Study of the bioactive properties, radical scavenging activity and phenolic profiles of extracts Crocus cancellatus subsp. Mazziaricus (Herb.) B. Mathew, 1982
Автор: Deniz Nahide, Aydin Cigdem, Mammadov Ramazan
Журнал: Бюллетень науки и практики @bulletennauki
Рубрика: Биологические науки
Статья в выпуске: 6 т.9, 2023 года.
Бесплатный доступ
Human beings have used plants as medicine for various health cause of years. Plants are widely used in the traditional medicine of different countries and are a source of strong drugs. To evaluate research some bioactive properties of the extract of the Crocus cancellatus subsp. mazziaricus (Herb.) B. Mathew, 1982 the antioxidant activities of these extracts were analyzed by means of cleaning methods (DPPH, ABTS scavenging activity), β-carotene/linoleic acid test system and FRAP activity. Phenolics and flavonoid contents as the equivalents of gallic acid, quercetin respectively. The phenolic content of the extracts was analyzed using HPLC. Brine shrimp lethality test was applied to analyze cytotoxic activity. The obtained results indicated that the highest phenolic compound ferulic acid with 2376.2±0.00 µg/g was in extracts. The highest total phenolic and flavonoid were found in the ethanolic extracts. The aerial part-methanol extract exhibited the highest antioxidant capacity and the corm acetone extract the highest amount of ferric reducing power activity. Among the four different extracts, the acetone extract showed the highest amount of radical scavenging both corm and aerial parts. The brine shrimp lethality assay of bulb ethanol extract has showed good cytotoxic with LC50 of 320.535 μg/mL. This study will be a source for future studies.
Antioxidants, bioactive properties, radical scavenging activity, hplc
Короткий адрес: https://sciup.org/14127996
IDR: 14127996 | УДК: 581.13 | DOI: 10.33619/2414-2948/91/04
Текст научной статьи Study of the bioactive properties, radical scavenging activity and phenolic profiles of extracts Crocus cancellatus subsp. Mazziaricus (Herb.) B. Mathew, 1982
Бюллетень науки и практики / Bulletin of Science and Practice
UDC 581.13
The Crocus genus belongs to the large Iridaceae family and consists of about 85 species of flowering plants [1]. Crocus sativus L, commonly known as saffron, is originally grown in Iran and Spain. Saffron has a long medicinal history as part of traditional [2].
Crocus species are characterized by slender grass-like leaves with white, yellow or purple flowers; plants are native chiefly to the Mediterranean region, but Crocus spp. are widely cultivated [3]. The Crocus genus is known mainly for the valuable aromatic and medicinal cultivated species C. sativus (saffron), which is of great economic importance [4]. Saffron has been used as a drug in folk medicine, particularly in traditional Indian folk medicine, where it has been used for the treatment of various kinds of mental illnesses among other uses without any toxic side effects [5]. The chemical composition of saffron was studied in detail by a number of authors. Crocus cancellatus which belongs to the Iridaceae family, commonly known as saffron, is a medicinal plant with various bioactivities including antioxidant, anticancer and antibacterial properties. In vitro studies revealed the antioxidant activity of C. cancellatus subsp. damascenus stigmas extract. The extract showed DPPH radical scavenging activity with the IC 50 value of 34.6 μg/mL, which was 6.2-fold lower than the positive control ascorbic acid (the IC 50 value of 5.0 μg/mL). The radical scavenging activity was confirmed by ABTS test with IC 50 values of 34.6 and 21.6 μg/mL and a good ferric reducing ability (53.9 μM Fe(II)/g). The authors reported on the high content of phenolic compounds (namely, caffeic acid and ferulic acid), which probably contribute to good activity [6]. The active constituents of Crocus spp. raw materials, namely crocetin, crocin and phenolic compounds are potential candidates for the treatment of various diseases [7].
Nowadays, synthetic antioxidants such as BHA, BHT and TBHQ are widely used in the food industry. However, it is arguable whether these compounds are safe and their use in food products is questioned [8]. In recent years there has been an interest in determining total phenolics (as natural antioxidant) content and their antioxidant activities in various plants specifically by-products of agriculture and even marine microorganisms [9, 10]. Phenolic compounds of edible and inedible plants have multiple biological effects such as anti-inflammatory, bactericidal as well as antioxidant properties because of their ability to neutralize free radicals [11]. Herbs are used in many industries such as medicine, food, fragrance and cosmetics. Crude extracts of herbs and spices have been reported to be rich in phenolics and attracted more attention in the food industry because of their antioxidant capacity [12]. Phytochemicals such as phenolics and flavonoids have antioxidant activities and can be used for scavenging excess free radicals present in the body [13]. Antioxidants have been recognized as potential therapeutics for preventing different human diseases [14]. Human beings have used plants as medicine for diverse health issues for thousands of years. Plants are widely used in the traditional medicine of different countries and are a source of many potent and powerful drugs [15]. Since ancient times human beings depend on plants for meeting various daily needs such as food, medicine and for construction and other purposes. Plants are considered an integral part of daily life.
The aim of this study was to determine the antioxidants, the phenolic compounds, total phenolic and flavonoid content, bioactive properties (blood chemistry-ALT, AST, GGT, urea) and cytotoxic activities of different parts of Crocus cancellatus subsp. mazziaricus (Herb.) B. Mathew, 1982 extracts are examined by employing various in vitro assay systems in order to understand the usefulness of this plant as well as in medicine and pharmacology. This is the first study on the antioxidant capacity and biological activities of the C. cancellatus subsp . mazziaricus extracts.
