Study on reaction of 2-allylthiobenzimidazole with bromine
Автор: Ilinykh E.S., Kim D.G.
Журнал: Вестник Южно-Уральского государственного университета. Серия: Химия @vestnik-susu-chemistry
Рубрика: Органическая химия
Статья в выпуске: 3 т.7, 2015 года.
Бесплатный доступ
It has been found by 1Н NMR method that bromination of 2-allylthio-benzimidazole proceeds to give the bromocyclization products (benzimidazo-[2,1 b]thiazolium and benzimidazo[2,1-b][1,3]thiazinium bromides) and two isomeric products of bromine addition to the double bond of allyl moiety.
2-mercaptobenzimidazole, 2-allylthiobenzimidazole, bromocyclization, bromonium and thiiranium ions, 1н nmr spectroscopy
Короткий адрес: https://sciup.org/147160316
IDR: 147160316 | УДК: 547.781
Текст научной статьи Study on reaction of 2-allylthiobenzimidazole with bromine
The chemistry of benzimidazole and particularly 2-mercaptobenzimidazole and its derivatives has received considerable attention because of their synthetic and biological importance. Natural compounds (e.g. vitamin В 12 ) and various medicines (e.g. dibazole, omeprazole, mebendazole, afobazole, etc. ) contain benzimidazole moiety in their structure. 2-Mercaptobenzimidazole is widely used in industrial application as a stabilizer and an antioxidant in rubber [1, 2], an adsorbent for some metals [3–5] and a corrosion inhibitor [6]. Besides, many kinds of biological activities have been reported for various N,S-derivatives of 2-mercaptobenzimidazole [7].
The chemistry of fused heterocyclic systems obtained from benzimidazole, as well as synthetic paths to thiazolo[3,2- a ]benzimidazoles and their chemical properties, has been described in the reviews [8, 9]. However, literature data on electrophilic heterocyclization of S-allyl derivatives of 2-mercapto-benzimidazole ( 1 ) and 5-ethoxy-2-mercaptobenzimidazole by the treatment with iodine and bromine are limited [10–13]. In the present paper we study the interaction of 2-allylthiobenzimidazole ( 2 ) with bromine and show unexpected results that are different from earlier data [11].
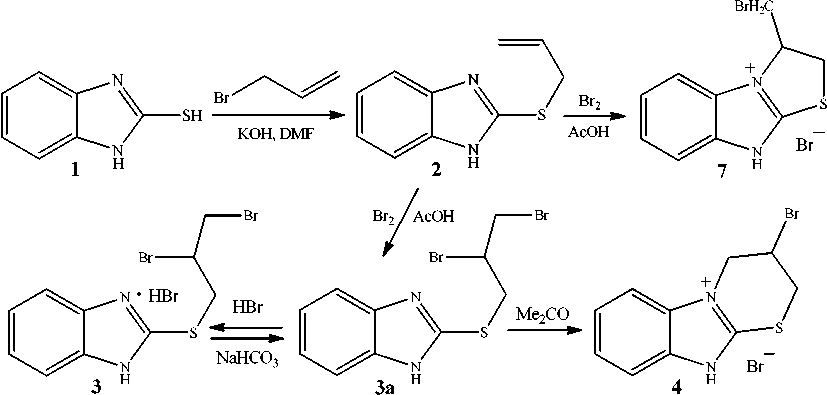
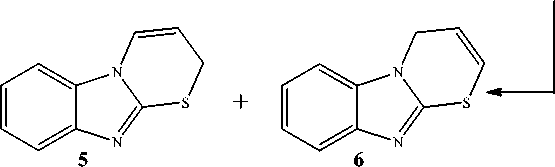
As demonstrated previously by Korotkikh et al [11], the reaction between 2-allylthiobenzimidazole 2 and bromine in acetic acid leads to bromine addition to the double bond and formation of 2-(2,3-dibromopropyl)thiobenzimidazole hydrobromide ( 3 ) precipitated from the reaction mixture. However, the authors do not explain the origin of HBr in the reaction. The treatment of hydrobromide 3 with mild alkaline agents produces 2-(2,3-dibromopropyl)thiobenzimidazole ( 3а ), which undergo cyclization into 3-bromo-2,3,4,10-tetrahydrobenzimidazo[2,1- b ][1,3]thiazinium bromide ( 4 ) and isomeric 2 Н -benz-imidazo[2,1- b ][1,3]thiazine ( 5 ) and 4 Н -benzimidazo[2,1- b ][1,3]thiazine ( 6 ) at room temperature in acetone or acetonitrile solution or in the melt (Scheme 1). The mixture of thiazines 5 and 6 is also obtained by refluxing bromide 4 in methanol solution of alkali.
