Structure and electronic properties of fluorographene and fluorographite crystals
Автор: Belenkov M.E., Chernov V.M., Butakov A.V.
Рубрика: Физика
Статья в выпуске: 1 т.17, 2025 года.
Бесплатный доступ
The paper presents the study of the structure and electronic properties of two-dimensional and three-dimensional crystals composed of monolayers of graphene polymorphs L6, L4-8, L3-12, L4-6-12, L5-7 functionalized by fluorine atoms with various types of attachment of fluorine atoms. It reveals that the interlayer distances in the studied crystals vary within a wide range from 4,73 to 5,96 Å and volumetric densities vary from 2,43 to 3,98 g/cm3. The value of the band gap in three-dimensional fluorographite crystals is on average 0,4 eV less than the band gap in the corresponding two-dimensional fluorographenes. The studied three-dimensional structures demonstrated a pattern: with an increase in the interlayer distance, the energy of the interlayer interaction decreases, and the volumetric density increases. The band gap of the fluorographenes and fluorographites decreases with an increase of sublimation energy. The paper establishes a correlation between the degree of C-C bond length variation of and the presence or absence of repulsive flagpole forces between the attached fluorine atoms. It proves that during functionalization, the values of the average C-C bond length increase for monolayers L6, L4-8, L3-12, L4-6-12 and L5-7. The values of the average lengths of C-C bonds practically do not change during the formation of fluorographite from fluorographene. The variation of C-C bond lengths in fluorographites is minimal for F-L6T1 and equal to 0,2 %, while the maximum is observed for F-L4-6-12T1, which is 10,7 %.
Functionalization, fluorographite, fluorographene, polymorphism, attachment type, band gap
Короткий адрес: https://sciup.org/147247580
IDR: 147247580 | УДК: 538.911 | DOI: 10.14529/mmph250105
Текст научной статьи Structure and electronic properties of fluorographene and fluorographite crystals
Graphite, which is a three-dimensional (3D) crystal, consists of monolayers of two-dimensional (2D) graphene bounded to each other by van der Waals forces [1]. A 3D crystal can also be constructed from monolayers of functionalized 2D graphene. Obviously, the properties of such a three-dimensional crystal will differ from the properties of 2D monolayer functionalized graphene, just as the properties of graphite differ from the properties of graphene [2–4].
In [5–12], calculations of the structure and electronic properties of fluorine-functionalized 2D and 3D crystals formed on the basis of various types of fluorographene polymorphs L 6 , L 3-12 , L 4-6-12 , L 5-7 and L 4-8 were carried out. In order to identify new patterns, this work carries out a comparative analysis and generalization of the results obtained in these works.
Calculations and methods
In this work, computer modeling of the structure and electronic properties of two-dimensional 2D fluorographene crystals and three-dimensional 3D fluorographite crystals was carried out. Fluorographene layers were obtained by fluoridation of graphene polymorphs L 6 , L 3-12 , L 4-6-12 , L 5-7 и L 4-8 . Calculations of the structural and energy characteristics of 2D fluorographene crystals were carried out using the DFT-GGA method using the open source software package Quantum Espresso [13].
As a result of calculations, it turned out that out of a possible 17 layers of fluorographene, 15 monolayers of fluorographene F-L6, F-L 3-12 , F-L 4-6-12 , F-L 5-7 and F-L 4-8 , shown in fig. 1, are stable. Stable layers were composed of 3D fluorographite crystals. The atom-atom potential method was used to calculate the three-dimensional structures of fluorographite. These structures were further used as the basis for calculations of the energy characteristics of the resulting fluorographites using the DFT-GGA method [13].
