Synthesis and structure of tetraphenylantimony β-isatoximate
Автор: Artemeva E.V., Sharutina O.K.
Журнал: Вестник Южно-Уральского государственного университета. Серия: Химия @vestnik-susu-chemistry
Рубрика: Химия элементоорганических соединений
Статья в выпуске: 1 т.10, 2018 года.
Бесплатный доступ
(β-Isatoximato)tetraphenylantimony (1) was obtained by the reaction of pentaphenylantimony with β-isatoxime. X-ray diffraction analysis showed that the antimony atom in complex 1 had distorted trigonal-bipyramidal coordination.
Pentaphenylantimony, β-isatoxime, dephenylation, (β-isatoximato)tetraphenylantimony, molecular structures, x-ray diffraction analysis
Короткий адрес: https://sciup.org/147160418
IDR: 147160418 | УДК: 546.865+547.1.13+547.304.6+547.53.024+548.312.5 | DOI: 10.14529/chem180106
Текст научной статьи Synthesis and structure of tetraphenylantimony β-isatoximate
It is known from the literature sources that pentaarylantimony reacts with inorganic and organic acids HX (X = OR, OAr, OSO 2 R, OC(O)R, Hal, NO 3 ) to form antimony derivatives Ar 4 SbX in high yield [1–6]. In some articles the dephenylation of pentaphenylantimony was studied in the reaction with oximes [7–10], leading to formation of tetraphenylantimony oximes. It has been found that when ketox-imes are used, the separation of two phenyl groups and the formation of triphenylantimony dioximates are sometimes observed [7].
Another method for the preparation of tetraaryl antimony oxymates is based on a substitution reaction with the use of tetraarylantimony halides. Thus, Ar 4 SbX derivatives were synthesized (Ar = Ph, 4-ClC 6 H 4 ) by the reaction of tetraarylantimony bromide with N-hydroxy-demethylcantharimide with the addition of Et 3 N; the reaction products were characterized by IR and NMR spectroscopy methods [11]. Similarly, organoantimony arylhydroxamates of the general formula (4-CH 3 C 6 H 4 ) 4 SbX were synthesized, the structure of the compounds thus obtained was established by the X-ray diffraction method [12]. By the means of MTT and SRB, it was found that those compounds had antitumor effect [11, 12].
Tetraarylantimony oximates were obtained in high yield as a result of a ligand redistribution reaction of pentaphenyl- and penta( p -tolyl)antimony with triarylantimony dioximates [7, 13].
According to X-ray diffraction analysis data, antimony atoms in tetraaryl antimony oximates, as a rule, have distorted trigonal-bipyramidal coordination with the iminoxy group oxygen atom in the axial position. Short distances between the iminoxy group nitrogen atom and the antimony atom are observed [7–10, 13].
The present paper describes further study of the interaction of pentaphenylantimony with oximes, in particular, β-isatoxime, and the establishment of the molecular structure of the obtained product.
ExperimentalSynthesis of β-isatoximato tetraphenylantimony (1)
a) 300 mg of pentaphenylantimony (0.59 mmol) and 96 mg (0.59 mmol) of β-isatoxime were dissolved in benzene, sealed in an ampoule and heated for several hours in a water bath until the precipitate dissolved. The mixture was held for several days at 20 °C. After removing the solvent, the solid residue was recrystallized from chloroform with addition of heptane. Yellow crystals with the mass of 0.348 g (99 %) and melting point of 193 °C were obtained.
IR spectrum, ν, сm–1: 3148, 3065, 1705, 1612, 1591, 1551, 1508, 1474, 1458, 1435, 1375, 1340, 1296, 1291, 1182, 1151, 1096, 1057, 1020, 997, 972, 785, 754, 729, 692, 650, 582, 492, 457, 419.
Similarly, an experiment was carried out with 1:2 molar ratio of the reactants. The solid residue was recrystallized from ethanol.
IR spectra were recorded on a Shimadzu IRAffinity-1S FTIR spectrometer (pellets with KBr; 4000 - 400 cm–1).
X-ray diffraction analysis of crystalline substance 1 was performed on a Bruker D8 QUEST automatic four-circle diffractometer (Mo K α - emission, λ = 0.71073 Å, graphite monochromator).
Data collection and editing, unit-cell parameters refinement, and correction for absorption were carried out in SMART and SAINT-Plus software. All calculations aimed at solving and refining the structure of compound 1 were performed in SHELXL/PC software [14, 15]. Structure 1 was determined by direct methods and refined with LS method in the anisotropic approximation for non-hydrogen atoms. The selected crystallographic data and the structure refinement results are listed in Table 1. Selected bond lengths and bond angles are summarized in Table 2.
The full tables of atomic coordinates, bond lengths, and bond angles were deposited with the Cambridge Crystallographic Data Centre (CCDC 1814675 for compound 1 ; .
