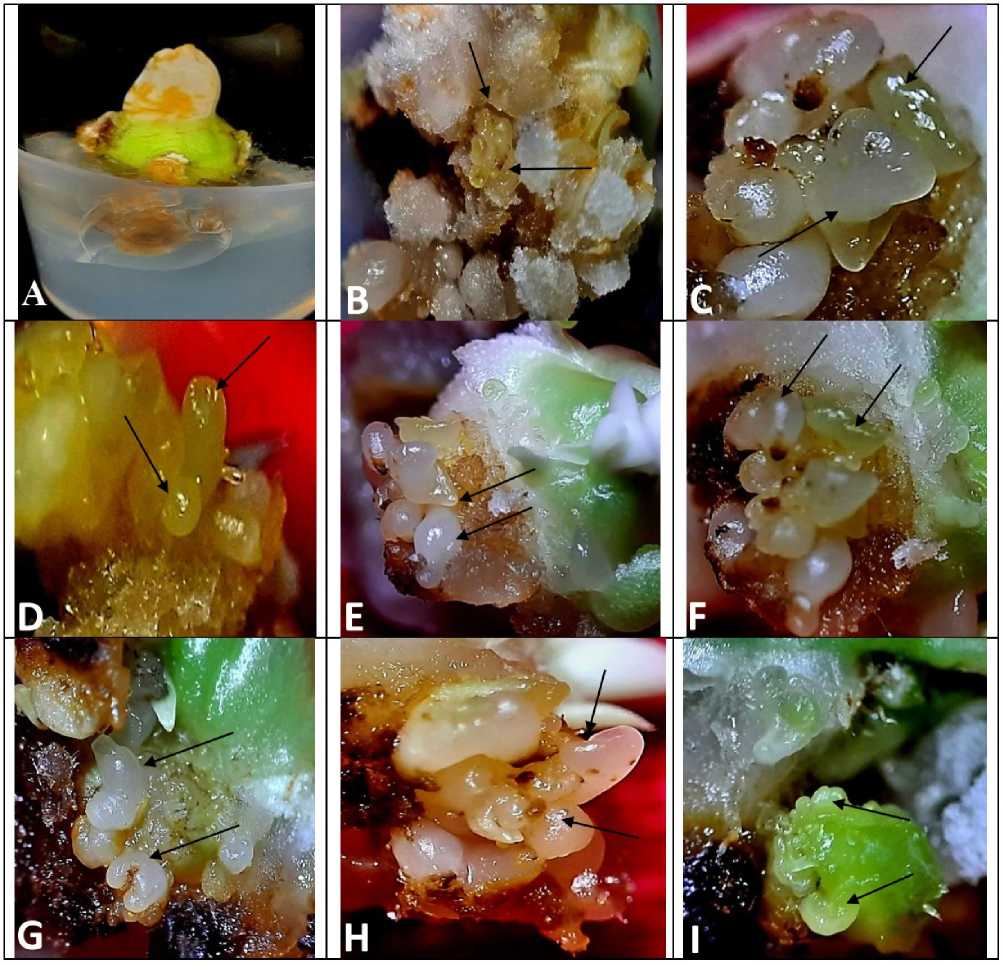
Figure 1. Peanut ( Arachis hypogaea L.); Cotyledon culture - effects of NaCl-salt stress on callus induction and somatic embryogenesis- (A) Cotyledon explant tissue shows slight proliferation followed by gradual necrosis on MS-basal medium (B) explant tissues shows profuse friable callus formation followed by differentiation of somatic embryos (arrow-marked) on MS- medium supplemented with 5mg/L of 2,4-D (C) Differentiation of well-defined somatic embryos at various stages (arrow-marked) on induced callus treated with 10mg/L of 2,4-D (D) Somatic embryogenesis (arrow-marked) on MS- medium supplemented with 15mg/L of 2,4-D (E) Induction of embryogenic (arrow-marked) callus in MS-medium supplemented with 2,4-D (10mg/L) and 10mM of NaCl (F) Somatic embryogenesis (arrow-marked) in induced callus on MS- medium fortified with 2,4-D (10mg/L) and 25mM of NaCl (G) Induction of embryogenic callus on 2,4-D (10mg/L) and 50mM of NaCl (H) Formation of embryogenic callus followed by browning of callus on MS- medium fortified with 2,4-D (10mg/L) and 100mM of NaCl (I) Somatic embryogenesis and gradual necrosis of induced callus on MS-medium with 2,4-D (10mg/L) and 150mM of NaCl-treatments (After 2-3 weeks of culture initiation).

