Synthesis and structure of bis(thiophene-2-aldoximato)-tris(5-bromo-2-methoxyphenyl)antimony
Автор: Artemeva E.V.
Журнал: Вестник Южно-Уральского государственного университета. Серия: Химия @vestnik-susu-chemistry
Рубрика: Химия элементоорганических соединений
Статья в выпуске: 1 т.12, 2020 года.
Бесплатный доступ
Synthesis of bis (thiophene-2-aldoximato) tris (5-bromo-2-methoxyphenyl)antimony (1) has been carried out by the oxidative addition reaction of tris (5-bromo-2-methoxyphenyl)antimony with thiophene-2-aldoxime in the presence of tert -butyl hydroperoxide with the 1:2 molar ratio of the reactants. The compound has been characterized by IR spectroscopy and X-ray diffraction analysis. According to the X-ray diffraction analysis data, in the crystal there are two types of crystallographically independent the molecules, geometrical parameters of which are slightly different. Coordination polyhedron of antimony atoms in a molecule is a distorted trigonal bipyramid. The sum of the СSbC angles equals 360°, the values of the individual angles differ from the theoretical 120° by no more than 8.6(8)°. The axial OSbO angle is 175.8(4)°. The OSbC angles vary within the range 85.8(6)º-97.6(6)º. The average value of the Sb-C bond lengths is 2.13(2) Å. The Sb-O distances equal 2.08(1) Å. The distances between the Sb atom and N atoms of the iminoxy groups are 2.80(2)-2.94(2) Å. The distances between the N and O atoms do not depend on the distances between the Sb and N atoms; they are equal to 1.39(2)-1.43(2) Å. In the molecules there are contacts between the Sb and O atoms of methoxy groups, the corresponding distances are within the range of 3.13(1)-3.23(1) Å. The molecules in a crystal are connected by intermolecular hydrogen bonds between the aromatic H and Br (2.883 Å), S (2.992 Å) and N (2.715 Å) atoms. In the molecules there are intramolecular short contacts between the iminoxy group O atom and S (2.72(1)-2.80(1) Å), as well as the methoxy group O atom (2.93(2)-3.03(2) Å).
Tris(5-bromo-2-methoxyphenyl)antimony, thiophene-2-aldoxime, oxidative addition, structure, x-ray diffraction analysis, ir spectroscopy
Короткий адрес: https://sciup.org/147233154
IDR: 147233154 | УДК: 546.86+547.53.024+548.312.5 | DOI: 10.14529/chem200104
Текст научной статьи Synthesis and structure of bis(thiophene-2-aldoximato)-tris(5-bromo-2-methoxyphenyl)antimony
It is known that triarylantimony dioximates are biologically active compounds, having antibacterial, antifungal [1] and antitumor [2, 3] activity. Various triarylantimony dioximates Ar 3 SbX 2 (Ar = Ph, p -Tol, о -Tol, m -Tol, 3-F-C 6 H 4 , 4-F-C 6 H 4 ; Х = ОNCHR, ОNCRR') were obtained by substitution [1, 2, 4‒7] and oxidative addition reactions, with the molar ratio of triaryl antimony and oxime 1:2 [8‒18]. Synthesis of tris (5-bromo-2-methoxyphenyl)antimony oximates has been described in a few papers only [17‒19]. Obviously, such compounds have not been studied enough, and a further investigation is required.
The present work concerns the study of the interaction of tris (5-bromo-2-methoxyphenyl)antimony with thiophene-2-aldoxime in the presence of tert -butyl hydroperoxide at 1:2:1 molar ratio of the reactants, and the structure determination of the reaction product.
ExperimentalSynthesis of bis(thiophene-2-aldoximato)tris(5-bromo-2-methoxyphenyl)antimony (1).
Tris (5-bromo-2-methoxyphenyl)antimony (0.1 g, 0.14 mmol) and thiophene-2-aldoxime (0.037 g, 0.29 mmol) were dissolved in 10 ml of diethyl ether, then 70 % aqueous solution of tert -butyl hydroperoxide (0.019 g, 0.14 mmol) was added. The mixture was kept for 24 h at 20 °C. After the solvent evaporation, the solid residue was recrystallized from amyl acetate. 0.151 g (97 %) of colorless crystals of 1 with MP 136 °C was obtained.