We have earlier reported iodo- and bromocyclization of 3-allylthio-1,2,4-triazoles [14–16]. Notably, bromination of 3-allylthio-1,2,4-triazole and 2-allylthiobenzthiazole with the structure similar to that of compound 2 leads to predominant annelation of thiazole ring. In order to make these issues clear, we have attempted to study the reaction between 2-allylthio-benzimidazole 2 and bromine in more detail.
Experimental
1H NMR spectra were recorded for DMSO- d 6 or CDCl 3 solutions of compounds on a Bruker DRX-400 instrument (400 МHz) using Me 4 Si as the internal standard.
2-Allylthiobenzimidazole (2). To the solution of 2-mercaptobenzimidazole 1 (0.300 g, 2 mmol) in DMF (5 mL) a solution of КОН (0.112 g, 2 mmol) in water (2 mL) and 3-bromopropene (0.18 mL, 2 mmol) were added. After 24 h, the reaction mixture was poured into water (50 mL) and the produced white precipitate was filtered off, washed with water and dried. The precipitate was recrystallized from the hexane–chloroform mixture (1:1) to give compound 2 as a white solid. Yield 0.266 g (70 %), mp 137 оС.
Bromination of 2-allylthiobenzimidazole (2). To a solution of 2-allylthiobenzimidazole 2 (0.190 g, 1 mmol) in acetic acid (3 mL) at 0–5 °С a solution of bromine (0.05 mL, 1 mmol) in acetic acid (3 mL) was added dropwise. The produced white precipitate was filtered off and washed with acetone. Yield 0.300 g (the mixture of compounds 3, 4, 7 and 8). The solvent was removed from the filtrate; the residue was treated with acetone, after removing acetone a white solid was obtained. Yield 0.103 g (the mixture of compounds 3–8).
Results and Discussion
Initial 2-allylthiobenzimidazole 2 was synthesized by the reaction of 2-mercapto-benzimidazole 1 with 3-bromopropene in the DMF–KOH medium (Scheme 1).
Scheme 1. Synthesis and bromination of 2-allylthiobenzimidazole (2)
We have studied the interaction of allyl sulfide 2 with bromine in acetic acid at the allylsulfide 2 :bromine ratio of 1:1.5 under conditions similar to those in [11]. Study of the precipitated product by 1H NMR method have made it possible to reveal not only hydrobromide 3 , but also benzimidazothiazi-nium bromide 4 and the second bromocyclization product, 3-bromomethyl-2,3-dihydro-9 Н -benzimidazo-[2,1- b ]thiazolium bromide ( 7 ) resulting from the thiazole ring annelation. From 1H NMR data, the ratio of compounds 3 , 4 and 7 is ~ 1.0 : 0.3 : 0.1. There is a logical question, why only hydrobromide 3 is revealed by the authors of paper [11]. In our opinion, this is due to the fact that 1H NMR studies were carried out by us on a Bruker instrument (400 МHz), whereas the authors of [11] used a Gemini 200 instrument (200 МHz).
Apparently, bromide 4 was produced via intramolecular nucleophilic substitution of adduct 3a .
The adduct of bromine as hydrobromide 3 was likely formed due to a partial cleavage of HBr from bromides 4 and 7 as a result of their interaction with 2-(2,3-dibromopropyl)thiobenzimidazole 3а . Thus, bromides 4 and 7 were converted into bases which remained in the solution. This fact may also explain a high prevalence of hydrobromide 3 in the mixture of obtained salts 3 , 4 and 7 .
