Synthesis and structure of bis(4-bromophenoxy)triphenylantimony
Автор: Sharutin V.V., Sharutina O.K.
Журнал: Вестник Южно-Уральского государственного университета. Серия: Химия @vestnik-susu-chemistry
Рубрика: Химия элементоорганических соединений
Статья в выпуске: 1 т.8, 2016 года.
Бесплатный доступ
Interaction of triphenylantimony with 4-bromophenol in the presence of tert-butylhydroperoxide in ether has led to the synthesis of bis(4-bromophenoxy)triphenylantimony, whose structure has been established by X-ray diffraction analysis. The antimony atom has distorted trigonal-bipyramidal coordination with aroxy groups in axial positions. The bond lengths equal: 2.094(4), 2.108(5), 2.109(5) Å for Sb-C; 2.062(4) and 2.066(3) Å for Sb-O. The axial angle OSbO equals 172.41(1)°.
Bis(4-bromophenoxy)triphenylantimony, synthesis, molecular structure, x-ray analysis
Короткий адрес: https://sciup.org/147160347
IDR: 147160347 | УДК: 546.865+547.53.024+547.563.4+548.312.5 | DOI: 10.14529/chem160109
Текст научной статьи Synthesis and structure of bis(4-bromophenoxy)triphenylantimony
It is known that reactions of triarylantimony with phenols in the presence of hydrogen peroxide lead to formation of triarylantimony diaroxide [1, 2]. Oxidative addition reactions with the participation of phenols and some other oxidizing agent were not studied previously. In the present paper bis (4-bromophenoxy)triphenylantimony ( 1 ) has been obtained by way of the oxidative addition reaction with the use of tert -butylhydroperoxide as the oxidizing agent; its molecular and crystal structure has been investigated.
Experimental
Synthesis of bis (4-bromophenoxy)triphenylantimony (1). The mixture of 0.353 g (1.00 mmol) triphenylantimony, 0.346 g (2.00 mmol) 4-bromophenol and 0.128 g 70% aqueous solution of tert butylhydroperoxide (1.00 mmol) in 10 mL ether was kept at room temperature for 24 h. The crystals formed were recrystallized from toluene. The yield was 0.391 g (85%) of compound 1 with m.p. 148 ° C. IR spectrum ( ν , cm–1): 1577, 1484, 1436, 1278, 1243, 1166, 1098, 1071, 1023, 998, 845, 830, 745, 731, 693, 643, 625, 519, 482, 461. Found, %: С 51.53, Н 3.65, Br 22.59. Calculated for C 30 H 23 Br 2 O 2 Sb, %: C 51.65, H 3.30, Br 22.95.
IR spectrum of compound 1 was recorded by the Bruker Tensor 27 IR spectrometer in paraffin oil between KBr pellets in the range 4000 - 400 cm - 1.
X-ray diffraction analysis of the crystal 1 was performed on the Bruker D8 QUEST automatic four-circle diffractometer (Mo K „ -emission, X = 0.71073 A, graphite monochromator). The data were collected and analyzed, the unit cell parameters were refined, and the absorption correction was applied using the SMART and SAINT- Plus programs [3]. All calculations for structure determination and refinement were performed using the SHELXL/PC programs [4]. The structures were determined by the direct method and refined by the least-squares method in the anisotropic approximation for non-hydrogen atoms. The position of hydrogen atoms was refined according to the riding model ( U iso (H) = 1.2U eq (C)). The main crystallographic data and refinement results for structure 1 are listed in Table 1, the geometric characteristics of the antimony atom coordination polyhedron are given in Table 2.
