Synthesis and halocyclization of allyl derivatives of 4,5-dihydro-1,3-thiazol-2-thione
Автор: Tarasova N.M., Kim D.G.
Журнал: Вестник Южно-Уральского государственного университета. Серия: Химия @vestnik-susu-chemistry
Рубрика: Органическая химия
Статья в выпуске: 2 т.7, 2015 года.
Бесплатный доступ
2-Propenyl-(2-methyl-1-propenyl-, 2-butenylthio- and (3-methylbutenyl-)thio4,5-dihydro-1,3-thiazoles are synthesized by alkylation of 4,5-dihydro-1,3-thiazol2-thione. These products were researched in halocyclization reactions with iodine and bromine.3-(halomethyl)-2,3,5,6-tetrahydrothiazolo[2,3-b]thiazolium and 6-halo-3,5,6,7-tetrahydro-2H-thiazolo[2,3-b]thiazinium halides were formed. The structure of all the synthesized compounds was confirmed by 1H NMR and gas chromatography-mass spectrometry.
5-dihydro-1, 3-thiazol-2-thione, 2-propenylthio-4, 5-dihydrothiazol, 2-(2-methyl-1-propenyl)thio-4, 2-butenylthio-4, 2-(3-methyl-butenyl)thio-4, 3-(halomethyl) 2, 6 tetrahydrothiazolo[2, 3-b]thiazolium, 6 halo 3, 7 tetrahydro-2h-thiazolo[2, 3-b]thiazinium halides, gas chromatography-mass spectrometry, 1h nmr, 3-b]тиазолия, 3-b]тиазиния
Короткий адрес: https://sciup.org/147160313
IDR: 147160313 | УДК: 547.789.13
Текст научной статьи Synthesis and halocyclization of allyl derivatives of 4,5-dihydro-1,3-thiazol-2-thione
Derivatives of 1,3-thiazoline have antimicrobial and antihelmintial activity, besides they are the effective immunomodulatory agents [1–3]. 2-Propenylthio-4,5-dihydro-1,3-thiazole was synthesized previously from alkyl derivatives of 4,5-dihydro-1,3-thiazol-2-thione ( 1 , 1,3-thiazoline-2-thione) [4]. The aim of the present study is the synthesis of new S-alkenyl derivatives of 4,5-dihydro-1,3-thiazol-2-thione and the investigation of their halocyclization reactions.
Discussion of results
We have found that 4,5-dihydro-1,3-thiazol-2-thione reacts with 3-bromopropene ( 2а ), 3-chloro-2-methylpropene ( 2b ), 4-bromobutene ( 2c ), 1-bromo-3-methyl-2-butene ( 2d ) to give 2-propenylthio-4,5-dihydrothiazole (2-allylthio-1,3-thiazoline) ( 3а ), 2-(2-methyl-1-propenyl)thio-4,5-dihydrothiazole ( 3b ), 2-butenylthio-4,5-dihydrothiazole ( 3c ), 2-(3-methylbutenyl)thio-4,5-dihydrothiazole ( 3d ) respectively (scheme 1) . Compounds 3а – d have been synthesized after treatment in ethanol with sodium ethylate as a base. Note that in similar conditions the mixture of 2-(2-methyl-2-propenyl)thio-5-methyl-1,3,4-thiadiazole with 2-(2-methyl-1-propenyl)thio-5-methyl-1,3,4-thiadiazole at the ratio 1:2 have been obtained from 5-methyl-1,3,4-thiadiazole-2-thione and methallylchloride 2b [5]. In this case the yield of compound 3b is 62%.