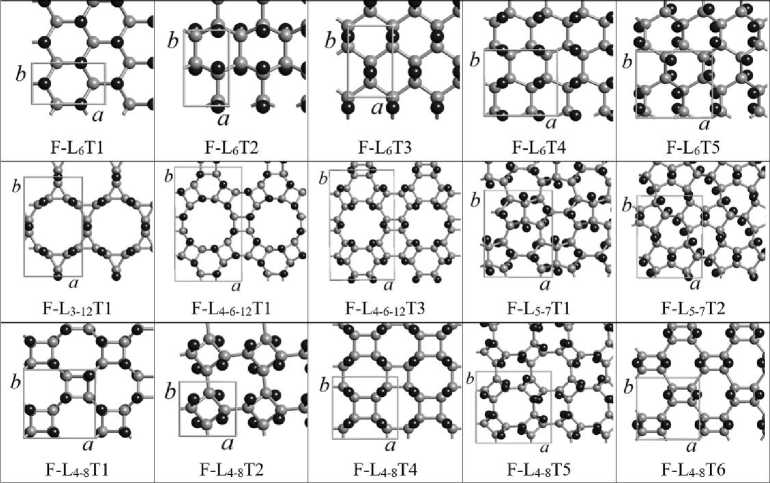
Fig. 1. Fluorographene monolayers used for building 3D-fluorographite crystals: F-L6, F-L3-12, F-L4-6-12, F-L5-7 и F-L4-8
Results and discussions
Table 1 shows the structural parameters: dL – interlayer distance, S – length of the shear vector and ρ – bulk density; and energy characteristics of the resulting 2D fluorographene crystals and 3D fluorographite crystals: EVdW – energy of van der Waals bonds between layers; Esub2D and Esub3D are the sublimation energy of fluorographenes and fluorographites, respectively; Eg2D and Eg3D are the band gaps of fluorographenes and fluorographites, respectively.
Table 1
Interlayer distance d L , shear vector length S, bulk density ρ, Van der Waals energy E VdW , sublimation energy of fluorographenes E sub2D and fluorographites E sub3D , band gap of fluorographenes E g2D and fluorographites E g3D
|
Crystal |
S , Å |
d L , Å |
ρ , g/cm3 |
E VdW ·102, eV |
E sub2D , eV |
E sub3D , eV |
E g2D , eV |
E g3D , eV |
|
F-L 6 T1 |
0,00 |
5,39 |
3,26 |
3,04 |
14,32 |
14,37 |
3,321 |
2,727 |
|
F-L 6 T2 |
1,09 |
4,73 |
3,98 |
4,81 |
14,19 |
13,94 |
3,390 |
3,114 |
|
F-L 6 T3 |
1,52 |
5,75 |
3,26 |
2,93 |
14,31 |
14,33 |
3,557 |
3,108 |
|
F-L 6 T4 |
1,68 |
5,60 |
3,30 |
3,19 |
14,08 |
14,19 |
4,195 |
3,666 |
|
F-L 6 T5 |
1,06 |
5,46 |
3,23 |
3,02 |
14,20 |
14,22 |
3,044 |
2,505 |
|
F-L 3-12 T1 |
1,47 |
5,76 |
2,43 |
1,85 |
13,77 |
13,83 |
3,43 |
3,032 |
|
F-L 4-6-12 T1 |
0,05 |
5,49 |
2,59 |
2,38 |
13,84 |
13,89 |
3,193 |
2,827 |
|
F-L 4-6-12 T3 |
3,46 |
5,77 |
2,64 |
2,37 |
13,80 |
13,83 |
4,150 |
3,682 |
|
F-L 5-7 T1 |
3,71 |
5,50 |
3,21 |
2,90 |
13,85 |
13,90 |
4,09 |
3,663 |
|
F-L 5-7 T2 |
3,36 |
5,57 |
3,39 |
3,13 |
14,17 |
14,24 |
3,32 |
3,126 |
|
F-L 4-8 T1 |
2,78 |
5,46 |
2,91 |
2,65 |
13,98 |
14,04 |
3,211 |
2,781 |
|
F-L 4-8 T2 |
0,00 |
5,18 |
2,97 |
3,08 |
13,36 |
13,44 |
4,958 |
4,599 |
|
F-L 4-8 T4 |
1,74 |
5,55 |
3,12 |
3,11 |
14,05 |
14,12 |
3,946 |
3,413 |
|
F-L 4-8 T5 |
3,14 |
5,96 |
2,75 |
2,23 |
13,56 |
13,60 |
4,686 |
4,322 |
|
F-L 4-8 T6 |
1,74 |
5,57 |
3,09 |
3,13 |
14,01 |
14,08 |
4,877 |
4,257 |
The average interlayer distance by types dL = 5,4 Å in fluorographites F-L6T1 – F-L6T5, as it turned out, practically coincides with the distance d L = 5,3 Å obtained in an experiment of X-ray diffraction analysis [14] on a fluorographite sample, synthesized in the Institute of Inorganic Chemistry of the Siberian Branch of the Russian Academy of Sciences in Novosibirsk according to the method described in [15]. From table 1 it can be seen that the difference between the minimum dL = 4,73 Å in F-L6T2 and the maximum d L = 5,96 Å in F-L 4-8 T5 values of the interlayer distance is quite significant – 1,23 Å (40 %).