IR spectrum, ν, сm–1: 3438, 3096, 3065, 3003, 2961, 2932, 2837, 1572, 1472, 1437, 1420, 1375, 1351, 1283, 1269, 1254, 1209, 1180, 1144, 1092, 1049, 1016, 912, 862, 823, 808, 741, 711, 667, 619, 602, 555, 525, 467, 436.
Found, %: С 39.94, Н 2.81. For C 62 H 52 Br 6 N 4 O 10 S 4 Sb 2 calculated, %: С 40.00, Н 2.75.
IR spectra of compound 1 were recorded on a Shimadzu IRAffinity-1S FTIR-spectrometer; samples were prepared by pelletting with KBr (absorption region 4000–400 cm–1).
Химия элементоорганических соединений
X-ray diffraction analysis of crystalline substance 1 was performed on a Bruker D8 QUEST automatic four-circle diffractometer (Mo K α -emission, λ 0.71073 Å, graphite monochromator).
Data collection and editing, unit-cell parameters refinement, and correction for absorption were carried out in SMART and SAINT-Plus software [20]. All calculations aimed at solving and refining the structure of compound 1 were performed in SHELXL/PC [21] and OLEX2 software [22]. Structure 1 was determined by direct methods and refined with the least squares method in the anisotropic approximation for non-hydrogen atoms. Selected bond lengths and bond angles of 1 are summarized in Table 1.
Crystal Data for C 62 H 52 N 4 O 10 Br 6 Sb 2 S 4 ( M 1864.28 g/mol): triclinic, space group Pī, a 9.565(10) Å, b 17.472(18) Å, c 24.42(3) Å, α 97.25(7)°, β 92.12(8)°, γ 98.46(6)°, V 3999(8) Å3, Z 2, μ Mo 3.827 mm‒1, D calc 1.548 g/cm3, 29317 reflections measured, 5456 unique reflections ( R int 0.0522), the number of refinement variables 800, GOOF 1.118 , R factors for F 2 > 2 σ ( F 2): R 1 0.0580, wR 2 0.1635, R factors for all reflections R 1 0.0680, wR 2 0.1700.
The full tables of atomic coordinates, bond lengths, and bond angles were deposited with the Cambridge Crystallographic Data Centre (CCDC 1901674 for compound 1; ; .
Table 1
Selected bond lengths and bond angles in structure 1
|
a |
|||||
|
Bond |
d , Å |
Angle |
ω , deg |
Angle |
ω , deg |
|
Sb(1)–С(1) |
2.15(2) |
O(4)Sb(1)O(5) |
175.8(4) |
O(4)Sb(1)C(11) |
87.3(6) |
|
Sb(1)–С(11) |
2.14(2) |
C(1)Sb(1)C(11) |
120.6(7) |
O(4)Sb(1)C(21) |
85.8(6) |
|
Sb(1)–С(21) |
2.11(2) |
C(1)Sb(1)C(21) |
112.5(7) |
O(5)Sb(1)C(1) |
86.1(6) |
|
Sb(1)–O(4) |
2.08(1) |
C(11)Sb(1)C(21) |
126.9(7) |
O(5)Sb(1)C(11) |
92.7(6) |
|
Sb(1)–O(5) |
2.08(1) |
O(4)Sb(1)C(1) |
97.6(6) |
O(6)Sb(1)C(21) |
90.8(6) |
|
N(1)‒C(35) |
1.30(3) |
O(4)N(1)C(35) |
110(1) |
||
|
N(2)‒C(45) |
1.28(3) |
O(5)N(2)C(45) |
113(2) |
||
|
b |
|||||
|
Sb(2)–C(51) |
2.16(2) |
O(I)Sb(2)O(J) |
175.2(5) |
O(I)Sb(2)C(61) |
87.8(6) |
|
Sb(2)–C(61) |
2.12(2) |
C(51)Sb(2)C(61) |
125.9(8) |
O(I)Sb(2)C(71) |
85.2(6) |
|
Sb(2)–C(71) |
2.13(2) |
C(51)Sb(2)C(71) |
122.5(7) |
O(J)Sb(2)C(51) |
89.4(6) |
|
Sb(2)–O(I) |
2.10(1) |
C(71)Sb(2)C(81) |
111.4(8) |
O(J)Sb(2)C(61) |
87.6(6) |
|
Sb(2)–O(J) |
2.04(1) |
O(I)Sb(2)C(51) |
92.2(6) |
O(J)Sb(2)C(71) |
97.7(6) |
|
N(3)‒C(85) |
1.23(3) |
O(I)N(3)C(85) |
112(2) |
||
|
N(4)‒C(95) |
1.21(4) |
O(J)N(4)C(95) |
113(2) |
||
Results and Discussion