Figure 2. Peanut ( Arachis hypogaea L.); Cotyledon culture - effects of NaCI- salt stress on somatic embryo germination and plantlets regeneration- (A) Culture shows somatic embryo germination followed by plantlets regeneration on MS-medium fortified with BAP (1.5mg/L) and IAA (1.5mg/L) without NaCl- salt (b) MS-medium with BAP (0.5mg/L), IAA (1.5mg/L) and NaCl (100mg/L) showing differentiation of typical somatic embryos and initiation of somatic embryo germination- after 1st week of transfer (C) Somatic embryo germination on MS-medium with BAP (1.0mg/L), IAA (1.5mg/L) and NaCl (100mg/L)-After 3rd week of culture initiation (D) MS-medium with BAP (1.5mg/L), IAA (1.5mg/L) and NaCl (100mg/L) shows somatic embryo germination (E) MS-medium with BAP (1.5mg/L), IAA (1.5mg/L) and NaCl (100mg/L) showing plantlets regeneration-After 5–6th week of culture (F) MS-medium with BAP (2.0mg/L), IAA (1.5mg/L) and NaCl (100mg/L) showing inhibitions in somatic embryo germination- After 7th week of culture initiation (G) Root induction in regenerated shoot on %-MS-medium supplemented with kinetin (2.0mg/L), NAA (1.5mg/L) and NaCl (100mM)-After 9th week of culture initiation (h) In Vitro precocious flowering in regenerated shoot on %-MS-medium with kinetin (2.0mg/L), NAA (1.5mg/L) and NaCl (100mM)-After 11th week of culture initiation (I) Acclimatization of in vitro raised plantlet in plastic cup soil (After 13th week of culture initiation).
Table 2. Peanut ( Arachis hypogaea L.); Cotyledon culture – effects of various concentrations of BAP on percentage of embryogenic callus showing shoot regeneration and number of regenerated shoots/embryogenic callus on MS-medium supplemented with or without NaCl-salt (100mM) and IAA (1.5mg/L).
|
Concentration of BAP (mg/L) with IAA (1.5mg/L) |
% of Embryogenic Callus showing Shoot Regeneration (Mean±SE) |
No. of Regenerated Shoots/ Embryogenic Callus (Mean±SD) |
Concentration of BAP (mg/L) with IAA (1.5mg/L) and NaCl (100mM) |
% of Embryogenic Callus showing Shoot Regeneration (Mean±SE) |
No. of Regenerated Shoots/ Embryogenic Callus (Mean±SD) |
|
Control-BM |
0 |
0 |
Control-BM |
0 |
0 |
|
0.5 |
68.1±0.04 |
2.4±0.09 |
0.5 |
44±0.1 |
2.24±0.01 |
|
1.0 |
75.2±0.13 |
3.45±0.15 |
1.0 |
58±0.1 |
2.69±0.07 |
|
1.5 |
84±1.7 |
3.98±0.2 |
1.5 |
70.2±0.59 |
2.76±0.5 |
|
2.0 |
66±0.2 |
2.60±0.03 |
2.0 |
60.3±0.15 |
2.09±0.01 |
Table 3 .Peanut ( Arachis hypogaea L.); Cotyledon culture – effects of various concentrations of kinetin on percentage of regenerated shoots showing root regeneration on ½-MS-medium supplemented with or without NaCl-salt (100mM) and NAA (1.5mg/L).
Transplantation of Regenerants
In vitro regenerated peanut plantlets during cotyledon culture were brought under gradual hardening and acclimatization procedures in growth chamber. 13th week-old regenerated plantlets with root and shoot were further transferred in plastic cup containing sterilized mixture of red soil (20%), vermiculite (40%), and compost (40%) for hardening under greenhouse conditions FIG. 2I ). These plantlets were found to grow successfully in plastic cup conditions and further were transferred to garden soil.
DISCUSSION
Almost all physiological processes like respiration, photosynthesis, nitrogen fixation and other metabolic and enzymatic processes are known to be affected by soil salinity and cause stunted growth leading to a significant reduction in crop productivity (Shabala and Munns, 2012; Acosta-Motos et al., 2017). Soil salinity, usually NaCl, may also reduce plant growth by ion known as susceptible to salinity and other stresses resulting the severe loss in productivity (Meena et al., 2012).
