Study on the influence of the additives for the thermophysical properties of paraffin-containing dispersed systems
Автор: Rezida G. Rakhmatullina, Gulnara U. Yarmukhametova, Albina R. Maskova, Alexei A. Rusinov
Журнал: Nanotechnologies in Construction: A Scientific Internet-Journal @nanobuild-en
Рубрика: Development of new materials
Статья в выпуске: 3 Vol.15, 2023 года.
Бесплатный доступ
Introduction. Particular interest for research has dispersed systems. Dispersed systems are multiphase systems, consisting of a quantity of smallest particles, evenly distributed in the liquid, gaseous or solid medium. Such systems are the majority of the real bodies around us: soil, bodies of plant and animal life, clouds and fogs, many industrial products, such as building materials, metals, polymers, paper, leather, fabrics and foodstuffs. Disperse systems have many unusual physical properties, which require separate study and are of great importance in practice. Methods and materials. This article explores the influence ion-forming additives on the thermophysical properties of paraffin-containing disperse systems. The widespread use of ion-forming additives is associated with a number of positive features. Ion-forming additives is a new type of modifiers of crystalline solid phase and liquid crystals, significantly affect the process of micelle formation of paraffin in dispersed paraffin-containing environment. Therefore, studies on their effect on the processes of nucleation and growth of crystals are of particular interest. During the research, samples were prepared with various ion-forming additives and obtained temperature dependences of dielectric permittivity for dispersed systems petrolatum – methyl ethyl ketone – water solution of NaCl and KCl. Polynomial models are selected as the model type, which are widely used in the processing of various data, including experimental, and are also an effective tool for solving a wide range of scientific and technical problems. Results and discussion. It has been established that an increase in the degree of crystallinity of the solid phase with the introduction of additives is associated with a decrease in surface tension and increase in secondary nucleation. Resulting universal regression dependence of permeability allows us to describe the experimental results with the reliability of the approximation 0.99. Conclusion. The analysis of the obtained results shows reliability of the considered regression models and their applicability in practice, as well as in further studies of the dynamics of the process in disperse systems and the process of nucleation and growth of crystals.
Dispersed systems, the dielectric constant, ion-forming additives, coefficient of determination, polynomial model, x-ray diffraction analysis, degree of crystallinity, response function
Короткий адрес: https://sciup.org/142238291
IDR: 142238291 | DOI: 10.15828/2075-8545-2023-15-3-251-257
Текст научной статьи Study on the influence of the additives for the thermophysical properties of paraffin-containing dispersed systems
Original article
D ispersed systems represent uniform distribution in the form of very small particles (dispersion) of one body (dispersed phase) in another (dispersion medium). The properties of such systems are determined by their degree of dispersion, particle size of the dispersed phase, and the presence of a highly developed interfacial surface. The particles of the dispersed phase are aggregates, made up of many molecules or ions. A huge variety of objects of dispersed systems classified according to the degree of
dispersion, state of aggregation, interfacial interaction between particles.
The world around us, like ourselves, consists of dispersed systems. They are ubiquitous in nature and can be found in various technological processes. Knowledge of patterns, inherent in dispersed systems, it is necessary to obtain various materials with desired properties: polymers, medicines, foodstuffs, lubricants, cement, ceramics, paper, as well as in solving problems of environmental protection. Therefore, the study of such systems is of particular interest. A special place in disperse systems is
DEVELOPMENT OF NEW MATERIALS occupied by solutions of macromolecular substances and solutions of surfactants.
Methods and ways for obtaining disperse systems are very different. Dispersed systems can be obtained by dispersion and condensation methods. Dispersion methods include methods of mechanical, ultrasonic, electric, etc. fragmentation of the phase substance in the medium with the presence of a stabilizer, contained in the system or introduced into it from outside. The most common stabilizer is the electrolyte. Condensation methods include methods of “physical” or “chemical” condensation, that is, condensation, which is a consequence of a physical or chemical process occurring in the system [1–5].
