Synthesis and structure of aroxy-tetra- p-tolylantimony (4-MeC 6H 4) 4SbOC 6H 2Br 2-2,6-(t-Bu)-4
Автор: Andreev P.V., Sharutin V.V., Sharutina O.K.
Журнал: Вестник Южно-Уральского государственного университета. Серия: Химия @vestnik-susu-chemistry
Рубрика: Химия элементоорганических соединений
Статья в выпуске: 1 т.8, 2016 года.
Бесплатный доступ
Penta-p-tolylantimony interaction with 2,6-dibromo(4-tert-butyl)phenol has led to syn-thesis of the complex tetra-p-tolylantimony 2,6-dibromo(4-tert-butyl)phenoxide, whose structure has been established by X-ray diffraction. The antimony atom in the molecule (4-MeC 6H 4) 4SbOC 6H 2Br 2-2,6-(t-Bu)-4 has distorted trigonal-bipyramidal coordination with the aroxy group in axial position. The bond Sb-O is 2.229(2) Å, the axial angle CSbO is 176.82(10)°.
Tetra-p-tolylantimony, 6-dibromo(4-tert-butyl)phenoxide, synthesis, molecular structure, x-ray analysis
Короткий адрес: https://sciup.org/147160343
IDR: 147160343 | УДК: 546.865+547.53.024+547.563.4+548.312.5 | DOI: 10.14529/chem160106
Текст научной статьи Synthesis and structure of aroxy-tetra- p-tolylantimony (4-MeC 6H 4) 4SbOC 6H 2Br 2-2,6-(t-Bu)-4
It is well known that in antimony derivatives of the general formula R 4 SbX the coordination polyhedron of the central atom and the character of its bonding with the electronegative ligand X is determined by the nature of this ligand and the organic radicals R. In the vast majority of R 4 SbX compounds the antimony atoms have trigonal-bipyramidal coordination in various degrees of distortion. Such compounds are of indisputable interest, as they make it possible to reveal the factors that determine the transition of SbC 4 fragment into the tetrahedral structure. The analysis of the published structural data for aroxy-tetraphenylantimony Ph 4 SbOAr has shown that the distortion of the central atom coordination polyhedron in them increases if in aroxy group there are electron-donating substituents [1–5].
In continuation of the study how the nature of phenol and aryl radicals at the antimony atom influences the geometric characteristics of aroxy-tetraarylantimony molecules we have synthesized and established the structure of new aroxy-tetra- p -tolylantimony: (4-MeC 6 H 4 ) 4 SbOC 6 H 2 Br 2 -2,6-( t -Bu)-4 ( 1 ).
Experimental
Synthesis of tetra- p -tolylantimony 2,6-dibromo(4- tert -butyl)phenoxide (1). The mixture of 0.29 g (0.5 mmol) penta- p -tolylantimony, 0.15 g (0.5 mmol) 2,6-dibromo(4- tert -butyl)phenol and 5 mL toluene was allowed to stand for 24 h at room temperature, then it was reduced to 1 mL and cooled. The yield was 0.35 g (87 %) of colorless crystals 1 with m.p. 209 ° C. IR spectrum ( v , cm-1): 1592, 1299, 1251, 1212, 1189, 1061, 1015, 866, 830, 797, 729, 575, 553, 486. Found, %: С 57.38; Н 5.07; Br 20.05. Calculated for C 38 H 39 OBr 2 Sb, %: C 57.50; H 4.92; Br 20.18.
IR spectrum was recorded with the use of the IR spectrometer Bruker Tensor 27 in KBr pellet in the range 4000–400 cm–1.
X-ray diffraction analysis of the complex 1 crystal was performed on the Bruker D8 QUEST automatic four-circle diffractometer (Mo K « -emission, X = 0.71073 A, graphite monochromator). The data were collected and analyzed, the unit cell parameters were refined, and the absorption correction was applied using the SMART and SAINT- Plus programs [6]. All calculations for structure determination and refinement were performed using the SHELXL/PC programs [7,8]. The structure 1 was determined by the direct method and refined by the least-squares method in the anisotropic approximation for nonhydrogen atoms. The position of hydrogen atoms was refined according to riding model ( U iso (H) = 1.2U eq (C)). The main crystallographic data and refinement results for structure 1 are listed in Table 1, the geometric characteristics of the antimony atom coordination tetrahedron are given in Table 2.