The difference between the minimum and maximum values of bulk density is also quite significant: 1,55 g/cm3 (50 %). From fig. 2 it can be seen that there is a one-to-one correspondence between the interlayer distance d L and the bulk density ρ – the larger d L , the smaller ρ .
The interlayer bond energy E VdW is maximum in fluorographite F-L 6 T2 ( E VdW = 4,81·10–2 eV) and is more than 2,6 times higher than the minimum value EVdW in fluorograpite F-L3-12T1 ( EVdW = 1,85·10–2 eV).
Физика
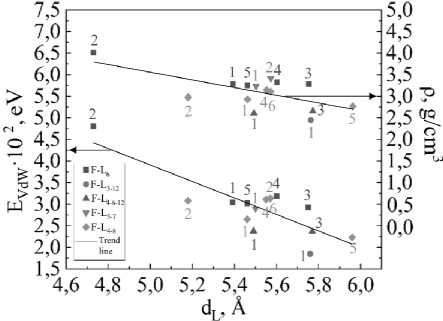
Fig. 2. The graph of interlayer distance dL versus bulk density ρ and Van der Waals energy EVdW
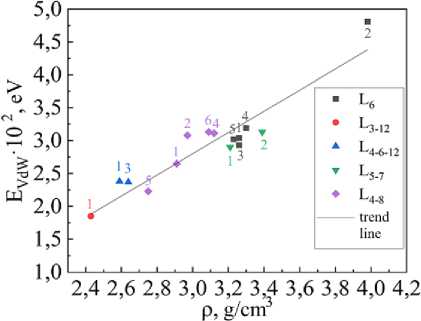
Fig. 3. The interlayer bond energy EVdW versus bulk density ρ
There is a connection between the van der Waals energy EVdW and the interlayer distance dL, as can be seen from fig. 2 – the greater EVdW, the smaller dL. According to fig. 3, there is also a connection between the Van der Waals energy EVdW and the density ρ, namely, the higher the Van der Waals energy, the higher the bulk density.
The sublimation energy of 3D fluorographite crystals, with the exception of F-L6T2 fluorographite, is slightly (0,5 %) higher than the sublimation energy of 2D fluorographene crystals. Fluorographene and fluorographite F-L 6 T1 have the highest sublimation energy, and fluorographene and fluorographite F-L 4-8 T2 have the lowest.
In fluorographites F-L 6 T1 and F-L 4-8 T2 there is no layer shift, that is, S = 0 (layer packing type AA),
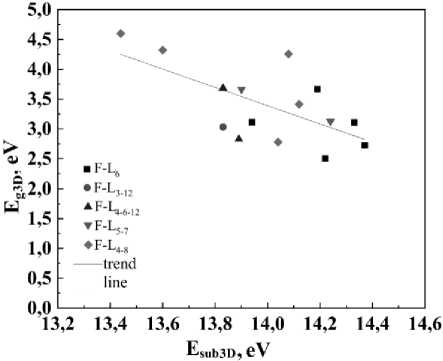
Fig. 4. The band gap Eg3D versus sublimation energy Esub3D in fluorographites
and in other fluorographites S is not equal to zero (layer packing type AB) and varies within a fairly wide range (from 0 to 3,71 Å). Note, that despite the same type of layer packing – type AA (no shear), fluorographites F-L6T1 and F-L4-8T2 are very different from each other in sublimation energies: for F-L 6 T1 E sub = 14,37 eV, while for F-L 4-8 T2 E sub = 13,44 eV.
It is interesting to note that, contrary to expectation, we did not find a link between the interlayer distance d L and the shift S .
According to [16], in pure (non-functionalized) graphite the interlayer distance in AA type packaging is greater than in graphite in AB type packaging. In the functionalized materials studied in this work – fluorographites – no similar correlation between the distance d L and the type of packaging was found.
The band gap of the studied fluorographites Eg3D varies widely from 2,51 eV (F-L6T5) to 4,60 eV (F-L4-8T2). It is on average 0,4 eV less than the band gap in fluorographene monolayers Eg2D .