Dispersed systems are formed during deparaffination and de-oiling in the process of cooling the raw material solution, in which the phase is solid hydrocarbons, and the medium is a solution of liquid components in the solvent used. In such systems, under the influence of electric fields electrokinetic phenomena occur, the study of which is very important in solving practical problems, associated with paraffin deposits in pipelines, separation of the dispersed phase in the form of a precipitate and electrocoagulation.
In deparaffination and de-oiling processes, based on the release of solid hydrocarbons by method of crystallization from a solution in selective solvents, of great importance is the cooling rate of suspensions. This is one of the main factors determining the size and degree of aggregation of crystals, on which the rate of phase separation also depends. When solid hydrocarbons are released in inhomogeneous electric fields the cooling rate of suspensions has practically no effect on the performance of the separation process, since the size of the crystals is not decisive due to the lack of a filtration step.
In the method of de-oiling of dispersed systems a new type of crystal structure modifiers is used. The latter allow to increase the filtration rate and the depth of de-oiling.
When de-oiling dispersed systems, an important role is played by the process of crystal formation. During the crystal formation of dispersed systems (petrolatums) many factors has influence, for example, composition of raw materials, solvent composition, temperature conditions of crystal formation. From how the solid phase is formed, the productivity of the plant for raw materials depends [6–15]. Structure modifiers have a significant effect on the solid phase, because of that, the study of their impact on processes is of both theoretical and practical interest.
METHODS AND MATERIALS
Petrolatum de-oiling method uses a new type of crystal structure rearrangement modifiers. The widespread use of ion-forming additives is associated with a number of positive features. Additives, in fact, are criminally active phenomena, concentrated on the interface, forming
thin adsorption layers or being captured by osmotic traps. In this case, the nature of the ionic interaction and the properties of the interfacial surfaces change sharply. As a result, it is possible to enhance the transition of a substance through the phase interface due to the compression of the electrical double layer.
Despite the existing theoretical prerequisites for explaining the mechanism of action of ion-forming additives in low molecular weight dispersed polymer systems [16–21], the complexity of these systems in some cases predetermines the ambiguous interpretation of the experimental results. To clarify the mechanism of action of additives, we undertook additional studies of these systems.
The purpose of the research is to study the effect of ion-forming additives on the thermophysical properties of paraffin-containing dispersed systems using the resonance method (Q-metry) and the regression modeling method.
Among the resonance methods used in this area, the Q-metry method is the simplest in terms of the nature of the work. Obtaining the studied compositions was carried out as follows: petrolatum at a temperature of 50оС to 65оС was mixed with a polar solvent (in our case with methyl ethyl ketone (MEK)). Sodium chloride dissolved in water was added to the solution at the same temperature. With constant stirring, cooling was carried out in a bulk crystallizer.
Dielectric measurements were performed at a fixed frequency 15 kHz using the resonance method. The measurements were carried out using a series equivalent circuit of a dielectric cell with a sample in accordance with its significant conductivity.
The functional dependence of temperature and dielectric permittivity of disperse systems with ion-forming additives was studied by regression modeling methods. As the type of model, polynomial models are chosen, which are widely used in the processing of various data, including experimental, and are also an effective tool for solving a wide range of scientific and technical problems. Their prevalence is due to the possibility of expanding the studied functional dependence in a Taylor series, which has a fast convergence, which allows limiting the number of expansion terms. Polynomial models are used to predict the value of a function at any point in the interval under study with a given degree of accuracy, which in turn gives the prospect of reducing the number of experiments [22–25].
RESULTS AND DISCUSSION
During the research, samples with various ion-forming additives were prepared and the temperature dependences of the dielectric permittivity for dispersed systems were obtained. On Fig. 1 and 2 present data on the dielectric constant versus temperature for dispersed systems petrolatum – MEK – water.
DEVELOPMENT OF NEW MATERIALS
From Fig. 1 and 2, it can be seen that with increasing temperature, a linear increase in the dielectric permittivity. A linear relationship can take place only when the electrical double layer moves with the surface, and its resulting electrical capacitance is determined by the capacitance of the electrical double layer.