Materials and methods
Plant material and extract preparation: Crocus cancellatus subsp . mazziaricus species were collected from Kazykbeli-Serinhisar and Alikurt-Bozkurt, Denizli (Turkey) in the autumn. The fresh corm and aerial parts of the plants samples were cleaned and dried in the shadow for extraction. Dried plant parts (corm and aerial parts) were extracted individually with different solvents such as ethanol, methanol, dH 2 O and acetone at room temperature in a water bath at 55°C for 6 h. After evaporation of the solvents on rotary evaporator ethanol, acetone and methanol extracts were obtained. After that, extracts were lyophilized to give the crude dry extract in a freeze-dryer. The powdered crude extracts were stored at -20°C until used [16].
Analysis of phenolic contents by HPLC: Phenolic compounds were evaluated by reversed-phase High Performance Liquid Chromatography (RP-HPLC, Shimadzu Scientific Instruments). The conditions utilized were as follows: C-18 column CTO-10ASVp, 4.6 mm × 250 mm, 5 μm; mobile phase was composed of solvent A (formic acid with 3 % methanol) and solvent B (100 % acetonitrile); injection volume 20 μL, gradient elution from 15 to 100 % B; run time 45 min and the flow rate was 1 mL/min. For analysis, the samples were dissolved in methanol, and 20 μL of this solution was injected into the column. The chromatograms were examined at 280 nm with an LC gradient detector. The phenolic compounds were recognized by comparing retention times and UV absorption spectra with those of pure standards. Gallic acid, 3,4-dihydroxy benzoic acid, 4-dihydroxy benzoic acid, chlorogenic acid, vanillic acid, caffeic acid, p-coumaric acid, ferulic acid and cinnamic acid were used as standard. Peaks were identified by comparing retention times and UV spectra with authentic standards. The amount of each phenolic compound was expressed as µg/g per gram of the extract.
Total phenolic content and total flavonoid content: the total phenolic content of extracts was determined using the Folin-Ciocalteu (FC) reagent with some modifications, as demonstrated by Slinkard and Singleton [17]. The total phenolic content was expressed as gallic acid equivalents (GAE) in mg/mL plant extract. Briefly, 0.75 mL of Folin-Ciocalteu reagent and 100 mL of sample
(5 mg/mL) were put into a test tube. The mixture was allowed to stand at room temperature for 5 min. 0.75 mL of 5% (w/v) Na 2 CO 3 was added to the mixture and then mixed gently. The mixture was homogenized and allowed to stand at room temperature for 90 min. Total polyphenol content was determined using a spectrophotometer at 760 nm. The Standard calibration curve was plotted using gallic acid.
The total flavonoid content of extracts was determined using the “aluminum chloride” method [18]. The total flavonoid content was determined on a standard curve using quercetin as a standard. For each extract, 1 mL of methanolic solution was mixed with 1 mL of aluminum trichloride (AlCl 3 ) in methanol (2%). The absorbance was read at 415 nm after 10 min against a blank sample consisting of 1 mL of methanol and 1 mL of plant extract without AlCl 3 . The mean of three readings was used and expressed as mg of quercetin equivalents (QE) per 100 mg of extract or fraction (mgQE/g).
Antioxidant activities: the total antioxidant capacity of the crude extracts was evaluated using the β-carotene-linoleic acid test system with slight modifications [19]. 0.2 mg of β-carotene in 1mL of chloroform was added to 20 μL of linoleic acid and 200 mg of Tween-20 emulsion mixture. The mixture was then evaporated at 40°C for 10 min by means of a rotary evaporator to remove chloroform. After evaporation of chloroform, 100 mL of distilled water saturated with oxygen, 4.8 mL of this emulsion was placed into test tubes which had 0.2 mg of the sample and 0.2 of the extract in them. For control, 0.2 mL of solvent (methanol, ethanol, acetone and dH 2 O) was placed in test tubes instead of the extract. As soon as the emulsion was added into the test tubes, initial absorbance was measured with a spectrophotometer to be at 470 nm. The measurement was carried out at 0.5 h intervals for 2 h. All samples were assayed in triplicate. BHT (butylated hydroxytoluene) was used as standard. The antioxidant activity was measured in terms of the successful bleaching of β-carotene by using the following equation:
AA: [ -(A0-At/A0 o -At o )]×100
where AA is the total antioxidant activity, A0 is the initial absorbance of the sample, At is the initial absorbance of the control, A0 o is the sample’s absorbance after 120 min, and At o is the control’s absorbance after 120 min.
Free radical scavenging activity of the extracts was determined using the free radical DPPH [20]. 4 mL of the DPPH’s 0.004% methanolic solution was mixed with 1 mL (0.2-1.0 mg/mL) of the extracts, and their absorbances were measured to be at 517 nm after incubation for 30 min at room temperature the absorbance value of the samples were evaluated against empty control group (where all determinants except the test compound were present). Lower absorbance of the reaction mixture indicates higher free radical scavenging activity. Every test was treated three times and the averages as determined. Free radical scavenging activity was measured using the equation below:
Scavenging activity =[(A 0 -A 1 /A 0 )]×100
where A 0 is the absorbance of the control (blank, without extract) and A 1 is the absorbance in the presence of the extract. The results were expressed as IC 50 (the concentration required to inhibit 50% of the DPPH).
The reducing power (FRAP) of the extracts was determined according to the method described by [21]. The different concentrations (40-150 μg/mL) of extracts were mixed with 2.5 mL of phosphate buffer (0.2 M, pH = 6.6) and 2.5 mL of potassium ferricyanide [K3Fe(CN)6]. The mixture was incubated at 50°C for 20 min. A portion (2.5 mL) of TCA (10%) was added to the mixture, which was then centrifuged for 10 min at 3000 rpm (MSE Mistral 2000, UK). The supernatant of the solution (2.5 mL) was mixed with 2.5 mL of distilled water and 0.5 mL of FeCl3 (0.1%). The Fe3+/Fe2+ transformation was investigated in the presence of extracts or standards and the absorbance values were measured at 700 nm in a spectrophotometer. Phosphate buffer (pH 6.6) was used as a blank solution. BHT was used as standard. The FRAP was expressed as Trolox equivalents in mg/g of samples used.