н
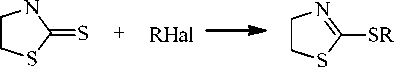
1 2a-d За-d
2a BrCH 2 CH=CH 2 ; 2b ClCH 2 C(CH 3 )=CH 2 ; 2c BrCH 2 CH 2 CH=CH 2 ; 2d BrCH 2 CH=C(CH 3 ) 2
Scheme 1. The alkylation of 4,5-dihydro-1,3-thiazol-2-thione
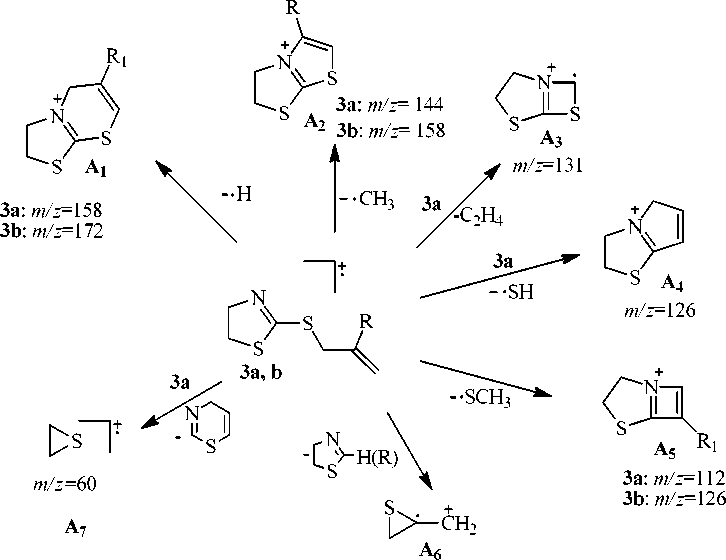
There is the molecular ion peak in the mass spectra (table 1, scheme 2, 3, 4) of compounds 3a–d. The fragmentation processes with the elimination of methyl radical and the formation of the stable thia-zolo-thiazolium system for compounds 3а, b (scheme 2), as well as the formation of the thiazolo-thiazinium system for compounds 3с, d (scheme 3, 4), are characteristic for all compounds. The peak
[М–СН 3 ]+ is maximum for compounds 3а, b . The hydrogen radical is eliminated from the molecular ion of compounds 3а - с with the formation 3,5-dihydro-2 H -thiazolo[2,3- b ][1,3]thiazinium cation ( А 1 ) for 3а, b and 5-methylene-3,5,6,7-tetrahydro-2H-thiazolo[2,3-b][1,3]thiazinium cation ( А 2 ) for 3с . The fragmentation of the molecular ion with the elimination of •SCH 3 radical and the formation of the stable carbocations 4-thia-1-azabicyclo[3.2.0]hepta-1(5),6-dienium (А 5 ) for compounds 3а, b and 7-methylene-4-thia-1-azabicyclo[3.2.0]hept-1(5)-enium ( B 8 ) for sulfide 3с is characteristic for compounds 3а – с .
Table 1
Mass-spectometry data of the investigated compounds
|
Compound |
Characteristic ions: m/z (I %) |
|
3а (С 6 H 9 NS 2 ) |
159 [М]+• (10), 158 [М–Н]+ (7), 146 (9), 144 [М – СН 3 ]+ (100), 131 [М – С 2 Н 4 ]+• (6), 126 [М – SН]+ (5), 112 [М – SCH 3 ]+ (8), 98 (9), 87 (6), 73 (15), 72 [М – С 3 SNН 3 ]+• (47), 61 (11), 60 [М – С 4 SNН 5 ]+• (57), 59 (37), 55 (6), 45 (29), 41 (39) |
|
3b (С 7 H 11 NS 2 ) |
173 [М]+• (˂ 1), 172 [М – Н]+ (˂ 5), 160 (10), 159 (9), 158 [М – СН 3 ]+ (100), 126 [М - SCH 3 ]+ (7), 112 (11), 87 (7), 86 (6), 72 [М – С 3 SNН 3 CH 3 ]+• (12), 61 (6), 60 (19), 59 (20), 55 (33), 39 (12) |
|
3c (С 7 H 11 NS 2 ) |
173[М]+• (9), 172 [М – Н]+ (19), 158 [М – СН 3 ]+ (8), 147 [М – С 2 Н 2 ]+• (5), 145 [М – С 2 Н 4 ]+• (52), 144 [М – С 2 H 5 ]+ (52),140 [М – SН]+ (43), 132 [М – С 3 Н 5 ]+ (5), 126 [М – SСН 3 ]+ (9), 119 [М – С 4 Н 6 ]+• (86), 87 (6), 86 (7), 85 (8), 72 [М – С 4 Н 6 NSH 2 ]+• (25), 62 (6), 61 (44), 60 [М – С 5 H 7 NS]+• (100), 59 (40), 58 (10) ,56 (20), 55 (55), 54 (9), 53 (14), 47 (6), 46 (11), 45 (29) |
|
3d (С 8 H 13 NS 2 ) |
187 [М]+• (21), 172 [М – СН 3 ]+ (7), 158 (8), 154 [М – SН]+(32),144 [М – С 3 Н 7 ]+(5), 140 (˂ 5%), 119 [М – С 6 Н 8 ]+• (21), 120 [М – С 5 Н 7 ]+ (6), 101 (10),100 (5), 69 [М–NSС 3 Н 9 S]+ (100), 68 (6), 67 (11), 61 (11), 60 [М–NSС 6 Н 9 ]+•, 59 (13), 45 (8), 41 (65), 39 (12) |
Peaks with intensity less than 5 % are not taken into account.