As it is suggested that peanut cultivars tolerant to salinity are not available today (Pal et al ., 2021). Hence, results of present study reveal the establishment of somatic embryogenesis from cotyledon tissues under salinity conditions and also regeneration of salt tolerant plantlets in peanut legume oil crop.
Induction and Development of Somatic embryos
Peanut regeneration through somatic embryogenesis has significantly increased by using different explants. Regeneration via somatic embryogenesis from immature cotyledon explants (Baker et al., 1994), immature zygotic embryos (Jayabalan et al., 2004), immature embryonic axes (Eapen and George, 1993a), mature embryo axes (Baker et al., 1995), leaflets (Chengalrayan et al., 2001), epicotyls (Little et al., 2000), and young leaflets from aseptically germinated embryo axes (Baker and Wetzstein, 1992) has been reported in peanut (Bhanumati et al., 2005).
During this study, the somatic embryogenesis and plantlets regeneration have been achieved in peanut (cv TMV13) by using mature cotyledon explant. After 17th day of the cotyledon culture, the maximum frequency (60.0±2.11%) of embryogenic callus and also number of somatic embryos (5.84±1.04) per embryogenic callus was recorded in cotyledon explants that were growing in MS- medium fortified with low concentration of 2,4-D (10mg/L) while high concentration (40mg/L) of 2,4-D was turned out to be strongly inhibitory and proved as reasonably inefficient for somatic embryogenesis (20.3±0.11%). Moreover, present study is also in conformity with earlier study in peanut immature embryo culture where somatic embryogenesis could be initiated in the callus induction medium (Hazra et al ., 1989).
However, in contrast previous study indicates that mature cotyledon explant responded high frequency of somatic embryo formation with the high concentration of 2,4-D (40mg/L) within 30th days of culture (Richard et al ., 1992) while another study suggests that the high number of somatic embryo formation could be possible with the concentration of NAA (1.34µM) and BAP (13.2µM) (Chand et al ., 2002). Furthermore, MS medium with high concentration of 2,4-D was proved to be supportive for somatic embryo formation (Lee and Soh, 1993).
Similarly, normal somatic embryos formation could be observed with the higher level of 2,4-D concentrations (Levi and Sink, 1991). However, cotyledon explants could be effective only for Bulbil-like body (BLB) induction on MS-medium supplemented with various concentrations of 2,4-D (10mg/L, 20mg/L, 30mg/L, and 40mg/L) in comparison to the other explants such as root, stem, leaf and hypocotyls ( edong et al ., 2016).
Additionally, the presence of picloram (15mg/L– 35mg/L) in MS-medium supports plantlet regenerations via somatic embryogenesis (Jayabalan et al., 2009). The maximum number of somatic embryos per explant and also the maximum number of epicotyl explants showing somatic embryogenesis was observed in the concentration range (83.0 and 124.4µM) of picloram (Little et al., 2000).
Germination of Somatic embryos and Plantlets Regeneration
Traditionally, various explants have been used for shoot regeneration (Bhatnagar et al ., 2010; Srinivasan et al ., 2010). Since cotyledon explant is a natural source of nutrition to the developing embryo, so in comparison to other standard explants, such as, leaf, stem, and root culture, it has been proved to be the most potent tissue for in vitro regeneration (Matand et al ., 2013).
Moreover, shoot initiation from the somatic embryos could be possible in the regeneration medium (Roja Rani and Padmaja, 2005) instead of transferring of callus from callus induction medium to embryo induction medium. During another study, embryonic callus from root explants was induced by 2,4-D and kinetin followed by maturation with gibberellin and abscisic acid leading to plantlets regeneration on hormone free half-strength MS-medium (Pathak et al ., 2012).
Significantly, during present study on mature cotyledon culture in peanut cv. TMV13, maximum frequency (84±1.7%) of shoot regeneration could be obtained on MS-regeneration medium containing low concentration of BAP (1.5mg/L) in combination with IAA (1.5mg/L) but in contrast, higher concentrations of BAP (3.0mg/L,4.0mg/L, 5.0mg/L, and 6.0mg/L) in combination with TDZ (1.0mg/L) were found to be suitable for high regeneration frequency (80-98%) in genotypes- CG7, ICGV12991 and Red Valencia (Aman et al ., 2009; Limbua et al ., 2019).