It has been found that the oxidative addition reaction of tris (5-bromo-2-methoxyphenyl)antimony with thiophene-2-aldoxime in the presence of tert -butyl hydroperoxide at 1:2:1 molar ratio goes by a standard pathway with the formation of triarylantimony dioximate:
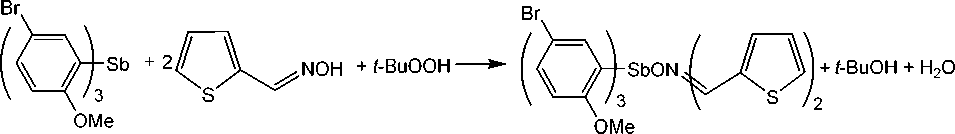
Compound 1 is a crystalline substance, highly soluble in aromatic hydrocarbons, resistant to moisture and air oxygen.
Structure 1 has been determined by X-ray diffraction analysis and confirmed by IR spectroscopy.
In the IR spectrum of compound 1 , there are absorption bands at 2932 cm–1 (thiophene C–H, st ), 708, 712 cm–1 (thiophene C–H, δ). Characteristic bands are observed at 1472 cm–1 (C=N bond),
1375 cm–1 (OH, δ), 912 cm–1 (N –O) [23]. Vibrations at 436 cm–1 indicate the presence of the Sb–C b ond in th e mole c ul e of c o mpound 1 [24]. The absorption bands at 1180 cm–1 and 1283 cm–1 correspond to vibrations of the C Ar –Br and C A r –OMe bonds, respectively [23].
According to X-ra y diff ra c t ion d a t a , in cr y stal 1 there are two types of crystallographically independent molecules a and b , the geometric parameters of which are equal w ith in t he e r ror limit s , t he re f o r e , in th e f ol lowi ng , we d isc u ss the s tr u c tu r al da ta of mole c ul e 1 a . The antimony atoms have a distorted trigonal-b ipyra mi da l c oordination with oxygen atoms in axial position s ( F ig . 1) . T he S bC 3 fr a g me nt ly ing in the e quat or ial pl a ne i s a lmost flat. The Sb atom deviates from the [C 3 ] plane toward t he a x ial oxy g e n a tom b y 0 . 001 Å ( a ) and 0.053 Å ( b ). The sum of the СSbC equatorial angles is 360° for bo th mole c u le s, the v a l ue s of the individual angles differ from the theoretical 1 20° by no mor e t ha n 8.6(8)°. The a xi a l OS bO ang le is 175.8(4)°. The OSbC angles vary within the rang e s 85. 8( 6 ) º –97.6(6)º. The NOON t o r s ion a ng le s a c c e pt la r g e v a lue s ( 1 40( 1)° ( a ), 117(1)° ( b )) because Sb interacts with N fr om diff e re nt sid e s. T h e a n g le s be tw e e n S bON p la nes e qua l 40. 54° ( a ), 63.42° ( b ).
The a v e r a g e v a lue s of the S b –C bond lengths are 2.13(2) Å ( a ) and 2.14(2) ( b ) Å. The Sb–O distances equal 2.08(1) Å wha t is approximately equal to the sum of covalent radi i o f S b a nd O a t oms (2. 07 ( 1) Å) . T h e S b·· · N dis ta nce s between the Sb atom and N atoms of imin oxy g r oups ( 2. 80( 2 ) , 2.94(2) Å ( a ), 2.82(2) Å ( b )) ar e c on sid e r ably le s s than the sum of Van der Waals radii of the Sb and N atoms (3.8 Å [25]). The N‒O di st ances do not depend on Sb···N distances and are e qual to 1. 43 ( 2) Å ( a ) and 1.39(2), 1.41(2) Å ( b ). T he g e ome tr ica l par a me ters of 1 are close to the ones for bis (thiophene-2-aldoximato)tri( o -tolyl)antimony [ 12 ]. The average values for N‒O (1.41 Å) and N‒C (1.26(4) Å) d is ta nce s, a s we l l a s ONC a ng le s ( 112 ( 2)°) in 1 are close to the ones for thiophene-2-aldehyde (1.394(3) Å, 1.269(8) Å, 111.6(3)°) as well [12].