On Fig. 3–5 shows changes in the temperature dependence of the dielectric constant of the system petrolatum – MEK – water solution of NaCl.
On Fig. 6–8 shows changes in the temperature dependence of the dielectric constant of the system petrolatum – MEK – water solution of KCl.
The results given above in the system petrolatum – MEK – water solutions of NaCl and KCl salts (Fig. 3–8) indicate changes in the dielectric permittivity.
It should be noted that there are slight changes in the dielectric permittivity before the phase transition and
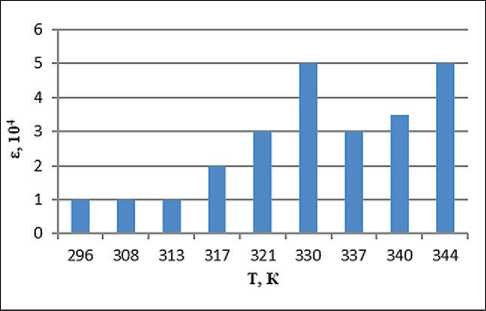
Fig. 3. Temperature dependence of the dielectric permittivity of the system with the addition of ion-forming additives of petrolatum 1:8, methyl ethyl ketone 100% and 0.5% water solution of NaCl
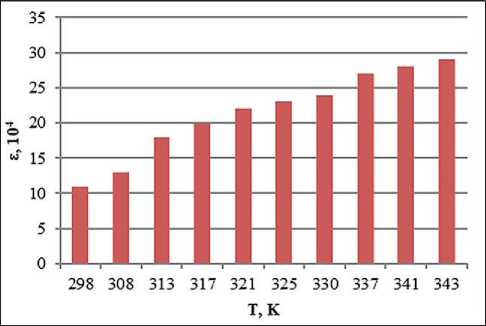
Fig. 1. Temperature dependence of the dielectric permittivity of the petrolatum system 1:8, methyl ethyl ketone 100% and 5% H2O
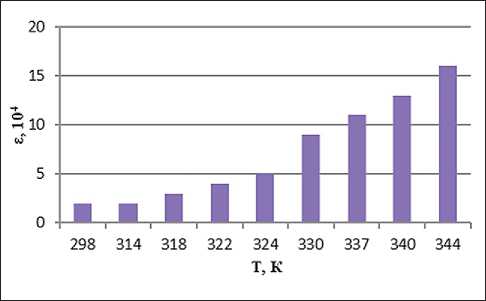
Fig. 4. Temperature dependence of the dielectric permittivity of the system with the addition of ion-forming additives of petrolatum 1:8, methyl ethyl ketone 100% and 1% water solution of NaCl
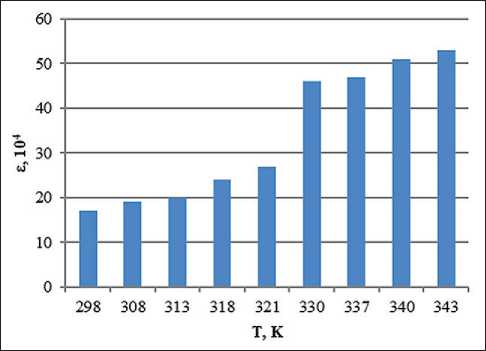
Fig. 2. Temperature dependence of the dielectric permittivity of the petrolatum system 1:8, methyl ethyl ketone 100% and 15% H2O
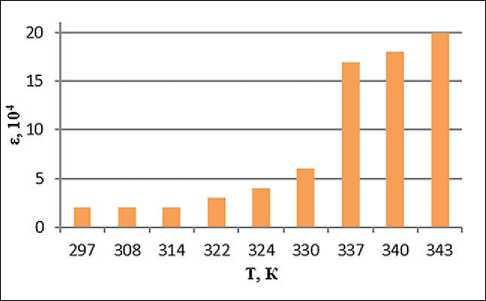
Fig. 5. Temperature dependence of the dielectric permittivity of the system with the addition of ion-forming additives of petrolatum 1:8, methyl ethyl ketone 100% and 2% water solution of NaCl
DEVELOPMENT OF NEW MATERIALS
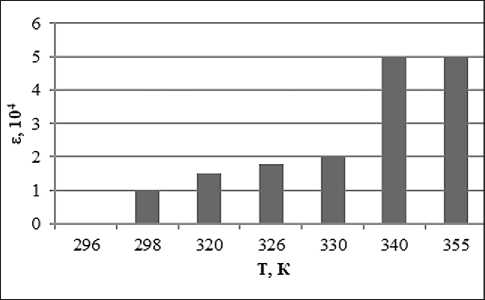
Fig. 6. Temperature dependence of the dielectric permittivity of the system with the addition of ion-forming additives of petrolatum 1:8, methyl ethyl ketone 100% and 0.5% water solution of KCl
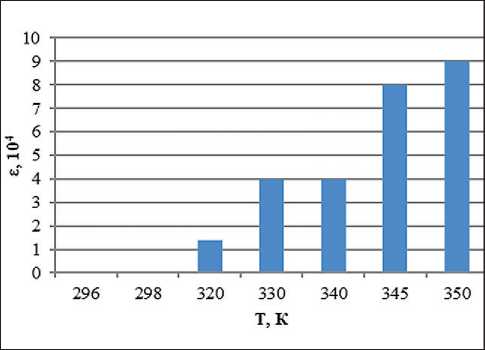
Fig. 7. Temperature dependence of the dielectric permittivity of the system with the addition of ion-forming additives of petrolatum 1:8, methyl ethyl ketone 100% and 1% water solution of KCl
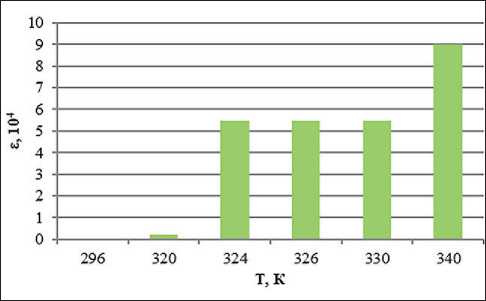