Moreover, other study reports for achieving the multiple shoot formation from somatic embryos on medium supplemented with BAP (8.9µM) and kinetin (14µM) (Chengalrayan et al ., 1997). During this study, in order to induce root formation or complete plantlet regeneration, shoots that were regenerated on various concentrations (0.5mg/L, 1.0mg/L, 1.5mg/L, and 2.0mg/L) of BAP with IAA (1.5mg/L) containing MS-nutrient media were transferred to ½-MS-root induction medium containing various concentrations of kinetin (1.0mg/L, 2.0mg/L, 3.0mg/L, and 5.0mg/L) along with
NAA (1.5mg/L). Results indicate that maximum frequency (55.6±1.5%) of regenerated shoots could show root initiation on ½-MS-medium supplemented with kinetin (2.0mg/L) along with NAA (1.5mg/L).
Previous study indicates the high frequency (93.3%) of plantlets regeneration could be possible from the cultures growing in medium supplemented with the NAA(1.34µM) in combination with various concentrations (2.2µM-8.8µM) of BAP while mean number of plantlet regeneration (15.4±0.88) was obtained with the medium supplemented with NAA (1.34µM) and BAP (4.4µM) (Chand et al ., 2002).
Effects of Salinity Stress on Somatic embryogenesis and Plantlets Regeneration
It is suggested that only the explants that are capable of tolerating induced stressful in vitro conditions can express embryogenic phenomenon (Rai et al ., 2011). Somatic embryos that were developed under salinity conditions in different hormones-containing medium, showed better germination in the presence of NaCl as compared with those developed on medium without NaCl (Rai et al ., 2010)
In contrast, present study indicates that salinity stress affects negatively on both somatic embryogenesis and plantlets regeneration. Cotyledon tissues growing in control conditions without NaCl-medium but supplemented with 2,4-D exhibited, normal embryogenic callus formation while explants cultured on NaCl-supplemented medium, showed gradual reduction in embryogenic callus formation and moreover, higher concentrations of NaCl (150mM and 200mM) were proved to be lethal and caused salinity stress inhibitions in terms of induction and development of somatic embryos.
In this study, reduced frequency of somatic embryogenesis (17.1±0.16%) and also number of somatic embryos per callus (2.6±0.5) was obtained from the concentration of 2,4-D (10mg/L) in presence of 150mM of NaCl-stressed condition. Significantly, 200mM of NaCl was turned out to be lethal and toxic for somatic embryogenesis. Moreover, other study reports that MS-medium supplemented with ABA and BAP combination gives best shoot regeneration from the cotyledon explants aviraj et al ., 2006).
Significantly, well-developed somatic embryos on transfer to MS-medium supplemented with gibberellic acid, indole-3-butyric acid, myo -inositol, and riboflavin exhibit best shoot production. During this study, maximum shoot-regeneration (70.2±0.59%) and also maximum number (2.76±0.5) of regenerated shoots per embryogenic callus was achieved on transfer of embryogenic callus grown on 2,4-D supplemented medium to shoot regeneration medium containing BAP (1.5mg/L) along with IAA (1.5mg/L) and NaCl (100mM).
Generally, IBA is known as a greater ability to promote rooting with less callus formation compared with other types of auxins (Drew et al ., 1993) and root regeneration in recent study was achieved on MS-medium containing ½ MS -salt with 2.0mg/L of IBA (Baker Al-Shara et al ., 2020)
However, during this study, roots were obtained in the well-developed regenerated shoots that were developed on medium supplemented with BAP (1.5mg/L), IAA (1.5mg/L) and NaCl (100mM). Thus, the maximum percentage (51±1.07%) of regenerated shoots could show root formation on ½- MS-medium fortified with (2.0mg/L) of kinetin in combination with NAA (1.5mg/L) and NaCl (100mM). However, other studies reveal that roots were induced from shoots that were treated with the concentration (5.4µM) of NAA (Roja Rani and Padmaja, 2005). It is moreover, suggested that NAA stimulates root regeneration better than IBA (Hoa et al ., 2021).
