Synthesis and structure of iridium complex [Ph 4Sb(DMSO)] +[IrCl 4(DMSO) 2] -
Автор: Sharutin V.V., Sharutina O.K., Senchurin V.S.
Журнал: Вестник Южно-Уральского государственного университета. Серия: Химия @vestnik-susu-chemistry
Рубрика: Металлоорганическая химия
Статья в выпуске: 4 т.7, 2015 года.
Бесплатный доступ
Iridium complex [Ph 4Sb(DMSO)] +[IrCl 4(DMSO) 2] - (1) has been synthesized by interaction of tetraphenylstibonium chloride with sodium hexachloroiridate(III) in water. From X-ray diffraction analysis data, two types of crystallographically independent cations [Ph 4Sb(DMSO)] + and anions [IrCl 4(DMSO) 2] - exist in the crystal. The antimony atoms in cations have distorted trigonal-bipyramidal surroundings with O-dimethyl sulphoxide ligand in axial position (equatorial angles CSbC 113.6(2)°-121.2(2)°, axial angles CSbО 178.33(18)°, 176.47(18)°, bond lengths Sb-С eq 2.106(6)-2.119(6) Å, Sb-С ax 2.126(6), 2.130(6) Å, Sb-О 2.545(4), 2.465(4) Å). In mononuclear octahedral anions the equatorial positions are occupied by chlorine atoms (angles ClIrCl- trans 178.69(7)°-179.56(7)°, SIrS- trans 178.35(7)°, 175.88(7)°; dimethyl sulfoxide ligands are coordinated with iridium atoms through sulfur atoms (Ir-S 2.2958(16)-2.3148(17) Å), bond lengths Ir-Cl vary in the range 2.3407(19)-2.3681(17) Å. The structural organization in the crystal is determined by interionic hydrogen bonds (H∙∙∙Cl 2.80-2.94 Å and H∙∙∙O 2.56-2.71 Å).
Tetraphenylstibonium chloride, гексахлороиридат(iii) натрия, sodium hexachloroiridate(iii), dimethyl sulfoxide, (dimethylsulfoxido)tetraphenylstibonium bis(dimethylsulfoxido)tetrachloroiridate(iii), synthesis, x-ray diffraction analysis, structure, бис(диметилсульфоксидо)тетрахлороиридат(iii) (диметилсульфоксидо)тетрафенилстибония
Короткий адрес: https://sciup.org/147160334
IDR: 147160334 | УДК: 547.243; | DOI: 10.14529/chem150410
Текст научной статьи Synthesis and structure of iridium complex [Ph 4Sb(DMSO)] +[IrCl 4(DMSO) 2] -
Introduction lonic complex compounds of iridium with anions of the type [IrHal4(S-DMSO)2]” are described in the literature as single examples [1–4]. Note that both organic cations and tetraorganylphosphonium cations [4] can act as counterions in the crystals of these compounds, at this the structural organization of iridium complexes is determined by the presence of solvent molecules in their structure. In the present paper the synthesis of (dimethylsulfoxido)tetraphenylstibonium bis(dimethylsulfoxido)tetrachloroiridate (III), the characteristic features of its structure are examined.
Results and Discussion
In order to synthesize new iridium complexes we studied the reactions of tetraphenylstibonium chloride with sodium hexachloroiridate(III) in aqueous solution.
It has been found that after addition of sodium hexachloroiridate(III) in water to the aqueous solution of tetraphenylstibonium chloride in equimolar amount, followed by solvent evaporation and recrystallization of the solid precipitate from dimethyl sulfoxide, yellow crystals are formed; it is (dimethylsul-foxiodo)tetraphenylstibonium bis (dimethylsulfoxido)tetrachloroiridate(III) [Ph 4 Sb]+[IrCl 4 (DMSO) 2 ]– ( 1 ):
-
1. H 2 O
-
2. DMSO
Ph 4 SbCl + Na 3 IrCl 6
[Ph 4 Sb(DMSO)]+[IrCl 4 (DMSO) 2 ]– + 3 NaCl 1
According to X-ray diffraction analysis, two types of crystallographically independent cations [Ph 4 Sb(DMSO)]+ and anions [IrCl 4 (DMSO) 2 ]– coexist in the crystal. The antimony atoms in cations have distorted trigonal-bipyramidal surroundings with O -dimethyl sulphoxide ligand in axial position (equatorial angles CSbC 113.6(2) ° -121.2(2) ° , axial angles CSbO 178.33(18) ° , 176.47(18) ° , bond lengths Sb-С eq 2.106(6)–2.119(6) Å, Sb–С ax 2.126(6), 2.130(6) Å, Sb–О 2.545(4), 2.465(4) Å) (Fig. 1).