Fig. 8. Temperature dependence of the dielectric permittivity of the system with the addition of ion-forming additives of petrolatum 1:8, methyl ethyl ketone 100% and 2% water solution of KCl
a sharp increase in its value in the region of the transition itself. Since in this case the electrical capacitance of the crystallites increases sharply due to the rapidly growing crystal faces, associated with a decrease in surface tension during the formation of osmotic traps. In a real situation, apparently, further growth is possible only due to the penetration of molecules on the crystal surface.
In the absence of water solutions of salts, the degree of crystallinity reaches a low 10–15%. In the presence of aqueous solutions of KCl and NaCl salts в системе MEK – petrolatum the degree of crystallinity increases and is, respectively 40% and 70%.
In this case, the degree of crystallinity especially increases in the presence of an water solution of KCl, in this case, large crystals grow, while small crystals form in an water solution of NaCl.
Polynomial regression model in general:
ε = a0 + a1T + a2T2 + a3T3 + a4T4 + a5T5 +
+ a6T6+ … + anTn + …, (1)
where a0, a1, a2, a3, …, an – coefficients of the model calculated by the method of least squares, 104/К; ε – the dielectric constant of dispersed systems with low molecular polymer additives, 104; Т – temperature, К.
To assess the quality of the constructed models and limit the terms of the expansion (1) used the coefficient of determination R2, which characterizing the degree of similarity between experimental and calculated values. Obviously, if the models do not correlate well with the original values, they are unlikely to have good predictive power. The coefficient of determination is ranked according to the Chaddock scale, according to which, at values of 0.7 and above, there is a close relationship between the response function ( ε ) and factor ( Т ).
Calculations of the main parameters (1) were carried out using the “Data Analysis” add-on in the Microsoft Excel environment [22–25]. In Table 1 shows the calculated coefficients of models and determinations.