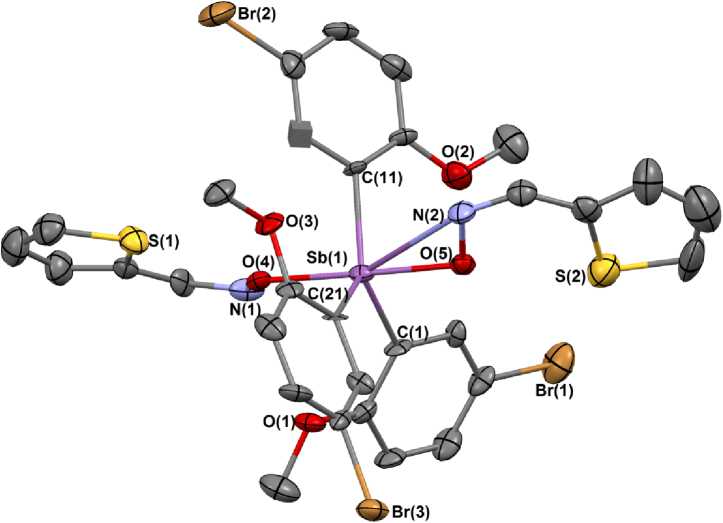
Fig. 1. Structure 1 a showing thermal ellipsoids at 30% probability. Hydrogen atoms have been omitted for clarity
In molecule 1 ther e ar e co nta cts Sb(1,2)···OMe, the corresponding distances eq ua l 3.18(1)–3.23(1) ( a ), 3.13(1)–3.23(1) Å ( b ).
Molecules a and b in crystal 1 are linked by intermolecular contacts H Ar ···Br (2.883 Å) and H Me ···S (2. 992 Å) ( Fig . 2) . Molec ules of b type are connected via H Ar ···N interactions (2.715 Å). In the mol e c ule s ther e ar e suc h in tr amole c u lar sho r t c on ta cts a s S ·· · O (2. 80(1) Å ( a ), 2.72(1), 2.74(1) Å ( b )), O MeO ···O (2.97(2), 2.93(2) Å ( a ), 3.03( 2 ) , 2. 9 3( 2 ) , 2. 7 9( 2) Å ( b )).
Химия элементоорганических соединений
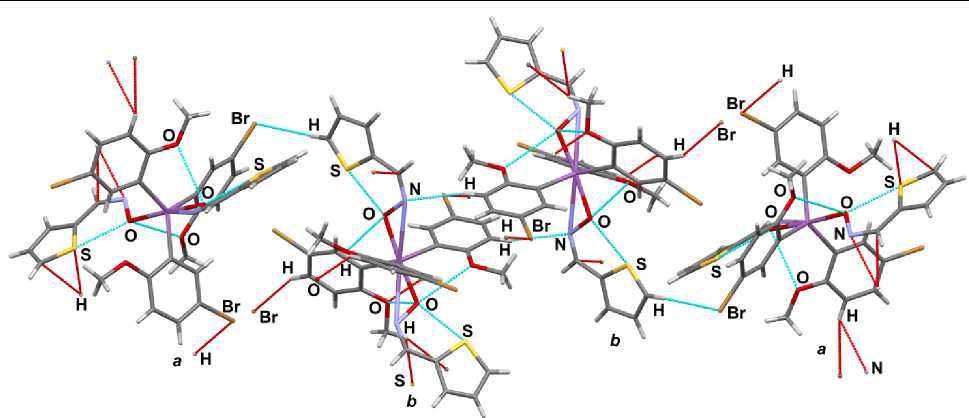
Fig. 2. The intermolecular interactions in 1
Conclusion
The ox idativ e a d di tio n rea c t ion of tris (5-bromo-2-methoxyphenyl)antimony with thiophene-2-aldox ime a t 1:2 mola r r a t i o lea ds t o f or ma tio n of tris (5-bromo-2-methoxyphenyl)antimony dioximate, the structural organization of which is due to hydrogen bonds and other short conta cts.