Experiments were performed according to Re et al. [22] with small modifications. ABTS and potassium persulfate were dissolved in distilled water to a final concentration of 7 mM and 2.45 mM respectively. These two solutions were mixed and allowed to stand in the dark at room temperature for 16 h before use to produce ABTS radical (ABTS•+). For the study of phenolic compounds the ABTS radical solution was diluted with distilled water to an absorbance of 1.00 at 734 nm. After the addition of 10 μL of sample to 4 mL of diluted ABTS solution, the absorbance was measured at 30 min. Free radical scavenging activity was measured using the equation below:
A%=(A control -A sample )/A control )×100
where A control is the absorbance of the blank control (ABTS solution without test sample) and A sample is the absorbance of the test sample. The results were expressed as IC 50 (the concentration required to inhibit 50% of the ABTS).
Cytotoxic activity: The brine shrimp lethality test (BSLT) was applied to analyze the possible cytotoxic activity of the extracts. A. salina eggs (10 mg) were incubated in 500 mL of seawater under artificial light at 28°C, pH 7-8. After incubation for 24 h, nauplii were collected with a Pasteur pipette and kept for an additional 24 h under the same conditions to reach the metanauplii (mature larvae) stage. Ten nauplii were drawn through a glass capillary and placed in each vial containing 4.5 mL of brine solution. In each experiment, 0.5 mL of the plant extract was added to 4.5 mL of brine solution and maintained at room temperature for 24 h under the light and then dead nauplii were counted [23]. Experiments were conducted along with control and five different concentrations (10-1000 µg/mL) of the extract in a set of three tubes. The data was performed by EPA Probit Analysis Program (version 1.5) to determine the LC 50 .
Bioactive properties: Male albino rats, weighing approximately 150-200 g, were obtained from the Pamukkale University, Faculty of Medicine, Experimental Research Center, Denizli, Turkey. The animals (Ethical approval was obtained from the Pamukkale University Animal Ethics Committee, Ethics Committee Decision No: PAUHDEK-2015/24) were allocated into four groups with seven rats in each group.
-
- Group I: Control animals received normal rat diet and water
-
- Group II: The plant ethanol extract at concentration of 0.5 and corm
-
- Group III: The plant ethanol extract at a concentration of 0.5 and the aerial part
-
- Group IV: The plant ethanol extract at a concentration of 1.0 and corm
-
- Group V: The plant ethanol extract at a concentration of 1.0 and the aerial part
After the experimental period, the animals were sacrificed under anesthesia, and blood samples were collected for the biochemical assays. Blood samples were taken by cardiac venipuncture on the 15th and 30th days after the initial treatment. Then, they were centrifuged at 1000 rpm for 10 minutes to collect serum and were stored at -20°C. Aspartate aminotransferase (AST), alanine aminotransferase (ALT), gamma-glutamyltransferase (GGT), and urea were measured for the determination of liver function.
Statistical analysis
All analyses and tests were run in triplicate and mean values were recorded. All the experimental data are presented as mean ± SEM of three individual samples. Data are presented as percentage of inhibition or radical scavenging on different concentrations of extracts IC 50 and LC 50 (the concentration required to scavenge 50% of free radicals) value was calculated from the dose-response curves. All of the statistical analyses were performed by means of Microsoft Office Excel 2010 software and Minitab. The results were evaluated using an unpaired t-test and a one way analysis of variance (ANOVA). The differences were regarded as statistically significant at p< 0.05.
Results and discussion
In the present study, the phenolic contents of the ethanol extracts of C. cancellatus subsp. mazziaricus were identified using the HPLC method, and these are listed in Table 1 and Figure 1. Some phenolic compounds were determined in the present study. In general, ferulic acid and cinnamic acid were the most predominant phenolic compounds found in the extracts. Ferulic acid was detected to be the major phenolic component in the extract, contributing about 2376.2±0.00 µg/g. Phenolic compounds could directly contribute to the antioxidant activity of plants and thus the identification and measurement of phenolic compounds in plants are found out as one of the important tools in understanding the value of plants for human health.