Fig. 4 shows the dependence of the band gap E g3D on the sublimation energy E sub3D in fluorographites. It can be seen that the band gap decreases with the increase in sublimation energy. A similar dependence is observed in fluorographenes.
Based on the band gap, the fluorographenes and fluorographites we are studying should be classified as semiconductors or dielectrics.
Table 2 shows the average lengths of C-C bonds in the carbon frameworks of pure polymorphs of layered (2D) (graphenes) and three-dimensional (3D) (graphites) structures and fluorine-functionalized polymorphs of fluorographene (2D) and fluorographite (3D) and their relative changes: d C0 is the average length of the C-C bond in the functionalized material (flurographene or fluorographite),
δ = C - C 0 ⋅ 100 % is the relative increase in the length of the C-C bond as a result of functionaliza- d C 0
tion, ∆ = Cmax - Cmin ⋅ 100 % – spread – the relative difference between the maximum dCmax and min-dC imum dCmin length of the C-C bond in the carbon framework.
Table 2
Average length of С-С-bond d C0 and d C , the relative increase of the length of the С-С-bond as a result of the functionalization δ , the spread of the lengths of С-С-bonds in the carbon frame ∆
|
Monolayer notation |
Pure polymorphs (graphenes and graphites) |
Functionalized polymorphs (fluorographenes and fluorographites) |
||||||
|
2D, 3D |
2D |
3D |
||||||
|
d C0 , Å |
Δ , % |
dC , Å |
δ , % |
Δ , % |
dC , Å |
δ , % |
Δ , % |
|
|
L 6 T1 |
1,43 |
0,2 |
1,57 |
9,8 |
0,1 |
1,57 |
9,8 |
0,2 |
|
L 6 T2 |
1,60 |
11,9 |
5,3 |
1,61 |
12,6 |
5,4 |
||
|
L 6 T3 |
1,58 |
10,5 |
1,1 |
1,58 |
10,5 |
1,8 |
||
|
L 6 T4 |
1,59 |
11,2 |
5,1 |
1,59 |
11,2 |
6,0 |
||
|
L 6 T5 |
1,59 |
11,2 |
1,9 |
1,59 |
11,2 |
3,1 |
||
|
L 3-12 T1 |
1,41 |
6,3 |
1,54 |
9,2 |
3,3 |
1,54 |
9,2 |
3,6 |
|
L 4-6-12 T1 |
1,45 |
7,6 |
1,59 |
9,7 |
10,3 |
1,59 |
9,7 |
10,7 |
|
L 4-6-12 T3 |
1,61 |
11,0 |
1,5 |
1,61 |
11,0 |
1,9 |
||
|
L 5-7 T1 |
1,41 |
8,9 |
1,57 |
11,3 |
4,4 |
1,56 |
10,3 |
8,8 |
|
L 5-7 T2 |
1,57 |
11,3 |
8,4 |
1,60 |
13,5 |
9,4 |
||
|
L 4-8 T1 |
1,44 |
6,3 |
1,57 |
9,0 |
6,8 |
1,57 |
9,0 |
6,7 |
|
L 4-8 T2 |
1,58 |
9,7 |
4,2 |
1,58 |
9,7 |
4,2 |
||
|
L 4-8 T4 |
1,58 |
9,7 |
4,9 |
1,58 |
9,7 |
4,9 |
||
|
L 4-8 T5 |
1,60 |
11,1 |
2,3 |
1,60 |
11,1 |
2,3 |
||
|
L 4-8 T6 |
1,58 |
9,7 |
3,8 |
1,58 |
9,7 |
3,8 |
||
A sufficiently large value of δ , indicating a significant increase in the average length of the C-C bond d C , indicates a deterioration in the structural properties of the studied graphenes and graphites during their functionalization.
In functionalized materials, during the transition from layered structures to three-dimensional ones, the values of dC and δ practically do not change, which indicates that the strength properties of layered and three-dimensional structures remain unchanged. In functionalized materials, the difference in the lengths of C-C bonds in the carbon frame ∆ during the transition from 2D to 3D crystals also practically does not change.