Acknowledgments
We ar e g r atef ul to V .V . S ha r u ti n f o r t he X -ray diffraction analysis of 1 and O.K. Sharutina for the a r t icle pr e p a ra t ion t o submissio n.
Список литературы Synthesis and structure of bis(thiophene-2-aldoximato)-tris(5-bromo-2-methoxyphenyl)antimony
- Bajpai, K. Synthesis and Reactions of o-Triorganoantimony Dioximates / K. Bajpai, R.C. Srivastava // Synth. Inorg. Met. - Org. Chem. - 1981. - V. 11, № 1. - P. 7-13.
- Yin, H. Synthesis, Spectroscopic and Structural Aspects of Triphenylantimony(V) Complex with Iinternally Functionalized Acetylferroceneoxime: Crystal and Molecular Structures of [C5H5FeC5H4C(CH3)=NO]2SbPh3 and C5H5FeC5H4C(CH3)=NOH / H. Yin, L. Quan, L. Li // Inorg. Chem. Com. - 2008. - № 11. - P. 1121-1124.
- Perspectives of Antimony Compounds in Oncology / P. Sharma, D. Perez, A. Cabrera et al. // Acta Pharm. Sinica, - 2008. - V. 29, № 8. - P. 881-890. DOI: 10.1111/j.1745-7254.2008.00818.x
- Gupta, A. Synthetic, Spectroscopic and Structural Aspects of Triphenylantimony(V) Complexes with Internally Functionalized Oximes: Crystal and Molecular Structure of [Ph3Sb{ON=C(Me)C5H4N-2}2] / A. Gupta, R.K. Sharma, R. Bohra // Polyhedron. - 2002. - V. 21, № 23. - P. 2387-2392. DOI: 10.1016/S0277-5387(02)01155-5
- Triorganoantimony(V) Complexes with Internally Functionalized Oximes: Syntetic, Spectroscopic and Structural Aspects [R3Sb(Br)L],[R3Sb(OH)L], and[R3SbL2], Cristal and Molecular Structures of [Me3Sb{ON=C(Me)C4H3O}2],[Me3Sb{ON=C(Me)C4H3S}2], 2-OC4H3C(Me)=NOH and 2- SC4H3C(Me)=NOH / A. Gupta, R.K. Sharma, R. Bohra et al. // J. Organometal. Chem. - 2002. - V. 645, № 2. - P. 118-126. DOI: 10.1016/S0022-328X(01)01338-9
- Jain, V.K. Synthesis and Spectroscopic Studies of Trialkyl Bis(iminoxy)stibines. / V.K. Jain, R. Bohra, R.C. Mehrotra // Inorg. Chim. Acta. - 1981. - V. 51. - P. 191-194.
- DOI: 10.1016/S0020-1693(00)88338-3
- Bis(2-hydroxybenzaldehyde Oximato-κO)triphenylantimony(V) / L. Dong, H. Yin, Wen L., D. Wang // Acta Crystallogr. - 2009. - V. 65E, № 11. - P. m1438.
- DOI: 10.1107/S1600536809043542
- Синтез и строение оксиматов трифенилсурьмы / В.А. Додонов, А.В. Гущин, Д.А. Горькаев и др. // Изв. РАН. Сер. хим. - 2002. - № 6. - С. 965-971.
- Шарутин, В.В. Синтез и строение диоксиматов трис(пара-толил)-, трис(3-фторфенил)- и трис(4-фторфенил)сурьмы / В.В. Шарутин, О.К. Шарутина, А.Н. Ефремов // Коорд. Хим. - 2017. - Т. 43, № 8. - С. 496-504.
- DOI: 10.7868/S0132344X17080072
- Синтез и строение бис(ацетофеноноксимата) трифенилсурьмы / В.В. Шарутин, О.К. Шарутина, О.В. Молокова и др. // Коорд. Хим. - 2002. - Т. 28, № 7. - С. 497-501.
- Синтез и строение оксиматов тетра- и триарилсурьмы / В.В. Шарутин, О.К. Шарутина, О.В. Молокова и др. // Коорд. Хим. - 2002. - Т. 28, № 8. - С. 581-590.