Химия элементоорганических соединений
Table 1
Crystallographic data and the experimental and structure refinement parameters for compound 1
|
Parameter |
Value |
|
Empirical formula |
C 38 H 39 Br 2 OSb |
|
Formula weight |
793.26 |
|
Т , К |
273(2) |
|
Crystal system |
Triclinic |
|
Space group |
Р ī |
|
a , Å |
10.7287(17) |
|
b , Å |
13.0075(18) |
|
c, Å |
14.446(2) |
|
α , deg |
74.905(7) |
|
β, deg |
75.639(8) |
|
γ , deg |
66.692(7) |
|
V , Å3 |
1763.5(5) |
|
Z |
2 |
|
ρ (calcd.), g/сm3 |
1.494 |
|
- 1 µ , mm |
3.075 |
|
F (000) |
792 |
|
Crystal size, mm |
0.690 x 0.590 x 0.240 |
|
Range of refraction indices |
- 13 ≤ h ≤ 13, - 14 ≤ k ≤ 16, - 18 ≤ l ≤ 18 |
|
Measured reflections |
19747 |
|
Independent reflections |
5586 (R int = 0.0345) |
|
Refinement variables |
402 |
|
GOOF |
1.031 |
|
R factors for F2 > 2 σ (F2) |
R 1 =0.0337, wR 1 =0.0808 |
|
R factors for all reflections |
R 1 =0.0498, wR 1 =0.0870 |
|
Residual electron density (min/max), e /Å3 |
0.882/ - 0.658 |
Table 2
Selected bond lengths ( d ) and bond angles ( ω ) in the structure of compound 1
|
Bond |
d , Å |
Angle |
ω , deg |
|
Sb(1)–С(1) |
2.114(3) |
C(1)Sb(1)О(31) |
176.82(10) |
|
Sb(1)–C(11) |
2.112(3) |
C(11)Sb(1)C(1) |
108.58(12) |
|
Sb(1)–C(21) |
2.111(3) |
C(21)Sb(1)C(1) |
126.19(12) |
|
Sb(1)–C(31) |
2.174(3) |
C(1)Sb(1)C(31) |
94.38(12) |
|
Sb(1)–O(1) |
2.229(2) |
C(21)Sb(1)C(11) |
123.25(12) |
|
O(1)–C(41) |
1.320(4) |
C(1)Sb(1)O(1) |
84.75(10) |
|
C(41)O(1)Sb(1) |
126.96(19) |
The full tables of atomic coordinates, bond lengths, and bond angles were deposited with the Cambridge Crystallographic Data Centre (CCDC 1048264; ; .
Results and Discussion
Aroxy-tetra- p -tolylantimony 1 has been produced via dearylation of penta- p -tolylantimony by 2,6-dibromo(4- tert -butyl)phenol at mild conditions with 87 % yield:
(4-MeC 6 H 4 ) 5 Sb + HOC 6 H 2 Br 2 -2,6-( t -Bu)-4 → (4-MeC 6 H 4 ) 4 SbOC 6 H 2 Br 2 -2,6-( t -Bu)-4 + CH 3 C 6 H 5
Compound 1 was crystallized from the toluene solution directly in the process of concentration.
According to X-ray diffraction analysis, in 1 the antimony atom has a distorted trigonal-bipyramidal coordination, trigonality degree τ=0.84 [9], with the oxygen atom of the aroxy group in axial position (Fig. 1).
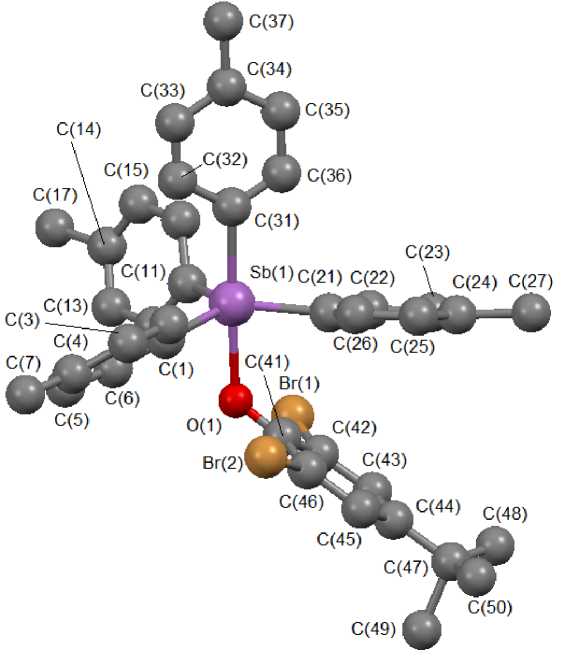
Fig. 1. The structure of compound 1
The atoms of the tert -butyl group C(48)–C(50) are disordered, they displace two positions with the ratio 0.62/0.38. The angle between the positions of the disordered fragment is equal to approximately 62 ° in relation to the bond C(44)-C(47). The axial angle OSbC equals 176.82(10) ° , the sum of the angles CSbC in equatorial plane is equal to 358.0(3)°. The antimony atom deviates from the equatorial plane [C 3 ] towards the axial carbon atom for 0.172 Å, which causes departure of the angle values between axial and equatorial bonds from the theoretical value 90 ° . The angles OSbCeq and CaxSbCeq change within the range 84.62(10) ° -86.83(10) ° and 93.46(12) ° -96.35(12) ° , respectively, the bonds Sb-Ceq coincide within the measurement accuracy and equal 2.111(3) - 2.114(3) A. The lengths Sb-Cax [2.174(3) A] are longer than equatorial ones, which is typical for trigonal-bipyramidal structures. The length Sb–O equals 2.229(2) Å, much higher than the sum of covalent radii of antimony and oxygen atoms (2.07 Å [10]).