Table 1
HPLC ANALYSIS OF EXTRACTS FOR PHENOLIC COMPOUND
|
Phenolic standard compounds |
Standard retention time (RT) (min) |
C. cancellatus subsp. mazziaricus (µg/g) |
|
1.Gallic acid |
7.8±0.00 |
174.6±0.00 |
|
2. 3,4-dihydroxy benzoic acid (protocathechuic acid) |
12.2±0.00 |
53.6±0.00 |
|
3. 4- dihydroxy benzoic acid |
16.9±0.00 |
140.3±0.00 |
|
4. Chlorogenic acid |
19.4±0.00 |
103.7±0.00 |
|
5. Vanillic acid |
21.7±0.00 |
186.4±0.00 |
|
6. Caffeic acid (3,4-dihydroxy cinnamic acid) |
24±0.00 |
39.6±0.00 |
|
7. p-coumaric acid |
29.3±0.00 |
47.4±0.00 |
|
8. Ferulic acid |
34.7±0.00 |
2376.2±0.00 |
|
9. Cinnamic acid |
70.7±0.00 |
283.5±0.00 |
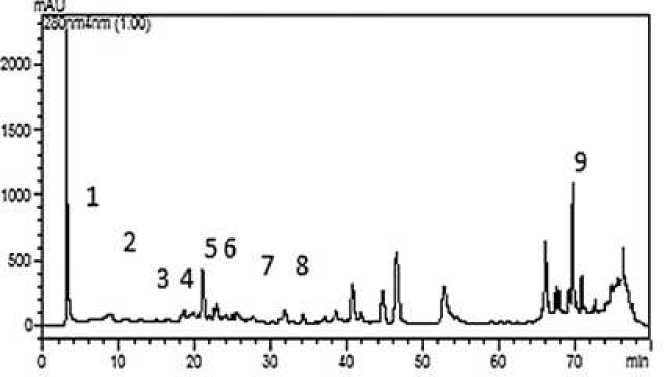
Figure 1. HPLC chromatograms of phenolic acids from the C. cancellatus subsp. mazziaricus ethanolic extracts. (1) Gallic acid; (2) 3,4-dihydroxy benzoic acid; (3) 4-dihydroxy benzoic acid; (4) chlorogenic acid; (5) vanillic acid; (6) caffeic acid; (7) p-coumaric acid; (8) ferulic acid; (9) cinnamic acid
The researchers reported that the p-Coumaric acid, apigenin-glucoside, rosmarinic acid, quercetin, and kaempferol are detected by HPLC-DAD in methanol extracts of C. baytopiorum which was an endemic species in Anatolia [24]. Tuberoso et al. [25] found that flavonoids are mainly represented by kaempferol, quercetin and isorhamnetin glycosides, with kaempferol 3-O-sophoroside being the most abundant.
Esmaeili et al. [26] evaluated total phenolic content in saffron corms in dormancy and waking stages. Gentisic and gallic acids were the highest and lowest phenolic compounds in dormant and waking corms, respectively. Hadizadeh et al. [27], isolated kaempferol from saffron petals and determined its structure by chemical and spectroscopic methods.
The total phenol content in the extracts ranged from 2.51±3.03 to 3.25±2.01mg GAE/mL (Table 2). The highest concentration of phenols was measured in the aerial part-ethanol extract (3.25±2.01 mgGAE/mL) (P<0.05). The concentration of flavonoids in the extracts of C. cancellatus ranged from 7.91±3.06 to 60.71±4.03 mg QE/g (Table 2). The concentration of flavonoids in the aerial part-ethanol extract was the highest (60.71±4.03 mg QE/g) (P<0.05). Flavonoids with multiple hydroxyl groups are more effective antioxidants than those with only one [28]. Zengin et al. [29] have determined the total phenolic compounds and total flavonoid compounds of methanol extracts in C. pallasii samples as 28.92±0.81 mg/g GAE/g, 31.44±0.29 mg RE/g extract. With regard to C . pallasii , the methanolic extract of the flowers possessed the highest TPC and TFC. Moreover, Wali et al. [30] investigated the total phenolic and flavonoid content observed in C. sativus which showed that the C. sativus stigma possessed higher phenolic, flavonoid content and consequently higher antioxidant activity as compared to C. sativus petals. Phenolic compounds will transfer hydrogen to free radicals to avoid the lipid oxidation chain reaction at the initial stage. The capacity of phenolic compounds to scavenge radicals is due to the effects of their phenolic hydroxyl groups.
Table 2
TOTAL PHENOLIC AND FLAVONOID CONTENTS OF THE EXTRACT
C. cancellatus subsp. mazziaricus
|
Plant extracts |
Total phenolic content (mg GAE/mL) |
Total flavonoid content (mgQE/g) |
|
corm - ethanol |
2.79±0.08 |
9.09±1.05 |
|
aerial part - ethanol |
3.25±1.04 |
60.71 ±0.08 |
|
corm - methanol |
2.94±1.05 |
7.91±0.07 |
|
aerial part - methanol |
2.86±0.06 |
48.91±1.09 |
|
corm - acetone |
2.59±0.04 |
12.39±0.10 |
|
aerial part - acetone |
2.63±0.10 |
34.00±0.05 |
|
corm - dH 2 O |
2.51±0.06 |
9.00±1.07 |
|
aerial part - dH 2 O |
2.63±0.08 |
23.85±1.09 |
The results showed that saffron stigma had an antioxidant activity that was lower than that of BHT and α-tocopherol. The total phenolic content of stigma was 6.55 mg gallic acid equivalent/g dry weight for methanolic saffron extract [31]. Goli et al. [12], showed that saffron petals had 3.42±0.2 mg phenolic content equivalent to gallic acid per g dry weight. This value was higher than the amount of phenolic acids (1.38 mg caffeic acid / g dry weight) reported by Termentzi and Kokkalou [32] for saffron petals harvested in Greece. Satybaldiyeva et al. [33] have reported total phenolic content in C. alatavicus. The highest phenolic contents were obtained in ethanol and methanol extracts from the aerial parts (72.29 and 62.37 mg GAE / g extract respectively).
Considering that antioxidants can act through different mechanisms, the antioxidant potential of C. cancellatus subsp. mazziaricus was determined using five different assays: β-Carotene-linoleic acid, DPPH, ABTS and Ferric-reducing antioxidant power (FRAP) assays (Table 3). In the present study, the potential of the plant to inhibit linoleic acid oxidation was evaluated using the β-Carotene/linoleic acid test system. This system depends on the principle that β-carotene discolors rapidly when no antioxidant is present as a result of the process in which free radicals produce hydroperoxides from linoleic acid. In the literature, there are some studies on the antioxidant activities, phenolic contents and some biological activities of Crocus species. Previous studies have also reported differences in the phenolic levels of different anatomical parts of several plant species. Many earlier reports have shown a significant correlation between total phenolic content and antioxidant activity [7, 34].