- Синтез и строение диоксиматов три(о-толил)сурьмы / В.В. Шарутин, О.К. Шарутина, Е.В. Артемьева, М.С. Макерова // Журн. общ. химии. - 2016. - Т. 86, № 12. - С. 2039-2044.
- Шарутин, В.В. Синтез и строение диоксиматов триарилсурьмы (3-FC6H4)3Sb[ON=CHC6H4(Br-2)]2 и p-Tol3Sb(ON=CHC4H3O)2 ∙ PhH / В.В. Шарутин, О.К. Шарутина, А.Н. Ефремов // Бутлер. сообщ. - 2016. - Т. 47, № 8. - С. 145-149.
- Синтез и строение диоксиматов трифенилсурьмы Ph3Sb(ON=CHR)2 (R = C6H4NO2-2, C6H4NO2-3, C6H4Br-2, C6H4Br-3, or C4H2ONO2-5) / В.В. Шарутин, О.К. Шарутина, Д.М. Габитова, С.Я. Шайхвалеева // Журн. неорган. химии. - 2017. - Т. 62, № 1. - С. 61-68.
- DOI: 10.7868/S0044457X17010172
- Синтез и строение диоксиматов триарилсурьмы / В.В. Шарутин, О.В. Молокова, О.К. Шарутина и др. // Журн. общ. химии. - 2004. - Т. 74, Вып. 10. - С. 1600-1607.
- Шарутин, В.В. Синтез и строение салицилальдоксиматов тетра- и трифенилсурьмы / В.В. Шарутин, О.К. Шарутина, О.В. Молокова // Журн. неорган. химии. - 2012. - Т. 57, № 6. - С. 902-907.
- Шарутин, В.В. Особенности взаимодействия трис(5-бром-2-метоксифенил)сурьмы с 2-оксибензальдоксимом. Строение бис(μ3-2-оксибензальдоксимато-О,О',N)-(μ2-оксо)-бис(5-бром-2-метоксифенил)дисурьмы / В.В. Шарутин, О.К. Шарутина // Журн. неорган. химии. - 2014. - Т. 59., № 11. - С. 1507-1511.
- DOI: 10.7868/S0044457X14110221
- Артемьева, Е.В. Синтез и строение комплексов Ar3Sb(ONCHC6H4NO2-2)2 · 0.5C6H6, Ar3Sb(ONCHC6H4NO2-3)2 · 2C6H6 и Ar3Sb(OC(O)CH2C6H4F-3)2 (Ar = C6H3OMe-2-Br-5) / Е.В. Артемьева, В.В. Шарутин, О.К. Шарутина // Журн. неорган. химии. - 2019. - Т. 64, № 11. - С. 1184-1190.
- DOI: 10.1134/S0044457X19110035
- Артемьева, Е.В. Синтез и строение β-изатоксимата тетрафенилсурьмы / Е.В. Артемьева, О.К. Шарутина // Вестник ЮУрГУ. Серия: Химия. - 2018. - Т. 10, № 1. - С. 48-54.
- DOI: 10.14529/chem180106
- Bruker (2000) SMART. Bruker molecular analysis research tool, versions 5.625 Bruker AXS, Madison, Wisconsin, USA.
- Bruker (2000) SAINTPlus data reduction and correction program, versions 6.02a, Bruker AXS, Madison, Wisconsin, USA.
- OLEX2: Complete structure solution, refinement and analysis program / O.V. Dolomanov, L.J. Bourhis, R.J. Gildea et al. // J. Appl. Cryst. - 2009. - V. 42. - P. 339-341.
- DOI: 10.1107/S0021889808042726
- Преч, Э. Определение строения органических соединений (Таблицы спектральных данных) / Э. Преч, Ф. Бюльманн, К. Аффольтер. - М.: Мир; БИНОМ. Лаборатория знаний, 2006. - 1735 с.
- Doak, G.O. The infrared spectra of some phenylsubstituted pentavalent antimony compounds / G.O. Doak, G.G. Long, L.D. Freedman // J. Organomet. Chem. - 1965. - V. 4, № 1. - P. 82-91.
- Бацанов, С.С. Атомные радиусы элементов / С.С. Бацанов // Журн. неорган. химии. - 1991. - Т. 36, № 12. - С. 3015-3037.