Notably, a thorough analysis of the 1H NMR spectra recorded for the resulting mixture has allowed us to identify not only abovementioned compounds 3 , 4 and 7 , but also the adduct of bromine different from compound 3 , namely, 2-[2-bromo-1-(bromomethyl)ethyl]thiobenzimidazole hydrobromide ( 8 ) (HBr was cleaved from bromides 4 and 7 ). It could be produced via intermediate formation of bromonium ion ( I ) that was able to undergo intramolecular rearrangement into thiiranium ion ( II ), which was then easily converted into the adduct of bromine of symmetrical structure 8 (Scheme 2). The intramolecular rearrangement of bromonium ion into thiiranium ion has first been studied in the bromination of methylallyl sulfide [17]. In addition, this rearrangement has also been revealed by us during the study of bromination of 3-allylthio-1,2,4-triazoles [16].
Ильиных Е.С., Ким Д.Г.
Исследование реакции 2-аллилтиобензимидазола с бромом
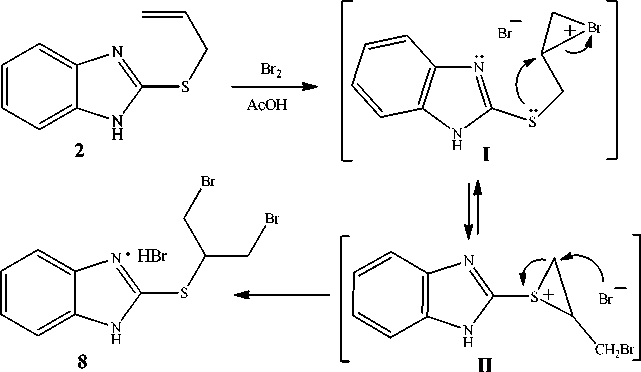
Scheme 2. The mechanism of formation of 2-[2-bromo-1-(bromomethyl)ethyl]thiobenzimidazole hydrobromide (8)
1H NMR spectral data of the synthesized compounds are shown in the table.
Table
1H NMR spectral data of the synthesized compounds
|
Compound |
1H NMR spectra, δ, ppm ( J , Hz) |
|
2 |
4.04 (2Н, d, 3 J = 6.8, –SCH 2 –); 5.07 (1H, m, =СН 2 ); 5.25 (1H, m, =СН 2 ); 6.03 (1Н, m, –С Н =); 7.11 (2Н, m, Н arom ); 7.46 (2Н, m, Н arom ) |
|
3 |
4.05 (2Н, m, –SCH 2 –); 4.10 (2Н, m, –CH 2 Br); 4.86 (1Н, m, –C H Br–); 7.50 (2Н, m, Н arom ); 7.73 (2Н, m, Н arom ) |
|
4 |
3.98 (1Н, m, –SC H A H B –); 4.02 (1Н, m,–SCH A H B –); 4.61 (1Н, m, –NC H M H N –); 4.73 (1Н, m,–NCH M H N –); 5.08 (1Н, m, –C H X Br–); 7.42 (2Н, m, Н arom ); 7.78 (2Н, m, Н arom ) |
|
7 |
4.05 (1Н, m, –C H A H B Br); 4.10 (1Н, m, –CH A H B Br); 4.13 (1Н, m, –SC H M H N –); 4.56 (1Н, m, –SCH M H N –); 5.19 (1Н, m, –+NC H X –); 7.44 (2Н, m, Н arom ); 7.76 (2Н, m, Нarom) |
|
8 |
3.94 (2Н, dd, 2 J = 11.0, 3 J = 6.6, -CH 2 Br); 3.98 (2Н, dd, 2 J = 11.0, 3 J = 6.6, -CH 2 Br); 4.63 (1Н, m, -SC H <); 7.52 (2Н, m, Н arom ); 7.73 (2Н, m, Н arom ) |
A set of proton signals of both bromocyclization products, bromides 4 and 7 , contained signals for aromatic protons shifted downfield and multiplet for the –C H X Br– proton ( δ 5.08 ppm) and the -+NC H Х – proton ( δ 5.19 ppm) , respectively, as well as the characteristic proton signals for such ABMNX spin systems.