According to the results of the calculations (Table 1), it follows that the coefficient of determination for the constructed models lies in the range from 0.76 to 0.99. This indicates a high relationship between the dielectric permittivity and temperature of the systems under study, as well as the applicability of models (1) for predicting intermediate values of the experiment (Fig. 9).
In Table 2 shows an example of calculating the predicted value of the dielectric permittivity of the MEK 100% + 10% H2O disperse system using model (1).
CONCLUSIONS
Using the resonance method and regression modeling, the influence of additives and the mechanism of ac-
DEVELOPMENT OF NEW MATERIALS
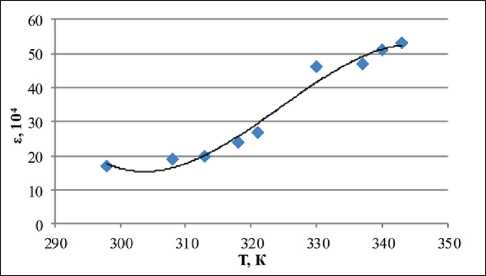
Table 1
Regression analysis results
|
Response function, ε |
Model Coefficients |
Determination coefficien, R2 |
|||
|
a0 |
a1 |
a2 |
a3 |
||
|
Dielectric permittivity of MEK 100% + 15% Н2О |
3562.3 |
–331.85 |
1.0272 |
–0.0011 |
0.98 |
|
Dielectric permittivity of MEK 100% + 10% Н2О |
3852.9 |
–37.675 |
0.1217 |
–0.0001 |
0.98 |
|
Dielectric permittivity of MEK 100% + 5% Н2О |
3764.8 |
–34.561 |
0.1052 |
–0.0001 |
0.99 |
|
Dielectric permittivity of P–M–water solution of NaCl 0,5% system |
3929.6 |
–37.07 |
0.1162 |
–0.0001 |
0.76 |
|
Dielectric permittivity of P–M–water solution of NaCl 1% system |
4901.2 |
–43.569 |
0,1277 |
–0.0001 |
0.99 |
|
Dielectric permittivity of P–M–water solution of NaCl 2% system |
–5549.2 |
56.932 |
–0.1941 |
0.0002 |
0.97 |
|
Dielectric permittivity of P–M–water solution of KCl 0,5% system |
65.752 |
–0.4773 |
0.0009 |
– |
0.86 |
|
Dielectric permittivity of P–M–water solution of KCl 1% system |
319.54 |
–2.1304 |
0.0036 |
– |
0.95 |
|
Dielectric permittivity of P–M–water solution of KCl 2% system |
418.11 |
–2.8334 |
0.0048 |
– |
0.82 |
Note: P – petrolatum, М – methyl ethyl ketone
Table 2
The results of the calculation of the dielectric permittivity of the system MEK 100% + 10% Н2О
Fig. 9. Polynomial model of dielectric permittivity and temperature of a dispersed system MEK 100% + 10% Н2О
DEVELOPMENT OF NEW MATERIALS for new technological processes of high-temperature deoiling of ceresins and paraffins.
A universal regression dependence of the permeability is obtained, which makes it possible to describe the experimental results with the reliability of the approximation 0.99.
The analysis of the obtained results showed the reliability of the considered regression models and their applicability in practice, as well as in further studies of the dynamics of the process in disperse systems and the process of nucleation and growth of crystals.
Список литературы Study on the influence of the additives for the thermophysical properties of paraffin-containing dispersed systems
- Vonetsky S.S. Colloid Chemistry Course. M.: Chemistry; 1964.
- Evstratova K.I., Kupina N.A., Malakhova E.E. Physical and colloidal chemistry: Textbook . Ed. K.I. Evstratova. M.: Higher shk.; 1990.
- Mushkambarov N.N. Physical and colloidal chemistry: Textbook. М.: GEOTAR – MED; 2001.
- Ershov Yu.A. Colloidal chemistry. physical chemistry of dispersed systems. Textbook for students of institutions of higher professional education studying in the specialty 060301 “Pharmacy” in the discipline “Physical and colloidal chemistry”. M.; 2012.