In Vitro Flowering/ Transplantation of Regenerants
Interestingly, present study indicates that combination of hormones required for root induction; kinetin (2.0mg/L) and NAA (1.5mg/L) with NaCl (100mM) in ½- MS medium was also proved to be efficient enough to induce precocious bud development followed by complete flowering (26.66±0.12%) after 11-weeks of culture initiation. However, higher concentrations of kinetin (3.0mg/L and beyond) in presence of NaCl (100mM) were turned out to be ineffective for flower induction in regenerated plantlets. However, other report reveals that lower concentrations of IAA and NAA in combination with cytokinins could be proved effective for flower bud induction Narasimhulu and Reddy 1984). Moreover, in recent study (Baghel and Bansal, 2014) on Guizotia abyssinica Cass. a multipurpose oil crop, the regenerated shoots were found to show in vitro flowering on MS-medium contained with combination of BAP (0.1mg/L) and GA3 (0.1mg/L)
During present study, in vitro regenerated salt-tolerant peanut plantlets were gradually acclimatized in paper cups containing sterilized mixture of red soil (20%), vermiculite (40%), and compost (40%) for hardening under greenhouse conditions. Later, 13-week-old acclimatized plantlets were transferred to garden soil conditions. Thus, present study offers an establishment of efficient peanut regeneration protocol under salinity stress conditions leading to achievement of salt-tolerant peanut crop. Moreover, this study would be a meaningful step in the direction of salt-tolerant legume crops improvements in general and peanut legume oil crop in particular.
CONCLUSION
This study indicates that cotyledon explant tissue shows maximum potential of somatic embryogenesis with MS-medium in presence of 2,4-D (10mg/L) and NaCl (100mM). Further, these somatic embryos were found to show high frequency of salt- tolerant shoot regeneration on MS-medium fortified with BAP (1.5mg/L), IAA (1.5mg/L) and NaCl (100mM). Moreover, rooting in regenerated shoots could be achieved on ½-MS with kinetin (2.0mg/L) and NAA (1.5mg/L) in presence of NaCl (100mM). Significantly, root induction medium in presence of NaCl (100mM) could be proved effective for precocious in vitro flowering in peanut. Later, in vitro raised salt-tolerant peanut plantlets were gradually acclimatized in paper cup under greenhouse followed by transfer to garden soil.
ACKNOWLEDGEMENTS
Список литературы Somatic embryogenesis, plantlet regeneration and in vitro flowering from cotyledon culture in a legume oil crop, peanut (Arachis hypogaea L.) under NaCl-stress conditions
- Acosta-Motos J.R., Ortuno M.F., Bernal-Vicente A., Diaz-Vivancos P., Sanchez-Blanco M.J. (2017) Plant Responses to Salt Stress. Adaptive Mechanisms. Agronomy, 7(1),18.
- Aman V., Malik C.P., Gupta V.K., Sinsinwar Y.K. (2009) Response of groundnut genotypes to plant growth regulator (BAP) to induce direct organogenesis. World Journal of Agricultural, Sciences, 5, 313-317.
- Baker C.M., Wetzstein H.Y. (1992) Somatic embryogenesis and plant regeneration from leaflets of peanut, Arachis hypogaea L., Plant Cell Rep., 11, 71-75.
- Baker C.M., Durham R.E., Burns R.A., Parrott W.A., Wetzstein H.Y. (1995) High frequency somatic embryogenesis in peanut (Arachis hypogaea L.) using mature dry seed, Plant Cell Rep., 15, 38- 42.
- Baker C.M., Wetzstein H.Y. (1994) Influence of auxin type and concentration on peanut somatic embryogenesis, Plant Cell Tissue Org. Cult., 36, 361-368.
- Baghel S., Bansal Y.K. (2014) Synergistic effect of BAP and GA3 on in vitro flowering of Guizotia abyssinica Cass.-A multipurpose oil crop, Physiol. Mol. Biol. Plants, 20(2), 241-247.
- Bhanumathi P., Ganesan M., Jayabalan N. (2005) A simple and improved protocol for direct and indirect somatic embryogenesis of peanut (Arachis hypogaea L.), Journal of Agricultural Technology, 1(2), 327-344.
- Baker A.S., Rosna M.T., Jamaludin M., Hashimah E., Asif K. (2020) Somatic Embryogenesis and Plantlet Regeneration in the Carica papaya L. cv. Eksotika, National center for Biotechnology information, 9(3), 360.