Noteworthy is the fact that there is a wide variety of C-C bond lengths in the carbon framework: from 0,2 % in F-L6T1 to 10,7 % in F-L4-6-12T1 in 3D-crystals. The reason for this is the following. In layers of both 2D fluorographenes and 3D fluorographites, fluorine atoms attached to neighboring carbon atoms in the C-C chain on one side of the layer (fig. 5, a ) are close to each other and are repelled by flagpole forces [17, 18]. Let’s call this option of attaching fluorine atoms to the carbon frame option a . Due to the repulsion of fluorine atoms, stretching occurs – elongation of the corresponding С-С bond. In another case, fluorine atoms attached to neighboring carbon atoms in the C-C chain on different sides of the layer
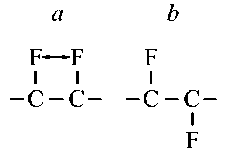
Fig. 5. Options for attaching fluorine atoms to the carbon frame: a – option a , b – option b
(fig. 5, b ) are far from each other and significant flagpole forces do not arise between them. Let’s call this option for addition of fluorine atoms option b .
In the FL6T1 layer, fluorine atoms are attached according to option b , so the C-C bonds in it are not stretched. Ultimately, it turns out that in the FL 6 T1 layer, just as in the original L 6 graphene, all C-C
Физика bonds have the same length, and the degree of spread in the lengths of C-C bonds ∆ up to a calculation error (0,2 %) is equal to zero.
In the FL 6 T2 layer, unlike the FL 6 T1 layer, there is not one, but two groups of fluorine atoms: one group is attached according to option a , the other according to option b , so that some C-C bonds are stretched, while others are not. Therefore, this layer has a large degree of spread of the lengths of C-C bonds: ∆ = 5,3 %.
In the FL 4-6-12 T3 layer there are two groups of C-C bonds: in one, short C-C bonds inside hexagonal cycles are stretched due to flagpole forces, in the other, long C-C bonds inside quadrangular cycles are not stretched. As a result, in the FL4-6-12T3 layer, the scatter in the lengths of C-C bonds turns out to be quite small ( ∆ = 1,5 %) and less than in the original non-functionalized graphene L 4-6-12 , where Δ = 7,6 %.
In the FL4-6-12T3 layer there are more C-C bonds stretched due to flagpole forces than in the FL 4-6-12 T1 layer. Therefore, the average length of C-C bonds in the FL 4-6-12 T3 layer, where d C = 1,61 Å, is greater than in the FL 4-6-12 T1 layer, where d C = 1,59 Å.
Conclusions
Computer modeling of the structure and electronic properties of two-dimensional and threedimensional crystals fluorographenes and fluorographites was carried out. Fluorographenes – fluorinefunctionalized graphene polymorphs F-L6, F-L3-12, F-L4-6-12, F-L5-7 and F-L4-8, of various types of attachment. Fluorographites consisted of parallel stacked monolayers of fluorographene polymorphs. It was found that in the resulting structures, interlayer distances and bulk densities vary widely from layer to layer. Bulk density, interlayer distance and interlayer van der Waals energy show linear correlation with one another, in which: the greater the interlayer distance, the lower the bulk density and interlayer interaction energy, and the greater the interlayer interaction energy, the greater the bulk density. The sublimation energy of 3D fluorographite crystals is practically not different from the sublimation energy of 2D fluorographene monolayers. The band gap in fluorographite crystals is on average 0,4 eV less than the band gap in fluorographene monolayers. Based on the band gap, the studied fluorographites can be classified as semiconductors or dielectrics. It was found that the band gap in both fluorographenes and fluorographites decreases with increase of sublimation energy. The relation between the degree of spread of the lengths of C-C bonds, the order of fluorine atom arrangement on sides of the carbon layer framework, and the presence or absence of repulsive flagpole forces has been established.
Список литературы Structure and electronic properties of fluorographene and fluorographite crystals
- Rêgo, C.R.C. Comparative Study of van der Waals Corrections to the Bulk Properties of Graphite / C.R.C. Rêgo, L.N. Oliveira, P. Tereshchuk, J.L.F. Da Silva // Journal of Physics: Condensed Matter. – 2015. – Vol. 27, no. 41. – P. 415502.
- Rohrer, J. Stacking and Band Structure of van der Waals Bonded Graphane Multilayers / J. Rohrer, P. Hyldgaard // Physical Review B. – 2011. – Vol. 83, no. 16. – P. 165423.