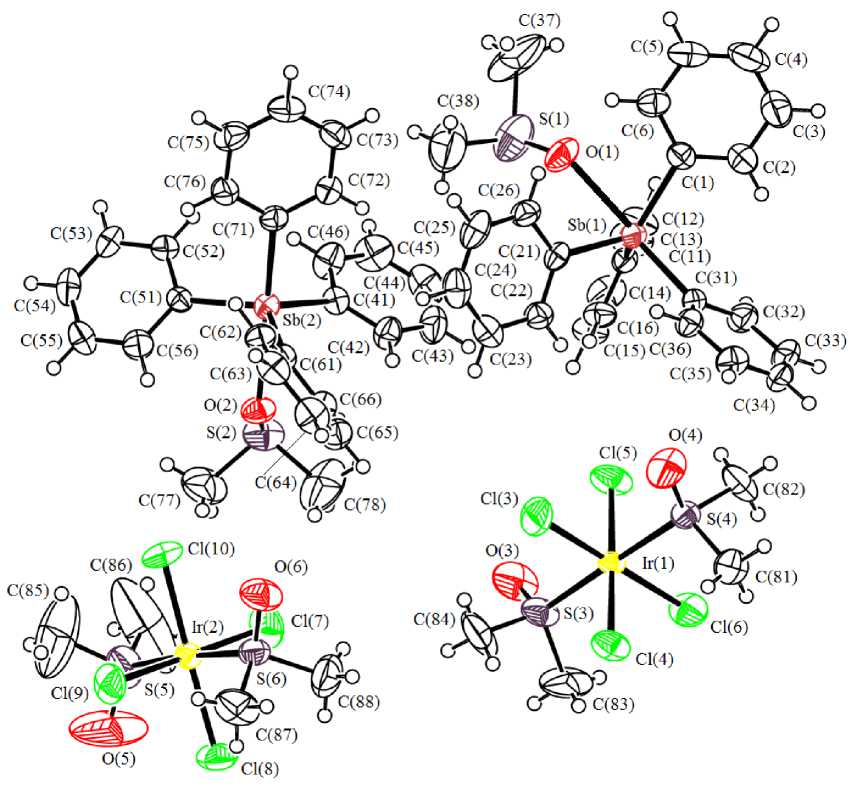
Fig. 1. The structure of compound 1
In mononuclear octahedral anions the equatorial positions are occupied by chlorine atoms (angles CllrCl- trans 178.69(7) ° -179.56(7) ° , SIrS- trans 178.35(7) ° , 175.88(7) ° ; dimethyl sulfoxide ligands are coordinated with iridium atoms through sulfur atoms (Ir - S 2.2958(16) - 2.3148(17) A), bond lengths Ir–Cl vary in the range 2.3407(19)–2.3681(17) Å. The structural organization in the crystal is determined by interionic hydrogen bonds (H∙∙∙Cl 2.80–2.94 Å and H∙∙∙O 2.56–2.71 Å), the strongest ones are shown in Fig. 2. Cations and anions are packed in stacks oriented along crystallographic axis a .
X-ray diffraction analysis of the complex 1 crystal was performed on the Bruker D8 QUEST automatic four-circle diffractometer (Mo K „ -emission, X = 0.71073 A, graphite monochromator). The data were collected and analyzed, the unit cell parameters were refined, and the absorption correction was applied using the SMART and SAINT- Plus programs [5]. All calculations for structure determination and refinement were performed using the SHELXL/PC programs [6]. The structure 1 was determined by the direct method and refined by the least-squares method in the anisotropic approximation for nonhydrogen atoms. The main crystallographic data and refinement results for structure 1 are listed in Table 1, the selected bond lengths and bond angles are given in Table 2.
The full tables of atomic coordinates, bond lengths, and bond angles were deposited with the Cambridge Crystallographic Data Centre (CCDC 1048264; ; .