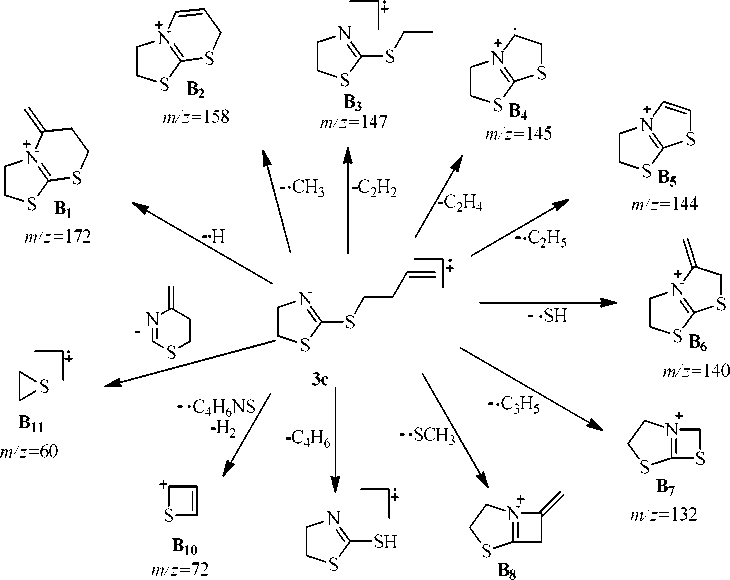
There are peaks corresponding to the elimination of a neutral ethylene molecule in the mass spectra of compounds 3а – с . Besides, there is the peak corresponding to the elimination of the acetylene and butadiene molecules in the spectrum of compound 3с .
m/z=T2
R = СН 3 ; 3а R 1 = H; 3b R 1 = CH 3
Scheme 2. Possible fragmentation processes of 2-propenylthio-4,5-dihydrothiazole (3а) and 2-(2-methyl-1-propenyl)thio-4,5-dihydrothiazole (3b)
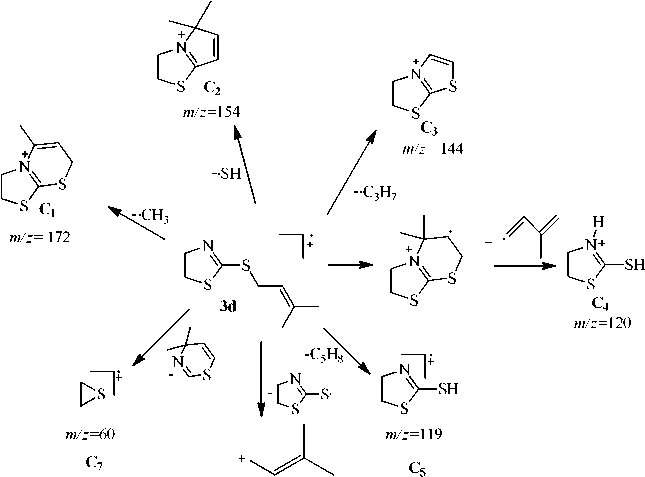
The peak of thiiranium radical cation ( В 11 ) which is formed by the elimination of the neutral 4-methylene-5,6-dihydro-4 H -1,3-thiazine molecule is maximum for compound 3с . Besides, this ion peak is present in spectra of compounds 3а, b , d , but its intensity is much lower. For compound 3а intensity corresponds to 54% and it is no more than 20 % for compounds 3b , d . The ion peak is m/z 69 corresponding to the cation С 6 which is formed in the result of the heterolytic disconnection of rhe C-S bond is maximal for compound 3d (scheme 4).