Table 3
ANTIOXIDANT ACTIVITIES OF METHANOL AND ACETONE EXTRACTS OF
C. cancellatus subsp. mazziaricus
|
Extracts |
ABTS IC 50 , mg/mL |
DPPH IC 50 , mg/mL |
FRAP (µM/g Trolox) |
β-caroten/linoleic acid (%) |
|
corm - ethanol |
6.228±0.16 |
4.123±1.05 |
3.710±0.13 |
97.5±0.09 |
|
aerial part - ethanol |
4.583±0.12 |
4.66±0.06 |
3.722±0.12 |
86.36±0.06 |
|
corm - methanol |
5.502±1.07 |
3.811±0.19 |
3.424±0.08 |
95.6±1.07 |
|
aerial part - methanol |
3.026±0.04 |
4.156±0.13 |
3.405±0.07 |
98.37±0.10 |
|
corm - acetone |
0.091±0.08 |
2.109±0.07 |
3.832±2.09 |
92.92±0.11 |
|
aerial part - acetone |
0.856±0.09 |
3.509±0.06 |
3.695±0.14 |
81.25±1.07 |
|
corm - dH 2 O |
8.385±0.10 |
4.535±0.04 |
3.510±1.12 |
78.94±0.06 |
|
aerial part - dH 2 O |
9.013±0.13 |
6.569±0.07 |
3.490±0.09 |
75.23±0.10 |
|
BHT |
0.010±0.16 |
28.13±0.96 |
7.598±0.05 |
99.84 ± 0.21 |
BHT = Butyl Hydroxy Toluene
According to the report by Acar et al . [24], antioxidant activities of the extracts from the Crocus species; C. flavus , C. biflorus and C. baytopiorum . The antioxidant activity of the extracts was evaluated by DPPH radical scavenging activity and βeta-carotene bleaching assays. Among the extracts, the highest free radical scavenging activity was observed for methanol. Methanol extract of C. flavus showed higher activity than other species of Crocus we studied (90.51%). The highest total antioxidant activity value was determined at a 0.8 mg/mL concentration of C. flavus methanol extract which showed 89.32% inhibition.
Satybaldiyeva et al. [35], have determined the total antioxidant and DPPH radical scavenging activities of C. alatavicus . Ethanol extracts from aerial parts and bulbs had good scavengers of DPPH radicals (65.5% and 54.08%, respectively) and the maximum antioxidant activities were observed in ethanol (61.34%) and methanol extracts (46.13%) from aerial parts. In another study, Shakeri et al. [36] reported the antioxidant activities of C. sativus L. and C. pallasii subsp. haussknechtii . The antioxidant activities of the extracts were assessed by the DPPH method. The DPPH radical scavenging activity of C. sativus L. corms methanol extract at a concentration of 200 μg/mL was significantly higher than that of C. pallasii.
Baba et al. [37] measured the free radical scavenging activity of ethanol and aqueous extracts of C. sativus L. corms by the DPPH test. The IC50 values for ethanol and aqueous extracts were 246.22±5.60 and 456±3.52 μg/mL, respectively. The antioxidant activity of an ethanol extract of C. sativus L. corms was higher than that of the aqueous extract. Based on the results of antioxidant activity, antioxidant agents in C. sativus L. are extracted by polar solvents such as methanol, ethanol and water. Polar extracts contain high levels of phenolic content [38]. The antioxidant activity of C. pallasii was investigated. The antioxidant abilities of the extracts were investigated via different antioxidant assays (metal chelating, radical quenching (ABTS and DPPH), reducing power (CUPRAC and FRAP) and phosphomolybdenum). As a result, it was observed that among C. pallasii extracts, the flower extract (18.42 and 42.53 mg TE/g, for DPPH and ABTS, respectively) was the most active radical scavenger [29].
Methanol extracts of Saffron petal ( C. sativus L.) were tested for their antioxidant activity with the β-carotene-linoleate and 1,1-diphenyl-2-picryl hydroxyl (DPPH). In model systems of β-carotene-linoleate and DPPH, the extract at 500 ppm concentration showed 91.4% and 74.2% antioxidant activity which was comparable with that of TBHQ (93.1% and 77.9%) at 100 ppm. The results showed that saffron petals could be considered a bioresource of phenolic compounds with high antioxidant activity.
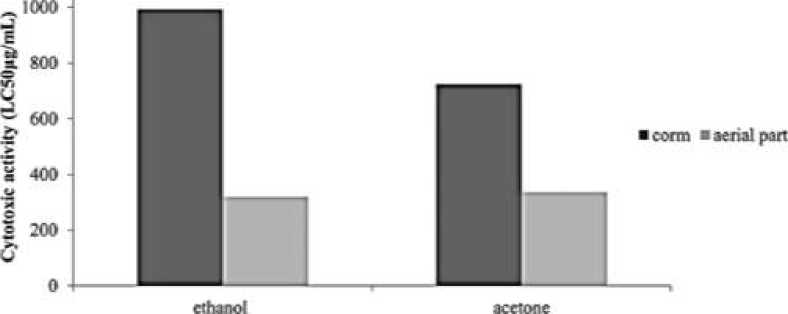
The brine shrimp lethality assay represents a rapid, inexpensive and simple bioassay for testing plant extracts bioactivity which in most cases correlates reasonably well with cytotoxic and anti-tumor properties. Results (LC 50 values) are shown in Figure 2 . Based on the results, the ethanol aerial part extract of C. cancellatus subsp. mazziaricus has shown good toxicity to brine shrimp nauplii, with an LC 50 of 320.535 μg/mL. In addition, the degree of lethality was found to be directly proportional to the concentration of the extract.