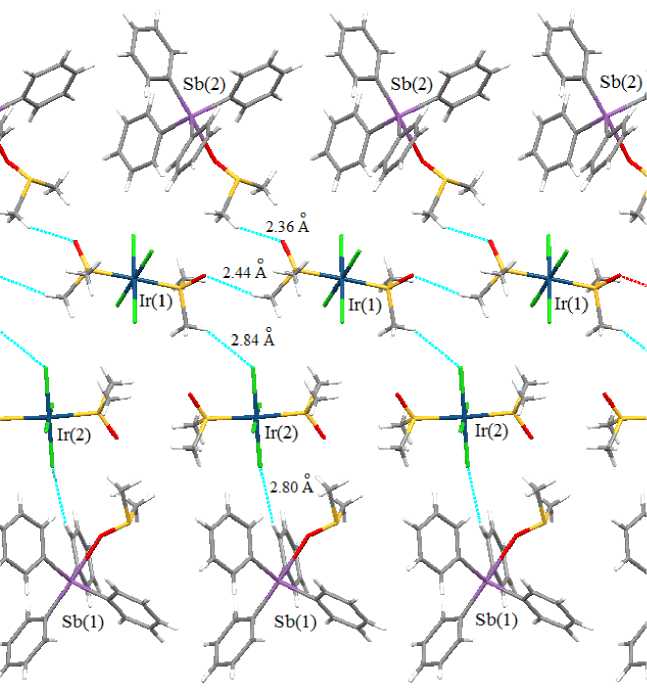
Fig. 2. Interionic bonds in the crystal of compound 1
Table 1
Crystallographic data and the experimental and structure refinement parameters for compound 1
|
Parameter |
Value |
|
Empirical formula |
C 60 O 6 S 6 Cl 8 Sb2Ir2H 76 |
|
Formula weight |
1997.07 |
|
Т , К |
296(2) |
|
Crystal system |
Triclinic |
|
Space group |
P-1 |
|
a , Å |
9.5963(6) |
|
b , Å |
19.2090(14) |
|
c, Å |
19.5688(14) |
|
α , deg |
91.109(3) |
|
β, deg |
90.549(2) |
|
γ , deg |
90.095(3) |
|
V , Å3 |
3606.4(4) |
|
Z |
2 |
|
ρ (calcd.), g/сm3 |
1.839 |
|
- 1 µ , mm |
4.935 |
|
F (000) |
1944.0 |
|
Crystal size, mm |
0.11 × 0.18 × 0.24 |
|
2 θ Range of data collection, deg |
5.98 - 41.32° |
|
Range of refraction indices |
-9 ≤ h ≤ 9, -19 ≤ k ≤ 19, -19 ≤ l ≤ 19 |
|
Measured reflections |
67394 |
|
Independent reflections |
7363 |
|
R int |
0.0325 |
Table 1 (end)
|
Parameter |
Value |
|
Refinement variables |
769 |
|
GOOF |
1.026 |
|
R factors for F2 > 2 σ (F2) |
R 1 = 0.0237, wR 2 = 0.0533 |
|
R factors for all reflections |
R 1 = 0.0286, wR 2 = 0.0560 |
|
Residual electron density (min/max), e /Å3 |
1.12/-0.93 |
Table 2
Selected bond lengths and bond angles in the structure of compound 1
|
Bond |
d , Å |
Angle |
ω , deg |
|
Ir(1) - S(3) |
2.2958(16) |
S(3)Ir(1)S(4) |
178.35(7) |
|
Ir(1) - S(4) |
2.3002(15) |
S(3)Ir(1)Cl(3) |
90.09(8) |
|
Ir(1) - Cl(3) |
2.3407(19) |
S(3)Ir(1)Cl(4) |
87.48(6) |
|
Ir(1) - Cl(4) |
2.3529(16) |
S(3)Ir(1)Cl(5) |
92.21(6) |
|
Ir(1) - Cl(5) |
2.3500(17) |
S(3)Ir(1)Cl(6) |
90.33(7) |
|
Ir(1) - Cl(6) |
2.3681(17) |
S(4)Ir(1)Cl(3) |
91.55(7) |
|
Ir(2) - S(5) |
2.311(2) |
S(4)Ir(1)Cl(4) |
92.71(6) |
|
Ir(2) - S(6) |
2.3148(17) |
S(4)Ir(1)Cl(5) |
87.62(6) |
|
Ir(2) - Cl(7) |
2.3493(18) |
S(4)Ir(1)Cl(6) |
88.03(6) |
|
Ir(2) - Cl(8) |
2.3498(17) |
Cl(3)Ir(1)Cl(4) |
90.30(8) |
|
Ir(2) - Cl(9) |
2.3546(17) |
Cl(3)Ir(1)Cl(5) |
88.89(8) |
|
Ir(2) - Cl(10) |
2.3396(17) |
Cl(3)Ir(1)Cl(6) |
179.56(7) |
|
Sb(1) - O(1) |
2.545(4) |
Cl(4)Ir(1)Cl(6) |
89.60(7) |
|
Sb(1) - C(1) |
2.117(6) |
Cl(5)Ir(1)Cl(4) |
179.14(7) |
|
Sb(1) - C(11) |
2.106(6) |
Cl(5)Ir(1)Cl(6) |
91.20(8) |
|
Sb(1) - C(21) |
2.106(6) |
S(5)Ir(2)S(6) |
175.88(7) |
|
Sb(1) - C(31) |
2.126(6) |
S(5)Ir(2)Cl(10) |
91.53(8) |
|
Sb(2) - O(2) |
2.465(4) |
Cl(7)Ir(2)Cl(9) |
179.22(7) |
|
Sb(2) - C(51) |
2.106(6) |
Cl(10)Ir(2)Cl(8) |
178.69(7) |
|
Sb(2) - C(71) |
2.130(6) |
Cl(10)Ir(2)Cl(9) |
90.30(8) |
|
Sb(2) - C(41) |
2.111(6) |
C(1)Sb(1)O(1) |
77.93(19) |
|
Sb(2) - C(61) |
2.119(6) |
C(1)Sb(1)C(31) |
100.4(2) |
|
O(4) - S(4) |
1.459(5) |
C(11)Sb(1)O(1) |
78.92(18) |
|
O(6) - S(6) |
1.464(5) |
C(11)Sb(1)C(1) |
116.7(2) |
|
S(1) - O(1) |
1.457(5) |
C(11)Sb(1)C(21) |
113.6(2) |
|
S(1) - C(38) |