®9 /и/-=126
/и/-=119
Scheme 3. Possible fragmentation processes of 2-butenylthio-4,5-dihydrothiazole (3с)
m/z=69
С6
Scheme 4. Possible fragmentation processes of 2-(3-methylbutenyl)thio-4,5-dihydrothiazole (3d)
In 1H NMR spectra of compounds 3а – d (Table 2) the proton signals of SCH 2 and NCH 2 groups of the thiazine cycle are observed in the range of 3.39 – 3.40 and 4.22 – 4.23 ppm, respectively. The proton signals of SCH 2 group of alkenyl radical are observed in the range of 3.79–3.80 ppm for all synthesized compounds except sulfide 3с . SCH 2 group triplet belonging to the S-alkenyl radical of compound 3с is observed in a weaker field at 3.19 ppm. This is probably due to the fact that SCH 2 group of compound 3c is removed at greater distance from the double bond compared to other sulfides 3а, b, d.
Table 2
Spectral data of the synthesized compounds
|
Compound |
1H NMR spectrum, δ , ppm ( J , Hz) |
|
3а |
3.41 (2H, t, J =8.0, SCH 2ц ); 3.79 (2H, dd, J =0.86, J =6.89, SCH 2. ); 4.23 (2H, t, J =7.99, NCH 2 ); 5.16 (1Н, d, J =10.06, =CH 2 ); 5.32 (1H, dd, J =1.31, J =16.96, =CH 2 ); 5.93 (1H, m, CH=) |
|
3b |
1.83 (3H, s, CH 3 ); 3.39 (2H, t, J =7.98, SCH 2ц ); 3.80 (2H, s, SCH 2 ); 4.22 (2H, t, J =7.98, NCH 2 ); 4.90 (1H, m, =CH 2 ); 5.01 (1H, s, =CH 2 ) |
|
3с |
2.46 (2H, m, -CH 2 -); 3.19 (2H, t, J =7.32, SCH 2 ); 3.40 (2H, t, J =7.98, SCH 2ц ); 4.22 (2H, t, J =7.98, NCH 2 ); 5.09 (2H, m, =CH 2 ); 5.82 (1H, m, =CH-) |
|
3d |
1.70 (3H, s, CH 3 ); 1.74 (3H, s, CH 3 ); 3.41 (2H, t, J =7.99, SCH 2ц. ); 3.80 (2H, d, J =7.88, SCH 2ц ); 4.23 (2H, t, J =7.99, NCH 2 ); 5.31 (1H, m, CH=) |
|
4a |
3.69 (1H, dd, J =2.76, J =11.27, CH 2 I); 3.75 (2H, m, SCH 2 ); 4.11 (2H, m, SCH 2 ); 4.15–4.20 (2H, m, +NCH 2 ); 4.31 (1Н, dd, J =9.84, J =12.09 CH 2 I); 4.70 (1Н, m, +NCH) |
|
4b |
3.71 (1Н, m, SCH 2 ); 3.86 (1Н, dd, J =2.32, J =12.74, SCH 2 ); 4.20 (1Н, m, +NCH 2 ); 4.36–4.45 (3Н, m, +NCH 2 ); 4.82 (1Н, m, CHI) |
|
4c |
3.87 (1H, dd, J =6.51, J =12.14, +NCH 2 ); 3.97 (1H, dd, J =2.71, J =11.62, CH 2 Br); 4.13 (1H, m, +NCH 2 ); 4.22–4.08 (4H, m, SCH 2 ); 4.32 (1H, dd, J =10.03, J =12.12, CH 2 Br); 4.97 (1H, m, +NCH) |
|
4d |
3.68 (1H, m, SCH 2 ); 3.74 (2H, t, J =8.56, SCH 2 ); 3.93 (1H, m, SCH 2 ); 4.45-4.31 (4H, m, +NCH 2 ); 5.16 (1H, m, CHBr) |
|
4e |
1.70 (3H, s, CH 3 ); 3.81 (2H, k, J =11.25, SCH 2 ); 4.02 (2H, m, SCH 2 ); 4.12 (4H, m, +NCH 2 , CH 2 I) |
|
4f |
1.66 (3H, s, CH 3 ); 4.03 (2H, dd, J =4.16, J =11.91, SCH 2 ); 4.11 (2H, m, SCH 2 ); 4.12 (2H, m, +NCH 2 ); 4.17 (2H, m, CH 2 Br) |
|
4g |