- Bhatnagar M., Prasad K., Bhatnagar-Mathur P., Narasu M.L., Waliyar F. (2010) An efficient method for the production of marker free transgenic plants of peanut (Arachis hypogaea L.), Plant cell Rep., 29, 495-502.
- Chand S., Sahrawat A.K. (2002) Somatic embryogenesis and plant regeneration from root segments of Psonalea corylifolia L. endangered medicinally important plant, In vitro Cellular and Developmental Biology -Plant, 38, 33-38.
- Cheng M., His D.C.H., Phillips G.C. (1992) In Vitro Regeneration of Valencia-type peanut (Arachis hypogaea L.) from cultured petioles, epicotyl section, and other seedling explants, Peanut Science, 19(2), 82-87.
- Chengalrayan K., Hazra S., Gallo Meagher M. (2001) Histological analysis of somatic embryogenesis and organogenesis induced from mature zygotic embryo derived leaflets of peanut (Arachis hypogaea L.), Plant Science, 161, 415-421.
- Chengalrayan K., Mhaske V.B., Hazra S. (1997) High frequency conversion of abnormal peanut somatic embryos, Plant Cell Rep., 16, 783-786.
- Drew R.A., McComb J.A., Considine J.A. (1993) Rhizogenesis and root-growth of Carica-papaya L. in vitro in relation to auxin sensitive phases and use of riboflavin, Plant Cell Tissue Organ Culture, 33, 17.
- Eapen S., George L. (1993) Somatic embryogenesis in peanut: influence of growth regulators and sugars, Plant Cell Tissue Organ Culture, 35, 151-156.
- Hazra S., Sathaye S.S., Mascarenhas A.F. (1989) Direct Somatic embryogenesis in peanut (Arachis hypogaea L.), Bio/Technology, 7, 949-951.
- Hoa P.T.B., Tue N.H., Trang P.T.Q., Hang L.T., Tien, N.Q.D., Loc N.H. (2021) An efficient protocol for in vitro regeneration of peanut (Arachis hypogaea L.) Cultivar l14, Journal Bioscience, 37. e37019 | ISSN 1981-3163.
- Jayabalan N., Anthony P., Davey M.R., Power J.B., Lowe K.C. (2004) Hemoglobin promotes somatic embryogenesis in peanut cultivars, Artificial cells Blood substitutes Biotechnology 32:147-157.
- Kaviraj C.P., Kiran G., Venugopal R.B., Kavi Kishor P.B., Rao S. (2006) Somatic embryogenesis and plant regeneration from cotyledonary explants of green gram [Vigna radiata (L) Wilezek]. -A recalcitrant grain legume, In Vitro Cellular Developmental Biology - Plant, 42, 134-138.
- Kim D.S., Lee I.S., Jang C.S., Hyun D.Y., Seo Y.W., Lee Y.I. (2004) Selection of cell 5-methyltryptophan resistant rice mutants from irradiated calli derived from embryos, Euphytica, 135, 9-19.
- Kedong X., Bingyan H., Kun L., Feiyan Q., Guangxuan T., Chengwei L., Xinyou Z. (2016) Peanut regeneration by somatic embryogenesis (SE), involving bulbil-like body (BLB), a new types of SE structure, Plant Cell Tissue Organ Culture, 125, 321328.
- Krishna G., Singh B.K., Kim E.K., Morya V.K., Ramteke P.W. (2015) Progress in genetic engineering of peanut (Arachis hypogaea L.)—A review, Plant biotechnology, 13(2), 147-162
- Lee K.S., Soh W.Y. (1993) Somatic embryogenesis and structural aberrancy of embryos in tissue cultures of Aralia cordata Thunb., Journal of plant Tissue Culture, 20, 77-83.
- Levi A., Sink K.C. (1991) Histology and morphology of asparagus somatic embryos, Hort. Sci., 26, 13221324.
- Limbbua P.G., Ngugi M.P., Oduor R.O. (2019) In Vitro Regeneration Protocol of Kenyan Adapted Groundnut (Arachis hypogaea L.) Genotypes using Cotyledonary Node Explants, Journal of Plant Biochemistry & Physiology, 7, 1.
- Little E.L., Magbanua Z.V., Parrot W.A.A. (2000) Protocol for repetitive Somatic embryogenesis from mature peanut epicotyls, Plant Cell Reports, 19, 351-357.