In the 1H NMR spectrum of compound 8 the –SC H < proton was revealed as multiplet at δ 4.63 ppm. Two-proton doublets of doublets of four –(CH 2 Br) 2 protons having a double integral intensity appeared at δ 3.94 and 3.98 ppm.
The filtrate obtained after separation of the mixture of products 3 , 4 , 7 , and 8 has been also studied (such study was not carried out by the authors of paper [11]). After evaporation of acetic acid we managed to obtain a crystalline precipitate which was the mixture of compounds 3 , 4 , 7 , and 8, as well as 2 H -thiazine 5 and 4 H -thiazine 6 (from 1H NMR data). Formation of thiazines 5 and 6 as by-products of this reaction has also been reported previously [11]. 1H NMR spectral data produced by us for the mentioned compounds are identical to the published ones [11].
Bromination of compound 2 has also been studied by us in the 1H NMR experiment. To its solution in CDCl3 a solution of an equimolar amount of bromine in CDCl3 was added, and after 1 hour 1H NMR spectrum of the reaction mixture was recorded. Compound 2 reacted with bromine at once as the spectrum recorded did not contain characteristic signals for the alkenyl protons. From 1H NMR data, bromine adducts 3 and 8 at the ratio of ~ 1:3 predominated in the studied reaction mixture. These results indicate that the bromocyclization products 4 and 7 were probably formed directly from intermediate bromine adducts 3 and 8.
To summarize, allyl sulfide 2 reacted with bromine to give the mixture of adducts 3a and 8 , as well as bromide 7 formed from adduct 8 . Compound 3a was partially converted to bromide 4 , and partly it reacted with bromide 7 to give hydrobromide 3 via cleavage of HBr from bromide 7 . Thiazines 5 and 6 were formed in minor amounts by the reaction between adduct 3a or 8 and bromide 4 followed by the removal of two HBr molecules.
Conlusions
It has been found that the interaction of 2-allylthiobenzimidazole 2 with bromine in acetic acid proceeds to give not a single product as reported in [11] but the mixture of two bromocyclization products, 3-bromo-2,3,4,10-tetrahydrobenzimidazo[2,1- b ][1,3]thiazinium and 3-bromomethyl-2,3-dihydro-9 Н -benzimidazo[2,1- b ]thiazolium bromides, and two products of bromine addition to the double bond of allyl moiety with symmetrical and asymmetrical structure. It has been found by 1H NMR experiment that at the initial stage of bromination of 2-allylthiobenzimidazole bromine adducts are formed, and then they are partially converted into the bromocyclization products.
This work was supported by the program «U.M.N.I.K. 1-14-11» of the Fund for Assistance to Small Innovative Enterprises in the scientific and technical sphere.
Список литературы Study on reaction of 2-allylthiobenzimidazole with bromine
- Preparation of Halloysite Nanotubes Supported 2-Mercaptobenzimidazole and Its Application in Natural Rubber/B. Zhong, Z. Jia, Y. Luo et al.//Compos. Part A. -2015. -Vol. 73. -P. 63-71 DOI: 10.1016/j.compositesa.2015.03.007
- Preparation of Silica-supported 2-Mercaptobenzimidazole and Its Antioxidative Behavior in Styrene-butadiene Rubber/B. Zhong, Q. Shi, Z. Jia et al.//Polym. Degrad. Stab. -2014. -Vol. 110. -P. 260-267 DOI: 10.1016/j.polymdegradstab.2014.09.008
- Moreira, J.C. Adsorption of Cu(II), Zn(II), Cd(II), Hg(II) and Pb(II), from Aqueous Solutions on a 2-Mercaptobenzimidazole -Modified Silicagel/J.C. Moreira, C.P. Luiz, G. Yoshitaka//Mikrochim. Acta II. -1990. -Vol. 102. -P. 107-115 DOI: 10.1007/BF01244293
- Pourreza, N. Determination of Mercury in Water and Fish Samples by Cold Vapor Atomic Adsorption Spectrometry after Solid Phase Extraction on Agar Modified with 2-Mercaptobenzimidazole/N. Pourreza, K. Ghanemi//J. Hazard. Mater. -2009. -Vol. 161. -No. 2-3. -P. 982-987 DOI: 10.1016/j.jhazmat.2008.04.043
- Pourreza, N. Nano-TiO2 Modified with 2-Mercaptobenzimidazole as an Efficient Adsorbent for Removal of Ag(I) from Aqueous Solutions/N. Pourreza, S. Rastegarzadeh, A. Larki//J. Ind. Eng. Chem. -2014. -Vol. 20. -No. 1. -P. 127-132 DOI: 10.1016/j.jiec.2013.04.016
- Scunning Microelectrochemical Characterization of the Anti-corrosion Performance of Inhibitor Films Formed by 2-Mercaptobenzimidazole on Copper/J. Izquierdo, J.J. Santana, S. González et al.//Prog. Org. Coat. -2012. -Vol. 74. -No. 3. -P. 526-533 DOI: 10.1016/j.porgcoat.2012.01.019
- 2-Mercaptobenzimidazole Derivatives Possessing Pharmacological Activity/S.B. Seredenin, J.A. Blendov, V.L. Saveliev et al.//US Patent 6376666, 2002.