- Multilayer Graphane Synthesized under High Hydrogen Pressure / V.E. Antonov, I.O. Bashkin, A.V. Bazhenov et al. // Carbon. – 2016. – Vol. 100. – P. 465–473.
- Graphane Sheets and Crystals under Pressure / X.-D. Wen, L. Hand, V. Labet et al. // Proceed-ings of the National Academy of Sciences. – 2011. – Vol. 108, no. 17. – P. 6833–6837.
- Беленков, М.Е. Трехмерная структура и электронные свойства кристаллов, сформированных из слоев гексагонального графена, функционализированного фтором / М.Е. Беленков, В.М. Чернов // Челябинский физико-математический журнал. – 2021. – Т. 6, № 3. – С. 363–374.
- Belenkov, M.E. Computer Simulation of Crystals Formed from 4-6-12 and 5-7 Fluorographene layers / M.E. Belenkov, V.M. Chernov // Journal of Physics: Conference Series. – 2021. – Vol. 1967. – P. 012058.
- Belenkov, M.E. Calculations of the Three-Dimensional Crystal Structures Consisting of 4-8 Graphene Layers Functionalized with Fluorine / M.E. Belenkov, V.M. Chernov // AIP Conference Pro-ceedings. – 2021. – Vol. 2402. – P. 020021.
- Belenkov, M.E. Structure and Electronic Properties of 4-6-12 Graphene Layers Functionalized by Fluorine / M.E. Belenkov, V.M. Chernov // Letters on Materials. – 2020. – Vol. 10, no. 3. – P. 254–259.
- Беленков, М.Е. Структура и электронные свойства полиморфных разновидностей фторо-графена / М.Е. Беленков, В.М. Чернов, Е.А. Беленков // Челябинский физико-математический журнал. – 2018. – Т. 3, № 2. – С. 202–211.
- Belenkov, M.E. Simulation of the Structure and Electronic Properties of Fluorographene Poly-morphs Formed on the Basis of 4-8 Graphene / M.E. Belenkov, V.M. Chernov, E.A. Belenkov // IOP Conference Series: Materials Science and Engineering. – 2019. – Vol. 537, Iss. 2. – P. 022058.
- Беленков, М.Е. Кристаллическая и электронная структура 3-12 графена, функционализированного фтором / М.Е. Беленков, В.М. Чернов // Физико-химические аспекты изучения кластеров, наноструктур и наноматериалов: межвуз. сб. науч. тр. (под общей редакцией В.М. Самсонова, Н.Ю. Сдобнякова). – Тверь: Твер. гос. ун-т, 2019. – Вып. 11. – С. 406–413.
- Беленков, М.Е. Ab initio расчеты кристаллической и электронной структуры полиморфов 5-7 фторографена / М.Е. Беленков, В.М. Чернов // Физико-химические аспекты изучения кластеров, наноструктур и наноматериалов. – 2020. – Вып. 12. – С. 326–337.
- Advanced Capabilities for Materials Modelling with Quantum ESPRESSO / P. Giannozzi, O. Andreussi, T. Brumme et al. // Journal of Physics: Condensed Matter. – 2017. – Vol. 29, no. 46. – P. 465901.
- Беленков М.Е. Рентгеноструктурный анализ фторографена / М.Е. Беленков // Наномате-риалы и Нанотехнологии: проблемы и перспективы. Cборник материалов VI Международной научной конференции для молодых ученых, Саратов, 15–16 мая 2017 года. – 2017. – С. 27–30.
- Fluorographene: A Two-Dimensional Counterpart of Teflon / R.R. Nair, W. Ren, R. Jalil et al. // Small. – 2010. – Vol. 6, Iss. 24. – P. 2877–2884.
- The Nature of Metastable AA' Graphite: Low Dimensional Nano- and Single-Crystalline Forms / J.K. Lee, J.G. Kim, K.P.S.S. Hembram et al. // Scientific Reports. – 2016. – Vol. 6. – P. 39624.
- Pumera, M. Graphane and Hydrogenated Graphene / M. Pumera, C.H.A. Wong // Chemical So-ciety Reviews. – 2013. – Vol. 42, Iss. 14. – P. 5987–5995.
- Samarakoon, D.K. Chair and Twist-Boat Membranes in Hydrogenated Graphene / D.K. Samarakoon, X.-Q. Wang // ACS Nano. – 2009. – Vol. 3, Iss. 12. – P. 4017–4022.