Table 1
Crystallographic data and the experimental and structure refinement parameters for compound 1
|
Parameter |
Value |
|
Empirical formula |
C 30 H 23 O 2 Br 2 Sb |
|
Formula weight |
697.05 |
|
Т , К |
296(2) |
|
Crystal system |
Monoclinic |
|
Space group |
P2 1 /c |
Table 1 (end)
|
Parameter |
Value |
|
a , Å |
15.5926(8) |
|
b , Å |
9.1094(4) |
|
c, Å |
20.5019(10) |
|
α , deg |
90.00 |
|
β, deg |
111.894(2) |
|
γ , deg |
90.00 |
|
V , Å3 |
2702.0(2) |
|
Z |
4 |
|
ρ (calcd.), g/сm3 |
1.713 |
|
- 1 µ , mm |
4.004 |
|
F (000) |
1360.0 |
|
Crystal size, mm |
0.29 × 0.28 × 0.05 |
|
2 θ Range of data collection, deg |
6.08 - 52.78° |
|
Range of refraction indices |
- 19 ≤ h ≤ 19, - 11 ≤ k ≤ 11, - 25 ≤ l ≤ 25 |
|
Measured reflections |
36895 |
|
Independent reflections |
0.0920 |
|
R int |
5523 |
|
Refinement variables |
316 |
|
GOOF |
1.045 |
|
R factors for F2> 2 σ (F2) |
R 1 = 0.0484, wR 2 = 0.1019 |
|
R factors for all reflections |
R 1 = 0.0839, wR 2 = 0.1177 |
|
Residual electron density (min/max), e /Å3 |
0.92/ - 0.66 |
Table 2
Selected bond lengths and bond angles in the structure of compound 1
|
Bond |
d, Å |
Angle |
ω , deg |
|
Sb(1) - O(1) |
2.062(4) |
O(1)Sb(1)O(2) |
172.41(15) |
|
Sb(1) - O(2) |
2.066(4) |
O(1)Sb(1)C(1) |
94.96(19) |
|
Sb(1) - C(1) |
2.109(5) |
O(1)Sb(1)C(11) |
82.85(17) |
|
Sb(1) - C(11) |
2.108(5) |
O(2)Sb(1)C(11) |
90.24(17) |
|
Sb(1) - C(21) |
2.094(5) |
O(2)Sb(1)C(21) |
87.78(18) |
|
Br(1) - C(34) |
1.907(6) |
C(11)Sb(1)C(1) |
122.9(2) |
|
Br(2) - C(44) |
1.903(6) |
C(21)Sb(1)C(1) |
112.4(2) |
|
O(1) - C(31) |
1.355(6) |
C(21)Sb(1)C(11) |
124.7(2) |
|
O(2) - C(41) |
1.341(6) |
C(31)O(1)Sb(1) |
135.1(3) |
The full tables of atomic coordinates, bond lengths, and bond angles were deposited with the Cambridge Crystallographic Data Centre (CCDC 1043441; ; .
Results and Discussion
Compound 1 has been obtained from triphenylantimony and 4-bromophenol in the presence of tert butylhydroperoxide (mole ratio 1:2:1, respectively) in ether (20 оС, 18 h).
Ph 3 Sb + 2 HOC 6 H 4 Br-4 + t -BuOOH → Ph 3 Sb(OC 6 H 4 Br-4) 2 + t -BuOOH + H 2 O
In order to get monocrystals that are suitable for X-ray structure investigation, compound 1 has been recrystallized from toluene.
According to X-ray diffraction analysis data, the antimony atom in 1 has distorted trigonal-bipyramidal coordination with the oxygen atoms of phenolate ligands in axial positions (Fig. 1).
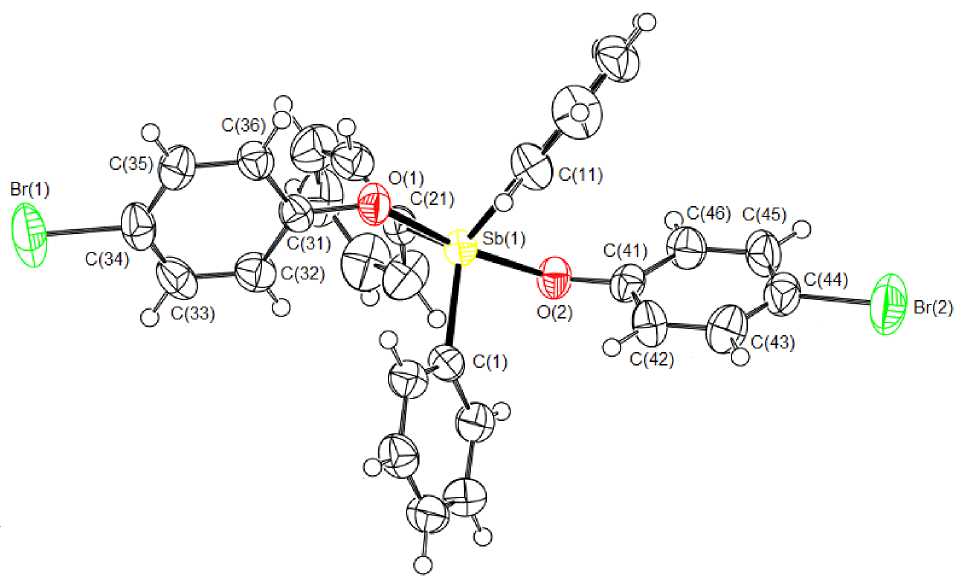
Fig. 1. The structure of compound 1
The axial angle OSbO equals 172.41(15) ° . Three equatorial phenyl groups exist in the least sterical-ly hindered propeller twist conformation. The antimony atom deviates from the equatorial plane [C 3 ] by 0.007 A. The sum of angles CSbC in the equatorial plane equal 360 ° , at that the values of individual equatorial angles (112.4(2) ° , 122.9(2) ° , and 124.7(2) ° ) differ noticeably. The planes of aroxy groups [C(31) - C(36)] and [C(41) - C(46)] make angles 81.45° and 53.22° with the equatorial plane [С3], respectively.