- Al-Rashood, K.A. Thiazolobenzimidazoles: Synthetic Strategies, Chemical Transformations and Biological Activities (Review)/K.A. Al-Rashood, H.A. Abdel-Aziz//Molecules. -2010. -Vol. 15. -No. 6. -P. 3775-3815 DOI: 10.3390/molecules15063775
- Dawood, K.М. Recent Advances on the Synthesis of Azoles, Azines and Azepines Fused to Benzimidazole/K.М. Dawood, N.M. Elwan, B.F. Abdel-Wahab//ARKIVOC. -2011. -(i). -P. 111 195.
- Ким, Д.Г. Реакция 2-алкенилтиобензимидазолов с иодом/Д.Г. Ким, В.В. Авдин, Л.В. Гаврилова//Химия гетероцикл. соед. -1997. -№ 8. -С. 1130-1132.
- Коротких, Н.И. Гетероциклизация 2-аллилтиобензимидазолов в производные бензимидазо-1,3-тиазинов/Н.И. Коротких, Г.Ф. Раенко, О.П. Швайка//Химия гетероцикл. соед. -1995. -№ 3. -C. 410-415.
- Реакции галогенциклизации и рециклизации: синтез тиирановых, тиетановых и селенетановых производных азолонов/Н.И. Коротких, А.Ф. Асланов, Г.Ф. Раенко и др.//Ж. орган. химии. -1999. -Т. 35, Вып. 5. -C. 752-761.
- Хемо-и региоселективность в реакциях галогенциклизации замещенных 2-алкенилтио-бензимидазолов/Н.Ю. Сливка, Ю.И. Геваза, В.И. Станинец и др.//Укр. хим. журн. -2003. -Т. 69, № 9-10. -C. 104-110.
- Ильиных, Е.С. Иодциклизация of S-аллильных производных 3-меркапто-4-метил-1,2,4-триазола/Е.С. Ильиных, Д.Г. Ким//Химия гетероцикл. соединений. -2011. -№ 5. -С. 766-769.
- Synthesis of Novel Fluorine-and Iodine-containing Triazolothiazines Based 3 (Alkenylthio)-5-(Trifluoromethyl)-4H-1,2,4-Triazole-3-Thiols/E.S. Il’inykh, D.G. Kim, M.I. Kodess et al.//J. Fluorine Chem. -2013. -Vol. 149. -P. 24-29 DOI: 10.1016/j.jfluchem.2013.01.025
- Ильиных, Е.С. Гетероциклизация алкенильных и пропаргильных производных 1,2,4-три-азол-3-тионов: автореф. дис. … канд. хим. наук/Е.С. Ильиных. -Екатеринбург, 2013. -24 с.
- Bland, J.M. An Intramolecular Bromonium to Thiiranium Ion Rearrangement/J.M. Bland, C.H. Stammer//J. Org. Chem. -1983. -Vol. 48. -No. 23. -P. 4393-4394 DOI: 10.1021/jo00171a049