2.25 (1H, m, -CH 2 -); 2.59 (1H, m, CH 2 ); 3.41 (2H, m, SCH 2 ); 3.53 (1H, dd, J =9.11, J =10.79, +NCH 2 ); 3.66 (1H, td, J 1 = J 2 = 9.13, J =11.12, +NCH 2 ); 3.74 (2H, m, SCH 2 ); 4.27 (1H, kd, J 1 = J 2 = 3.93, J =3.96, J =8.04, CH 2 I); 4.37 (1H, td, J 1 = J 2 = 9.11, J =12.25, CH 2 I); 4.71 (1H, m, +NCH) |
|
4h |
2.25 (1H, m, CH 2 ); 2.58 (1H, m, CH 2 ); 3.44 (2H, m, SCH 2 ); 3.71 (2H, m, SCH 2 ); 3.90 (1H, dd, J =7.65, J =11.11, +NCH 2 ); 3.99 (1H, dd, J =3.82, J =11.09, +NCH 2 ); 4.32 (2H, m, CH 2 Br); 4.70 (1H, m, +NCH) |
|
4i |
1.60 (3H, s, CH 3 ); 1.65 (3H, s, CH 3 ); 3.68 (2H, m, SCH 2 ); 3.73 (1H, dd, J =5.44, J =14.56, SCH 2 ); 4.12 (1H, dd, J =3.49, J =14.38, SCH 2 ); 4.45 (1H, td, J= 8.79, J =12.22, +NCH 2 ); 4.68 (1H, ddd, J =7.30, J= 8.67, J =12.24, +NCH 2 ); 5.14 (1H, dd, J =3.70, J =4.99, CHI) |
|
4j |
1.57 (3H, s, CH 3 ); 1.67 (3H, s, CH 3 ); 3.49 (1H, dt, J =4.48, J= 10.80, J =10.85, SCH 2 ); 3.72 (2H, dd, J =6.34, J =16.03, SCH 2 ); 4.14 (1H, dd, J =3.19, J =14.57, SCH 2 ); 4.46 (1H, td, J= 8.84, J =12.22, +NCH 2 ); 4.71 (1H, td, J= 7.94, J =12.27, +NCH 2 ); 5.25 (1H, dd, J =3.36, J =4.54, CHI) |
|
4k |
1.87 (3H, s, CH 3 ); 1.94 (3H, s, CH 3 ); 3.70 (2H, dd, J =1.38, J =10.36, SCH 2 ); 4.17 (2H, m, SCH 2 ); 4.27 (1H, dd, J =2.33, J =14.38, +NCH 2 ); 4.64 (1H, dd, J =2.27, J =10.81, +NCH 2 ); 5.50 (1H, m, +NCH) |
It was studied the halocyclization of synthesized sulfides (scheme 5). The interaction of compound 3а with halogens proceeds with the formation of the mixture of 3-(halomethyl)-2,3,5,6-tetrahydrothiazolo[2,3- b ]thiazolium polyhalide ( 4a, с ) and 6-halo-3,5,6,7-tetrahydro-2 Н -thiazolo[2,3- b ]thiazinium polyhalide ( 4b, d ), in the same way as 2-(propenylthio)-5-methyl-1,3,4-thiadiazole [6]. Monoiodide derivatives have been isolated by adding sodium iodide in acetone to the reaction mixture, monobromide derivatives have been isolated by the reaction of excess of bromine with acetone. It should be noted that bromocyclization reaction of compound 3а leads to lesser yield of the product with thiazine cycle (the ratio of 4с : 4d is 5:1), whereas the ratio of the products containing five- and sixmembered cycles is 2:1 for the bromocyclization reaction of 2-(allylthio)-5-methyl-1,3,4-thiadiazole.
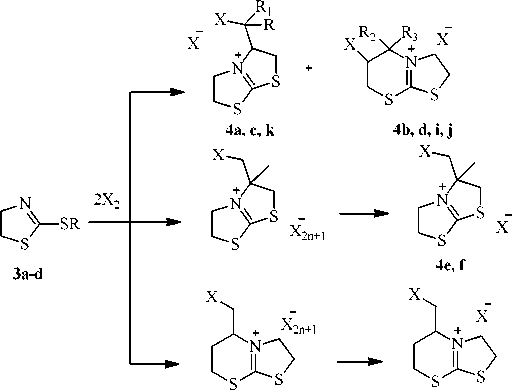
4g, h
4a : X=I, R=R 1 =H; 4b : X=I, R 2 =R 3 =H; 4c : X=Br, R=R 1 =H; 4d : X=I, R 2 =R 3 =H; 4e : X=I; 4f : X=Br;
4g : X=I; 4h : X=Br; 4i : X=I, R 2 =R 3 =CH 3 ; 4j : X=Br, R=R 1 =CH 3 ; 4k : X=Br, R 2 =R 3 =CH 3
Scheme 5. Halocyclization of S-derivatives of 4,5-dihydro-1,3-thiazol-2-thione
The halocyclization reaction of compound 3b proceeds with the formation of the single thiazolo-thiazolium system, which has been isolated as 3-(halomethyl)-3-methyl-2,3,5,6-tetrahydrothiazolo[2,3- b ]thiazolium halides 4e,f .