- Maina S.M., Quinata E., Kiran K.S., Simon T.G., Moses G. (2010) Surface sterilant effect on the regeneration efficiency from cotyledon explants of groundnut (Arachis hypogaea L.) genotypes adapted to Eastern and Southern Africa, African J. of Biotech., 9, 2866-2871.
- Matand K., Wu N., Wu H., Tucker E., Love K. (2013) More improved peanut (Arachis hypogaea L.) protocol for direct shoot organogenesis in mature dry - cotyledonary and root tissues, Journal of Biotech Research, 5, 24-34.
- Meena M.K., Singh A.K., Manibhushan, Upadhyaya A. (2012) Effect of Sulphur and Zinc on Rice Performance and Nutrient Dynamics in Plants and Soil of Indo Gangetic Plains, Journal of Agricultural Science, 4, 11.
- Murashige T., Skoog F. (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures, Physiol Plant, 15, 473-497.
- Narasimhulu S.B., Reddy G.M. (1984) In vitro flowering and Pod formation from cotyledons of groundnut (Arachis hypogaea L.), Theoretical and Applied Genetics, 69, 87-91.
- Radhakrishnan T., Murthy T.G.K., Chandran K., Bandyopadhyay A. (2000) Micropropagation in Peanut (Arachis hypogaea L.), Biologia Plantarum, 43(3), 447-450.
- Rai, M.K., Jaiswal V.S., Jaiswal U. (2010) Regeneration of plantlets of guava (Psidium guajava L.) from somatic embryos developed under salt- stress condition, Acta Physiologia Plantarum, 32, 10551062.
- Rai M.K., Shekhawat N.S., Harish, Gupta A.K., Phulwaria M., Ram K., Jaiswal U. (2011) The role of abscisic acid in plant tissue culture: a review of recent progress, Plant Cell Tissue Organ Culture, 106, 179-190.
- Richard E., Durham, Wayne A., Parrott, (1992) Repetitive somatic embryogenesis from peanut cultures in liquid medium, Plant Cell Reports, 11, 122-125.
- Pal K.K., Dey R., Sherathia D.N., Devidayal, Mangalassery S., Kumar A., Rupapara R.B., Mandaliya M., Rawal P., Bhadania R.A., Thomas M.,
- Patel M.B., Maida P., Nawade B.D., Ahmad S., Dash P., Radhakrishnan T. (2021) Alleviation of Salinity Stress in Peanut by Application of Endophytic Bacteria, Front Microbiol., 12.
- Roja Rani A., Padmaja G. (2005) A Protocol for high frequency plant conversion from somatic embryos of peanut (Arachis hypogaea L.), Plant Biotechnology, 7(3), 187-193.
- Sairam R.K., Tyagi A. (2004) Physiology and molecular biology of salinity stress tolerance in plants, Current Science, 86, 3.
- Pathak S., Mishra B.K., Misra P., Misra P., Joshi V.K., Shukla S., Trivedi P.K. (2012) High frequency of Somatic embryogenesis, regeneration and correlation of alkaloid biosynthesis with gene expression in Papaver somniferum L., Plant Growth Regulation, 68, 17-25.
- Srinivasan T., Kumar K., Kirti P. (2010) Establishment of efficient and rapid regeneration system for some diploid wild species of Arachis, Plant Cell Tissue Organ Culture, 101, 303-309.
- Swathy Anuradha T., Jami S.K., Datla R.S., Kirti P.B. (2006) Genetic Transformation of Peanut (Arachis hypogaea L.) using cotyledonary node as explant and a promoterless gus:J:nptII fusion gene based vector, Indian Academy of Science, 31, 235-246.
- Shabala S., Munns R. (2012) Salinity Stress: Physiological Constraints and Adaptive Mechanisms. In: Shabala S, Ed., Plant Stress Physiology CAB International Oxford, 59-93.
- Tuberoso C., Kowalczyk A., Sarritzu E., Cabras P. (2007) Determination of antioxidant compounds and antioxidant activity in commercial oilseeds for food use, Journal of Food Chemistry, 103, 1494-15501.
- Witcombe J.R., Hollington P.A., Howarth C.J., Reader S., Steele K.A. (2008) Breeding for abiotic stresses for sustainable agriculture, Philos. Trans. R. Soc. B., 363, 703-716.