Extracts
Figure 2. Cytotoxic activities of extracts by LC50 values
-
C. sativus L. (Saffron) is often used as a spice in various parts of the world and as a therapeutic agent for a range of diseases. A literature survey revealed that more than 100 bioactive compounds have been isolated from saffron stigma. Recent pharmacological analysis of saffron stigma and petals showed a broad range of biological activities like antioxidant, anticancer, cytotoxic, etc. Wali et al . [30], have reported the cytotoxic activity revealed by C. sativus L. petals. Cytotoxic activity was tested against MDA-MB-231 cell lines using the MTT method whereas, antidiabetic activity was evaluated using an alpha-glucosidase inhibition assay. Ethanol had the highest inhibition of MDA-MB-231 cells (12.98 ±2.53 mg/mL IC 50 ) (p < 0.05).
Satybaldiyeva et al. [33], have determined the brine shrimp cytotoxic activity of the ethanolic extract from the aerial part of C. alatavicus. The results reveal that the ethanolic extract from the aerial part of C. alatavicus exhibit high cytotoxicity with an LC50 of 15.71 μg/mL. Tokgun et al. [39], reported cytotoxic activity in human breast cancer cell line MCF-7 cells of the methanol extracts of C. antalyensis. The results reveal that the methanol extract has shown good toxicity, with an IC50 of 0.72 μg/mL. Here the study reported that C. antalyensis total extracts have strong cytotoxic effects on cellular proliferation, and this cytotoxicity results from p53-mediated apoptosis.
The effects of using water extract of C. cancellatus subsp. mazziaricus corm and aerial part on rats on the activities of ALT, AST, GGT and urea activities in the blood plasma were shown in Tables 4.
Table 4
MEAN VALUES OF ALT, AST, GGT (U/L) AND UREA (MG/DL) ACTIVITIES IN THE BLOOD OF RATS TREATED WITH WATER EXTRACT
|
Values |
Extracts |
Week 2 |
Week 4 |
||
|
0.5 mg/mL |
1.0 mg/mL |
0.5 mg/mL |
1.0 mg/mL |
||
|
ALT |
corm |
86.166±1.21 |
59.857±2.42 |
99.571±1.44 |
62.857±1.54 |
|
Aerial part |
83.857±1.32 |
64.857±1.24 |
67.858±1.21 |
102.00±1.65 |
|
|
AST |
corm |
239.428±1.23* |
121.142±1.48* |
179.855±2.22* |
120.00±2.54* |
|
Aerial part |
159.571±1.43 |
158.857±2.44 |
163.428±1.51* |
192.714±2.52 |
|
|
GGT |
corm |
108.571±2.20* |
67.285±1.56 |
88.00±1.66* |
70.285±1.59 |
|
Aerial part |
96.142±1.29* |
79.571±1.43* |
76.857±1.54* |
72.285±1.71 |
|
|
Urea |
corm |
44.300±3.24 |
48.400±1.55 |
45.900±1.30 |
47.885±2.76 |
|
Aerial part |
47.828±1.45 |
47.200±2.33 |
44.785±2.44 |
45.785±1.29 |
|
|
Control |
ALT |
81.00±1.06 |
83.043±0.07 |
||
|
AST |
123±1.10 |
125.052±1.05 |
|||
|
GGT |
40.142±1.09 |
65.081±0.09 |
|||
|
Urea |
54.171±1.07 |
56.003±1.07 |
|||
Values denote the mean ± standard deviation of measurements from n=35 animals; P˂0.05. AST=aspartate aminotransferase; ALT=alanine aminotransferase; GGT=gamma-glutamyltransferase; Control= control group, normal rat diet and water
In the liver AST, ALT and GGT show high activity and are most often determined if there is a suspicion of acute or chronic liver disease. The use of extract for 2 and 4 weeks produced a significant increase in the liver AST activity as compared to the control (p<0.05). Using the extract for 4 weeks resulted in a significant increase in the liver GGT activity with a corresponding significant increase in the activity of the serum enzyme compared to the control (p<0.05). Increased AST activity in the serum is a sensitive marker of liver damage, even if the damage is of subclinical nature. During the extract, values were significantly higher than in the beginning treated water extracts. This study is the first to report on the bioactive properties of the extracts of C. cancellatus subsp. mazziaricus species.
Conclusions
In the present study, analysis of the phenolic component, antioxidant activity, cytotoxic activity, bioactive properties and content of total phenol, flavonoid revealed that the ethanol, methanol, acetone and dH2O corm and aerial part extract of C. cancellatus subsp. mazziaricus can be a potent source of natural antioxidants. The results of the present study demonstrated that C. cancellatus subsp. mazziaricus constitute a rich source of antioxidants. The bioactive phytochemical compounds in C. cancellatus subsp. mazziaricus were found to exhibit potential antioxidant activity through in vitro model. The strong antioxidant potential of C. cancellatus subsp. mazziaricus may be due to the presence of diverse phytochemical compounds, mainly flavonoids and phenolic acids. Further research is required to determine the potential health benefits of these bioactive components in vivo and elucidate their mechanism of action. Therefore, this research study can be useful for further research studies and needs to be investigated with more detailed data.