The bond lengths Sb - C(1,11,21) equal 2.109(5), 2.108(5), 2.094(5) A; they approach the observed values of the equatorial bonds in the bis (2,4,6-tribromophenoxy)triphenylantimony molecule (2.095(6), 2.105(4), 2.106(4) A [1]). The lengths Sb - O(1,2) (2.062(4) and 2.066(4) A) are less than the similar bonds in bis (2,4,6-tribromophenoxy)triphenylantimony (2.091(6), 2.099(4) Å [1]), but more than those in bis (phenoxy)triphenylantimony (2.046(6), 2.056(4) Å [5]). Note that the sum of the covalent radii of the O and Sb atoms comprises 2.07 A [6]. The distances O(1) - C(31) and О(2) - С(41) in 1 equal 1.355(6) and 1.341(5) Å. In the bis (phenoxy)triphenylantimony and bis (2,4,6-tribromophenoxy)triphenylantimony molecules the similar bonds measure 1.353(2), 1.336(2) Å and 1.333(5), 1.334(5) Å, respectively.
Conclusions
Hence, the distortion of trigonal-bipyramidal coordination of the central atom in molecule 1 manifests itself in the noticeable deviation of axial and equatorial angles from their theoretical values, which is probably caused not by existence of intramolecular interactions, but by peculiarities of crystal arrangement. In the series of compounds bis (phenoxy)triphenylantimony, bis (4-bromophenoxy)-triphenylantimony, bis (2,4,6-tribromophenoxy)triphenylantimony the lengthening of the Sb–O bonds is observed, at that the expected regular shortening of the Sb–O bonds does not occur.
Список литературы Synthesis and structure of bis(4-bromophenoxy)triphenylantimony
- Синтез и строение бис(2,4,6-трибромофенокси)трифенилсурьмы/В.В. Шарутин, А.П. Пакусина, М.А. Пушилин и др.//Коорд. химия. -2002. -Т. 28, № 6. -С. 408-411.
- Синтез и строение дикарбоксилатов и диароксидов трис(4-N,N-диметиламино-фенил)сурьмы(V)/В.В. Шарутин, В.С. Сенчурин, О.К. Шарутина и др.//Журн. неорган. химии. -2011. -Т. 56, № 7. -С. 1129-1135.
- Bruker (1998). SMART and SAINT-Plus. Versions 5.0. Data Collection and Processing Software for the SMART System. Bruker AXS Inc., Madison, Wisconsin, USA.
- Bruker (1998). SHELXTL/PC. Versions 5.10. An Integrated System for Solving, Refining and Displaying Crystal Structures From Diffraction Data. BrukerAXSInc., Madison, Wisconsin, USA.
- Синтез и строение дифенокситрифенилсурьмы/В.В. Шарутин, А.П. Пакусина, О.В. Субачева и др.//Коорд. химия. -2003. -Т. 29, № 6. -С. 418-422.
- Бaцaнов, С.С. Атомные радиусы элементов/С.С. Бацанов//Журн. неорган. химии. -1991. -T. 36, № 12. -С. 3015-3037.