The halocyclization reaction of compound 3c proceeds with the formation of 3-(halomethyl)-3,5,6,7-tetrahydro-2 H -thiazolo[2,3- b ][1,3]thiazinium halides 4g,h .
The iodocyclization reaction of compound 3d proceeds with the annelation of the six-membered thiazine cycle and the formation of 6-iodo-5,5-dimethyl-3,5,6,7-tetrahydro-2 H -thiazolo[2,3- b ]thiazinium iodide in the same way as compound 3c . In its turn the interaction of compound 3c with bromine leads to the formation of the mixture of 3-(2-bromopropene)-2,3,5,6-tetrahydrothiazolo[2,3- b ]thiazolium bromide 4k and 6-bromo-5,5-dimethyl-3,5,6,7-tetrahydro-2 H -thiazolo[2,3- b ]thiazinium bromide 4j at the ratio of 1:5.
Experiment
The 1H NMR spectra of the synthesized compounds have been recorded on the Bruker DRX-400 (400 MHz) spectrometer in DMSO-d6, the internal standard is TMS. Mass-spectra (EI, 70 eV) have been recorded on the SHIMADZU GCMS QP-2010 Ultra gas chromatograph mass spectrometer.
2-Propenylthio-4,5-dihydrothiazole (2-allylthio-1,3-thiazoline) (3a), 2-(2-methyl-1-propenyl) thio-4,5-dihydrothiazole (3b), 2-butenylthio-4,5-dihydrothiazole (3c), 2-(3-methylbutenyl)thio-4,5-dihydrothiazole (3d).
-
1,3- Thiazoline-2-thione (1.19 g, 10 mmol) and 10 mmol haloalkene (3-bromopropene ( 2a ), 3-chloro-2-methylpropene ( 2b ), 4-bromobutene ( 2c ), 1-bromo-3-methyl-butene-2 ( 2d )) were added to the sodium solution (0.23 g) in 15 ml ethanol. The mixture was boiled during 3 hours, filtered off, evaporated of ethanol, extracted with diethyl ether. The substance was isolated as viscous yellow liquid. The product yields: 3а: (64 %); 3b (62 %); 3c (72 %); 3d (62 %).
3-(Iodomethyl)-2,3,5,6-tetrahydrothiazolo[2,3- b ]thiazolium iodide (4a), 6-iodo-3,5,6,7-tetrahydro-2 H -thiazolo[2,3- b ]thiazinium iodide (4b), 3-(iodomethyl)-3-methyl-2,3,5,6-tetrahydrothiazolo[2,3- b ]thiazolium iodide (4e), 3-(iodomethyl)-3,5,6,7-tetrahydro-2 H -thiazolo[2,3- b ][1,3]thiazinium iodide (4g), 6-iodo-5,5-dimethyl-3,5,6,7-tetrahydro-2 H -thiazolo[2,3- b ][1,3]thiazinium iodide (4i).
The compound 3a-d solution (1 mmol) in 3 ml alcohol was added to the iodine solution (0.5 g) in 5 ml alcohol, the solution was decanted after 72 hours, the remaining black substance was dissolved in acetone, then the double excess of NaI in acetone has added, the obtained precipitate was filtered off.
The substance was isolated as yellow crystalline powder. The product yields: 4а, b : 0.21 g; 4e : (51 %), m.p. 180-184 ° C (decomposition); 4g : (56 %), m.p. 158-160 ° C (decomposition); 4i : (46 %), m.p. 152 -154 ° С (decomposition).