Список литературы Study of the bioactive properties, radical scavenging activity and phenolic profiles of extracts Crocus cancellatus subsp. Mazziaricus (Herb.) B. Mathew, 1982
- Harpke, D., Meng, S., Rutten, T., Kerndorff, H., & Blattner, F. R. (2013). Phylogeny of Crocus (Iridaceae) based on one chloroplast and two nuclear loci: ancient hybridization and chromosome number evolution. Molecular phylogenetics and evolution, 66(3), 617-627. https://doi.org/10.1016/j.ympev.2012.10.007
- Rios, J. L., Recio, M. C., Giner, R. M., & Manez, S. (1996). An update review of saffron and its active constituents. Phytotherapy Research, 10(3), 189-193. https://doi.org/10.1002/(SICI)1099-1573(199605)10
- Erol, O., Can, L., & Küçüker, O. (2014). Crocus yaseminiae (Iridaceae) a new species from South Anatolia, Turkey. Phytotaxa, 188(2), 103-111. https://doi.org/10.11646/phytotaxa.188.2.4
- Beiki, A. H., Keifi, F., & Mozafari, J. (2010). Genetic differentiation of Crocus species by random amplified polymorphic DNA. Genet Eng Biotechnol J, 18, 1-10.
- Khalili, M., Fathi, H., & Ebrahimzadeh, M. A. (2016). Antioxidant activity of bulbs and aerial parts of Crocus caspius, impact of extraction methods. Pak J Pharm Sci, 29(3), 773-777.
- Loizzo, M. R., Marrelli, M., Pugliese, A., Conforti, F., Nadjafi, F., Menichini, F., & Tundis, R. (2016). Crocus cancellatus subsp. damascenus stigmas: chemical profile, and inhibition of α-amylase, α-glucosidase and lipase, key enzymes related to type 2 diabetes and obesity. Journal of Enzyme Inhibition and Medicinal Chemistry, 31(2), 212-218. https://doi.org/10.3109/14756366.2015.1016510
- Mykhailenko, O., Kovalyov, V., Goryacha, O., Ivanauskas, L., & Georgiyants, V. (2019). Biologically active compounds and pharmacological activities of species of the genus Crocus: A review. Phytochemistry, 162, 56-89. https://doi.org/10.1016/j.phytochem.2019.02.004
- Konczak, I., Zabaras, D., Dunstan, M., & Aguas, P. (2010). Antioxidant capacity and phenolic compounds in commercially grown native Australian herbs and spices. Food Chemistry, 122(1), 260-266. https://doi.org/10.1016/j.foodchem.2010.03.004
- Negro, C., Tommasi, L., & Miceli, A. (2003). Phenolic compounds and antioxidant activity from red grape marc extracts. Bioresource Technology, 87(1), 41-44. https://doi.org/10.1016/S0960-8524(02)00202-X
- Fu, L., Xu, B. T., Xu, X. R., Gan, R. Y., Zhang, Y., Xia, E. Q., & Li, H. B. (2011). Antioxidant capacities and total phenolic contents of 62 fruits. Food chemistry, 129(2), 345-350. https://doi.org/10.1016/j.foodchem.2011.04.079
- Temerdashev, Z. A., Frolova, N. A., & Kolychev, I. A. (2011). Determination of phenolic compounds in medicinal herbs by reversed-phase HPLC. Journal of Analytical Chemistry, 66, 407-414. https://doi.org/10.1134/S1061934811040150
- Goli, S. A. H., Mokhtari, F., & Rahimmalek, M. (2012). Phenolic compounds and antioxidant activity from saffron (Crocus sativus L.) petal. Journal of Agricultural Science, 4(10), 175. https://doi.org/10.5539/jas.v4n10p175
- Pietta, P. G. (2000). Flavonoids as antioxidants. Journal of natural products, 63(7), 1035-1042. https://doi.org/10.1021/np9904509
- Baldemir, A., Köroğlu, A., Altanlar, N., & Coşkun, M. (2018). A comparative study on the in vitro antioxidant and antimicrobial potentials of three endemic Ononis L. species from Turkey. Turkish Journal of Pharmaceutical Sciences, 15(2), 125. https://doi.org/10.4274/tjps.62533
- Nirmala, K. A., & Kanchana, M. (2018). Leucas aspera–A Review of its Biological activity. Systematic Reviews in Pharmacy, 9(1), 41-44. https://doi.org/10.5530/srp.2018.1.8
- Aydın, C., & Mammadov, R. (2017). Phenolic composition, antioxidant, antibacterial, larvacidal against Culex pipiens, and cytotoxic activities of Hyacinthella lineata s teudel extracts. International Journal of Food Properties, 20(10), 2276-2285.. https://doi.org/10.1080/10942912.2016.1236271
- Slinkard, K., & Singleton, V. L. (1977). Total phenol analysis: automation and comparison with manual methods. American journal of enology and viticulture, 28(1), 49-55. https://doi.org/10.5344/ajev.1977.28.1.49
- Arvouet-Grand, A., Vennat, B., Pourrat, A., & Legret, P. (1994). Standardization of propolis extract and identification of principal constituents. Journal de pharmacie de Belgique, 49(6), 462-468.
- Ismail, A., & Hong, T. S. (2002). Antioxidant activity of selected commercial seaweeds. Malaysian Journal of Nutrition, 8(2), 167-177.
- Wu, C., Chen, F., Wang, X., Kim, H. J., He, G. Q., Haley-Zitlin, V., & Huang, G. (2006). Antioxidant constituents in feverfew (Tanacetum parthenium) extract and their chromatographic quantification. Food Chemistry, 96(2), 220-227. https://doi.org/10.1016/j.foodchem.2005.02.024
- Oyaizu, M. (1986). Studies on products of browning reaction antioxidative activities of products of browning reaction prepared from glucosamine. The Japanese journal of nutrition and dietetics, 44(6), 307-315. https://doi.org/10.5264/eiyogakuzashi.44.307
- Re, R., Pellegrini, N., Proteggente, A., Pannala, A., Yang, M., & Rice-Evans, C. (1999). Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free radical biology and medicine, 26(9-10), 1231-1237. https://doi.org/10.1016/S0891-5849(98)00315-3
- Krishnaraju, A. V., Rao, T. V., Sundararaju, D., Vanisree, M., Tsay, H. S., & Subbaraju, G. V. (2005). Assessment of bioactivity of Indian medicinal plants using brine shrimp (Artemia salina) lethality assay. International Journal of Applied Science and Engineering, 3(2), 125-134. https://doi.org/10.6703/IJASE.2005.3(2).125
- Acar, G., Mercan Doğan, N., Duru, M. E., & Kıvrak, I. (2010). Phenolic profiles, antimicrobial and antioxidant activity of the various extracts of Crocus species in Anatolia. African Journal of Microbiology Research.