All observed geometrical parameters for the molecule of compound 1 (decrease of the sum of equatorial angles, significant deviation of the antimony atom from the equatorial plane, approximation of the values for axial and equatorial bonds) point at the tendency of the antimony trigonal-bipyramidal coordination to transform into tetrahedral one [11].
It should be noted that unlike compound 1 , tetraphenylantimony picrate ( 2 ) is an ionic compound.
Changing of the antimony atom coordination polyhedron in the series of tetraarylantimony aroxides can be explained by consideration of the Sb–O bond nature in respect to the phenolate anion basicity, which is determined by electron density redistribution in the aroxy group, depending on the substituent nature in it. The lower is the phenolate anion basicity, the less strong is the coordination bond Sb–O, and the structure of the compound Ar 4 SbOAr' approaches ionic character to a greater extent. Three nitro groups in 2 , with their negative inductive effect, contribute to stabilization of phenolate anion and decrease in its basicity, at that the group Ph 4 Sb transforms into the stable tetrahedral cation.
Conclusions
To summarize, the distortion of trigonal-bipyramidal configuration of aroxy-tetraarylantimony compounds and the lengthening of the Sb–O bond are both determined by electron acceptor properties of the aroxy group, which strengthen with increasing number of electron acceptor substituents in the aromatic ring.
Химия элементоорганических соединений
Список литературы Synthesis and structure of aroxy-tetra- p-tolylantimony (4-MeC 6H 4) 4SbOC 6H 2Br 2-2,6-(t-Bu)-4
- Синтез, строение и термическое разложение арокситетрафенилстиборанов/В.В. Шарутин, В.В. Жидков, Д.В. Муслин и др.//Изв. АН СССР. Сер. хим. -1995. -№ 5. -С. 958-963.
- Реакции пентаарилсурьмы с орто-замещенными фенолами/В.В. Шарутин, О.К. Шарутина, П.Е. Осипов и др.//Журн. общ. химии. -1997. -Т. 67. -№ 9. -С. 1528-1530.
- Арокситетраарильные соединения сурьмы. Синтез, строение и термическое разложение/В.В. Шарутин, О.К. Шарутина, П.Е. Осипов и др.//Журн. общ. химии. -2000. -Т. 70, № 6. -С. 931-936.
- Синтез и строение 2-третбутилфенокситетрафенилсурьмы/В.В. Шарутин, О.К. Шарутина, П.Е. Осипов и др.//Коорд. химия. -2001. -Т. 27, № 7. -С. 518-520.
- Синтез и строение 3-гидокси-4-ацетилфенокситетрафенилсурьмы/В.В. Шарутин, О.К. Шарутина, Е.А. Бондарь и др.//Журн. общ. химии. -2003. -Т. 73, № 3. -С. 376-379.
- Bruker (2000) SMART. Bruker Molecular Analysis Research Tool, Versions 5.625 Bruker AXS, Madison, Wisconsin, USA.
- Bruker (2000) SAINTPlus Data Reduction and Correction Program Versions 6.02a, Bruker AXS, Madison, Wisconsin, USA.
- Sheldrick, G.M. A Short History of Shelx/G.M. Sheldrick//Acta Cryst. A. -2008. -V. 64. -P. 112-122.
- Synthesis, Structure, and Spectroscopic Properties of Copper(II) Compounds Containing Nitrogen-Sulphur Donor Ligands; the Crystal and Molecular Structure of Aquacopper(II) Perchlorate/A.W. Addison, T.N. Rao, J. Reedijk et al.//J. Chem. Soc. Dalton Trans. -1984. -№ 7. -Р. 1349-1356.
- Бацанов, С.С. Атомные радиусы элементов/С.С. Бацанов//Журн. неорган. химии. -1991. -Т. 36, вып. 12. -С. 3015-3037.
- Шарутина, О.К. Молекулярные структуры органических соединений сурьмы (V)/О.К. Шарутина, В.В. Шарутин. -Челябинск: Издательский центр ЮУрГУ, 2012. -395 с.