3-(Bromomethyl)-2,3,5,6-tetrahydrothiazolo[2,3- b ]thiazolium bromide (4c), 6-bromo-3,5,6,7-tetrahydro-2 H -thiazolo[2,3- b ]thiazinium bromide (4d), 3-(bromomethyl)-3-methyl-2,3,5,6-tetrahydrothiazolo[2,3- b ]thiazolium bromide (4f), 3-(bromomethyl)-3,5,6,7-tetrahydro-2 H -thiazolo[2,3- b ][1,3]thiazinium bromide (4h), 6-halo-5,5-dimethyl-3,5,6,7-tetrahydro-2 H -thiazolo[2,3- b ][1,3]thiazinium bromide (4j), 3-(2-bromopropenyl)-2,3,5,6-tetrahydrothiazolo[2,3- b ]thiazolium bromide (4k).
The bromine solution (0.08 ml, 1.5 mmol) in 3 ml chloroform or CH 2 Cl 2 was added to the compound 3a-d solution (1 mmol) in 5 ml chloroform or CH 2 Cl 2 while stirring and cooling. The mixture left in hermetically closed flask in a refrigerator for 120 hours. The solution was decanted, the remaining orange viscous liquid was dissolved in acetone. The obtained white crystalline powder was filtered off. The product yields: 4с, d : 0.095 g; 4f : (57 %), m.p. . 214–216 °С (decomposition); 4h : (34 %), m.p. 150 –154 °С (decomposition); 4j, k : 0.18 g.
Conclusion
2-Propenylthio-4,5-dihydrothiazole (2-allylthio-1,3-thiazoline), 2-(2-methyl-1-propenyl)thio-4,5-dihydrothiazole, 2-butenylthio-4,5-dihydrothiazole, 2-(3-methylbutenyl)thio-4,5-dihydrothiazole have been obtained by the interaction of 4,5-dihydro-1,3-thiazole-2-thione with various haloalkene. 3-(Halomethyl)-2,3,5,6-tetrahydrothiazolo[2,3- b ]thiazolium, 6-halo-3,5,6,7-tetrahydro-2 H -thiazolo-[2,3- b ]thiazinium, 3-(halomethyl)-3-methyl-2,3,5,6-tetrahydrothiazolo[2,3- b ]thiazolium, 3-halomethyl-3,5,6,7-tetrahydro-2 H -thiazolo[2,3- b ][1,3]thiazinium, 6-halo-5,5-dimethyl-3,5,6,7-tetrahydro-2 H -thiazolo[2,3- b ][1,3]thiazinium halides and 3-(2-bromopropenyl)-2,3,5,6-tetrahydrothiazolo[2,3- b ]thiazolium bromide have been synthesized by the halocyclization reaction of the obtained sulfides. The structures of the synthesized compounds are proved by the gas chromatography–mass spectrometry and the nucleic magnetic resonance method 1H NMR.
Список литературы Synthesis and halocyclization of allyl derivatives of 4,5-dihydro-1,3-thiazol-2-thione
- Synthesis and antimicrobial evaluation of some new thiazoline and thiazolidinone derivatives incorporating triazolobenzimidazole/A.M. Mahmoud, O.I. Salem, S.G. Abdel Moty, A.M. Abdel Alim//Indian J. Pharm. Sci. -2013. -V. 75. -I. 5. -P. 545-¬556.
- Beard, C. 5(6)Benzene ring substituted benzimidazole-2-carbamate derivatives having antihelmintic activity. US Patent 3962437/C. Beard. -1976. -June 8.
- Wei, P.H.L. Modulating the immune response with 2-substituted-3-hydroxy-thiazolobenzo(and azabenzo)thiazolium salts and mesoionic didehydro derivatives thereof. US Patent 4275065/P.H.L. Wei, F.J. Gregory. -1981. -June 23.
- Hirai, K. Trans-iodopropenylation of alkyl halides: (E)-1-iodo-4-phenyl-2-butene/K. Hirai, Y. Kishida//Org. Synth. -1977. -V. 56. -P. 77.
- Судолова, Н.М. Синтез новых производных тиазолотиадиазолиевой системы/Н.М. Судолова, Д.Г. Ким//Бутлеровские сообщения. -2011. -Т. 26. -№ 11. -С. 76-80.
- Судолова, Н.М., Гетероциклизация S¬-производных 2-меркапто-5-метил-1,3,4-тиадиазола/Н.М. Судолова, Д.Г. Ким, О.В. Голова//Тез. докл. XX Российской молодежной научно-практической конференции «Проблемы теоретической и экспериментальной химии». Екатеринбург, 20-24 апреля, 2010. -Екатеринбург, 2010. -С. 462-463.