- Tuberoso, C. I., Rosa, A., Montoro, P., Fenu, M. A., & Pizza, C. (2016). Antioxidant activity, cytotoxic activity and metabolic profiling of juices obtained from saffron (Crocus sativus L.) floral by-products. Food Chemistry, 199, 18-27. https://doi.org/10.1016/j.foodchem.2015.11.115
- Esmaeili, N., Ebrahimzadeh, H., Abdi, K., & Safarian, S. (2011). Determination of some phenolic compounds in Crocus sativus L. corms and its antioxidant activities study. Pharmacognosy magazine, 7(25), 74. https://doi.org/10.4103/0973-1296.75906
- Hadizadeh, F., Khalili, N., Hosseinzadeh, H., & Khair-Aldine, R. (2003). Kaempferol from saffron petals. Science Direct Working Paper, (S1574-0331), 04. https://ssrn.com/abstract=2979832
- Gheldof, N., & Engeseth, N. J. (2002). Antioxidant capacity of honeys from various floral sources based on the determination of oxygen radical absorbance capacity and inhibition of in vitro lipoprotein oxidation in human serum samples. Journal of agricultural and food chemistry, 50(10), 3050-3055. https://doi.org/10.1021/jf0114637
- Zengin, G., Mahomoodally, M. F., Sinan, K. I., Picot-Allain, M. C. N., Yildiztugay, E., Cziáky, Z., ... & Ahemad, N. (2020). Chemical characterization, antioxidant, enzyme inhibitory and cytotoxic properties of two geophytes: Crocus pallasii and Cyclamen cilicium. Food Research International, 133, 109129. https://doi.org/10.1016/j.foodres.2020.109129
- Wali, A. F., Alchamat, H. A. A., Hariri, H. K., Hariri, B. K., Menezes, G. A., Zehra, U., ... & Ahmad, P. (2020). Antioxidant, antimicrobial, antidiabetic and cytotoxic activity of Crocus sativus L. petals. Applied Sciences, 10(4), 1519. https://doi.org/10.3390/app10041519
- Karimi, E., Oskoueian, E., Hendra, R., & Jaafar, H. Z. (2010). Evaluation of Crocus sativus L. stigma phenolic and flavonoid compounds and its antioxidant activity. Molecules, 15(9), 6244-6256. https://doi.org/10.3390/molecules15096244
- Termentzi, A., & Kokkalou, E. (2008). LC-DAD-MS (ESI+) analysis and antioxidant capacity of Crocus sativus petal extracts. Planta medica, 74(05), 573-581. https://doi.org/10.1055/s-2008-1074498
- Satybaldiyeva, D. N., Mursaliyeva, V. K., Mammadov, R., & Zayadan, B. K. (2016). Phenolic profiles and brine shrimp cytotoxicity of the ethanolic extract from the aerial part of Crocus alatavicus L. International Journal of Biology and Chemistry, 9(1), 38-41. https://doi.org/10.26577/2218-7979-2016-9-1-38-41
- Rahaiee, S., Moini, S., Hashemi, M., & Shojaosadati, S. A. (2015). Evaluation of antioxidant activities of bioactive compounds and various extracts obtained from saffron (Crocus sativus L.): a review. Journal of Food Science and Technology, 52, 1881-1888. https://doi.org/10.1007/s13197-013-1238-x
- Satybaldiyeva, D., Mursaliyeva, V., Rakhimbayev, I., Zayadan, B., & Mammadov, R. (2015). Preliminary phytochemical analysis and antioxidant, antibacterial activities of Crocus alatavicus from Kazakhstan. Notulae Botanicae Horti Agrobotanici Cluj-Napoca, 43(2), 343-348. https://doi.org/10.15835/nbha43210089
- Shakeri, R., Khorshidi, J., Radjabian, T., Lashkari, A., & Safavi, M. (2019). Cytotoxic and antioxidant activities of Crocus pallasii subsp. haussknechtii corms extracts compared with Crocus sativus. Research Journal of Pharmacognosy, 6(3). https://doi.org/10.22127/rjp.2019.89461
- Baba, S. A., Malik, A. H., Wani, Z. A., Mohiuddin, T., Shah, Z., Abbas, N., & Ashraf, N. (2015). Phytochemical analysis and antioxidant activity of different tissue types of Crocus sativus and oxidative stress alleviating potential of saffron extract in plants, bacteria, and yeast. South African Journal of Botany, 99, 80-87. https://doi.org/10.1016/j.sajb.2015.03.194
- Barchan, A., Bakkali, M., Arakrak, A., Pagán, R., & Laglaoui, A. (2014). The effects of solvents polarity on the phenolic contents and antioxidant activity of three Mentha species extracts. Int J Curr Microbiol App Sci, 3(11), 399-412.
- Tokgun, O., Akca, H., Mammadov, R., Aykurt, C., & Deniz, G. (2012). Convolvulus galaticus, Crocus antalyensis, and Lilium candidum extracts show their antitumor activity through induction of p53-mediated apoptosis on human breast cancer cell line MCF-7 cells. Journal of Medicinal Food, 15(11), 1000-1005. https://doi.org/10.1089/jmf.2012.0050