- Sunyaev Z.I. Oil dispersed systems. Mo.: MINGP im. Gubkin; 2011.
- Wenzel S.V. The use of lubricating oils in internal combustion engines. М.: Chemistry; 1999.
- Chernozhukov N.I., Krein S.E., Losikov B.V. Chemistry of mineral oils. М.: Gostoptekhizdat; 1999.
- Kazakova L.P., Krein S.E. Physical and chemical bases for the production of petroleum oils. М.: Chemistry; 1978.
- Nigmatullin R.G., Gainanov S.U., Telyashev G.G. Petrolatum deoiling method. Invention Patent RU 2052491 C1. 20.01.1996. Request № 93015322/04 от 23.03.1993.
- Rakhmatullina R.G., Maskova A.R., Garayshin A.I. Studies of relaxation processes of syndiotactic 1,2-polybutadiene. Bulletin of the Kazan State Technical University. A.N. Tupolev. 2021; 77 (1): 38–42.
- Patent 2027740 Russian Federation, C1. Method for deoiling slack and petrolatum. Karakuts V.N., Nigmatullin R.G., Zolotarev P.A., Telyashev G.G. № 5060336/04; req. 25.08.1992; publ. 27.01.1995.
- Pereverzev A.N., Bogdanov N.F., Roshchin Yu.N. Paraffin production. М.: Chemistry, 1973. 223 p.
- Usachev V.V. Carbamide dewaxing. М.: Chemistry; 1967.
- Rudakova N.Ya., Timoshina A.S., Cherepneva E.I. Paraffin production М.: Gostoptekhizdat; 1960.
- Patent. 2374301 Russian Federation, C1. The method of deparaffinization of oil raw materials. Pykhalova N.V., Kayralieva A.I., Shumeev A.M. № 2008124057/04; req. 11.06.2008; publ. 27.11.2009.
- Nigmatullin R.G., Zolotarev P.A., Saifullin N.R. Purification of oils in the ion field. Chemistry and technology of fuels and oils. 1995; 6: 34–36.
- Nigmatullin R.G. Deoiling of sludge with the use of an ionic modifier – iron sulfate. Chemistry and technology of fuels and oils.1997; № 2: 34–35.
- Nigmatullin I.R. Deparaffinization of paraffinic oil fractions using ion-forming additives. Oil refining and petrochemistry. Scientific and technological achievements and best practices. 2009; 11: 6-8.
- Severinovskaya O.V., Varzatsky O.A., Shul’ga S.V., Pokrovsky V.A., Gromovoi T.Yu. Application of an ionforming additive for mass-spectrometric analysis and identification of biological objects. Chemistry, Physics and Surface Technology. 2011; 2(3): 366–369.
- Antonov S.A., Bartko R.V., Matveeva A.I., Tonkonogov B.P., Kilyakova A.Yu., Filatov R.V., Dogadin O.B., Nikulshin P.A. The use of modifying additives in the process of solvent dewaxing. Chemistry and technology of fuels and oils. 2020; 4 (620):16–26.
- Maskova A.R., Yarmukhametova G.U., Rakhmatullina R.G., Sabitov I.N., Aminova G.K. Obtaining new additives for polyvinyl chloride compositions. Nanotechnologies in construction. 2022; 14(3):241–249. https://doi. org/10.15828/2075-8545-2022-14-3-241-249. EDN: BWZONW.
- Mathematical modeling. Theoretical basis. Materials for practical classes and independent work of students. Methodical instructions: educational and methodical complex. USPTU, department. PED; comp. G.U. Yarmukhametov. Ufa: USPTU; 2018.
- Middleton M.R. Analysis of statistical data using Microsoft Excel for Office XP. M.: BINOM. Knowledge Lab; 2005.
- Gmurman V.E. Guide to solving problems in probability theory and mathematical statistics. M.: Yurayt; 2011.
- Kolemaev V.A., Kalinina V.N. Probability Theory and Mathematical Statistics: Textbook (Higher Education Series). M.: Higher. shk.; 2000.