1.739(10) |
C(11)Sb(1)C(31) |
102.2(2) |
|
S(1) - C(37) |
1.785(11) |
C(21)Sb(1)O(1) |
80.45(19) |
|
S(2) - O(2) |
1.517(4) |
C(21)Sb(1)C(1) |
119.2(2) |
|
S(2) - C(77) |
1.763(8) |
C(21)Sb(1)C(31) |
100.2(2) |
|
S(2) - C(78) |
1.753(9) |
C(31)Sb(1)O(1) |
178.33(18) |
|
S(3) - O(3) |
1.450(5) |
C(51)Sb(2)O(2) |
82.50(18) |
|
S(3) - C(84) |
1.779(9) |
C(51)Sb(2)C(71) |
98.7(2) |
|
S(3) - C(83) |
1.758(8) |
C(51)Sb(2)C(41) |
114.4(2) |
|
S(4) - C(81) |
1.764(6) |
C(51)Sb(2)C(61) |
121.2(2) |
|
S(4) - C(82) |
1.769(7) |
C(71)Sb(2)O(2) |
176.47(18) |
|
S(5) - C(86) |
1.676(11) |
C(41)Sb(2)O(2) |
83.41(18) |
|
S(5) - O(5) |
1.477(6) |
C(41)Sb(2)C(71) |
99.0(2) |
|
S(5) - C(85) |
1.678(11) |
C(41)Sb(2)C(61) |
117.3(2) |
|
S(6) - C(88) |
1.765(7) |
C(61)Sb(2)O(2) |
77.58(18) |
|
S(6) - C(87) |
1.770(7) |
C(61)Sb(2)C(71) |
99.0(2) |
Conclusion
The structure of iridium complex [Ph 4 Sb(DMSO)]+[IrCl 4 (DMSO) 2 ]–, obtained from tetraphenylsti-bonium chloride and sodium hexachloroiridate (III) in water, followed by recrystallization from dimethyl sulfoxide, has been established by X-ray diffraction analysis.
Список литературы Synthesis and structure of iridium complex [Ph 4Sb(DMSO)] +[IrCl 4(DMSO) 2] -
- Haddad Y.M.Y., Henbest H.B., Trocha-Grimshaw J. Aspects of Catalysis. Part II. Dimethyl Sulphoxide Complexes of Iridium(III) Including Hydrides. Journal of the Chemical Society, Perkin Transactions 1, 1974, pp. 592-595.
- Messori L., Marcon G., Orioli P, Fontani M., Zanello P., Bergamoc A., Savac G., Murad P. Molecular Structure, Solution Chemistry and Biological Properties of the Novel , (I) and of the Orange Form of 4 , (II). Journal of Inorganic Biochemistry, 2003, vol. 95, iss. 1, pp. 37-46.
- Albertí F.M., Fiol J.J., García-Raso A., Torres M., Terrón A., Barceló-Oliver M., Prieto M.J., Moreno V., Molins E. Ruthenium(III) and Iridium(III) Complexes with Nicotine. Polyhedron, 2010, vol. 29, iss. 1, pp. 34-41. DOI: DOI: 10.1016/j.poly.2009.05.082
- Sharutin V.V., Sharutina O.K., Senchurin V.S., Somov N.V. Russian Journal of General Chemistry, 2015, vol. 85, no. 3, pp. 634-639. DOI: DOI: 10.1134/S1070363215030184
- SMART and SAINT-Plus. Versions 5.0. Data Collection and Processing Software for the SMART System. -Bruker AXS Inc. -1998. -Madison, Wisconsin, USA.
- SHELXTL/PC. Versions 5.10. An Integrated System for Solving, Refining and Displaying Crystal Structures From Diffraction Data. -Bruker AXS Inc. -1998. -Madison, Wisconsin, USA.

![Synthesis and structure of iridium complex [Ph 4Sb(DMSO)] +[IrCl 4(DMSO) 2] - Synthesis and structure of iridium complex [Ph 4Sb(DMSO)] +[IrCl 4(DMSO) 2] -](/file/cover/147160334/synthesis-and-structure-of-iridium-complex-ph-4sb-dmso-ircl-4-dmso-2.png)