Table 1
Crystallographic data and the experimental and structure refinement parameters for compound 1
|
Parameter |
Value |
|
1 |
|
|
Empirical formula |
C 64 H 50 N 4 O 4 Sb 2 |
|
Formula weight |
1182.65 |
|
Т , К |
293.15 |
|
Crystal system |
triclinic |
|
Space group |
P–1 |
|
a , Å |
10.106(12) |
|
b , Å |
10.340(14) |
|
c, Å |
26.82(3) |
|
α , deg |
83.36(6) |
|
β, deg |
79.45(4) |
|
γ , deg |
84.63(7) |
|
V , Å3 |
2729(6) |
|
Z |
2 |
|
ρ (calcd.) , g/сm3 |
1.4389 |
|
µ , mm–1 |
1.042 |
|
F (000) |
1190.0 |
|
Crystal size, mm |
0.42 × 0.13 × 0.1 |
|
2 θ Range of data collection, deg |
5.66 – 49.54 |
|
Range of refraction indices |
–11 ≤ h ≤ 11, –12 ≤ k ≤ 12, –31 ≤ l ≤ 31 |
|
Reflections collected |
45577 |
|
Independent reflections |
9232 |
|
R int |
0.0583 |
|
Refinement variables |
667 |
|
GOOF |
1.045 |
|
R factors for F2 > 2 σ (F2) |
R 1 = 0.0973, wR 2 = 0.2376 |
|
R factors for all reflections |
R 1 = 0.1105, wR 2 = 0.2444 |
|
Residual electron density (min/max), e /Å3 |
2.46/–2.94 |
Table 2
Selected bond lengths and bond angles in the structure of compound 1
|
Bond |
d , Å |
Angle |
ω , deg |
|
Sb(1)–С(1) |
2.128(13) |
C(11)Sb(1)O(1) |
177.9(4) |
|
Sb(1)–С(21) |
2.115(12) |
C(1)Sb(1)C(21) |
112.9(5) |
|
Sb(1)–С(31) |
2.116(13) |
C(1)Sb(1)C(31) |
117.2(5) |
|
Sb(1)–О(1) |
2.231(9) |
C(21)Sb(1)C(31) |
125.9(5) |
|
Sb(1)–С(11) |
2.167(12) |
N(1)O(1)Sb(1) |
108.5(7) |
|
О(1)–N(1) |
1.331(14) |
O(1)N(1)C(41) |
112.7(11) |
|
N(1)–C(41) |
1.330(16) |
C(1)Sb(1)O(1) |
81.1(4) |
Table 2 (end)
|
Bond |
d , Å |
Angle |
ω , deg |
|
O(2)–C(42) |
1.206(16) |
C(21)Sb(1)O(1) |
85.5(4) |
|
N(2)–C(42) |
1.388(17) |
C(31)Sb(1)O(1) |
83.2(4) |
|
Sb(2)–С(51) |
2.122(14) |
C(81)Sb(2)O(3) |
168.1(6) |
|
Sb(2)–С(61) |
2.122(14) |
C(51)Sb(2)C(61) |
121.4(6) |
|
Sb(2)–С(71) |
2.103(18) |
C(51)Sb(2)C(71) |
115.3(7) |
|
Sb(2)–О(3) |
2.195(11) |
C(61)Sb(2)C(71) |
120.8(6) |
|
Sb(2)–C(81) |
2.179(14) |
N(3)O(3)Sb(2) |
127.5(11) |
|
O(3)–N(3) |
1.300(18) |
O(3)N(3)C(91) |
111.0(15) |
|
N(3)–C(91) |
1.33(2) |
C(51)Sb(2)O(3) |
87.4(5) |
|
O(4)–C(92) |
1.21(2) |
C(61)Sb(2)O(3) |
74.9(5) |
|
N(4)–C(92) |
1.36(2) |
C(71)Sb(2)O(3) |
92.3(6) |
Results and Discussion
We have studied the reactions of pentaphenylantimony with β-isatoxime containing a large organic radical with several functional groups. It has been found that, regardless of the molar ratio of the reactants (1:1 or 1:2), the reaction product is (β-isatoximato)tetraphenylantimony:
Ph 5 Sb +
NOH
NO H
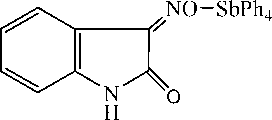
+ PhH.
Compound 1 is a crystalline substance, highly soluble in aromatic and aliphatic hydrocarbons, resistant to the air moisture and oxygen.
The resulting compound has been studied by IR spectroscopy and X-ray diffraction analysis.
In the IR spectrum of compound 1 there are absorption bands at 3148 and 3065 cm–1, characterizing the presence of the N–H bond, and a band at 1705 cm–1, which appears as a result of valence vibrations of the C=O bond. Characteristic bands are observed at 1551 cm–1 (C=N bond), 1020 cm–1 (N–O bond). In comparison with the initial oxime spectrum, the absorption bands in the spectrum of compound 1 are shifted to longer wavelengths. Vibrations at 419 cm–1 indicate the presence of the Sb–C bond in the molecule of compound 1 [16].
According to the X-ray diffraction analysis data, in the crystal of compound 1 there are two crystallographically independent molecules ( a and b ). Antimony atoms have distorted trigonal-bipyramidal coordination with the iminoxy group oxygen atom and the carbon atom of one of phenyl groups in the axial positions (Fig. 1).
The SbC 3 fragment lying in the equatorial plane is not flat. The Sb atom deviates from the [C 3 ] plane by 0.246 Å ( a ) and 0.198 Å ( b ) toward the axial carbon atom. The sums of the valence angles of С eq SbC eq in the equatorial planes are 356.0(5)° ( a ) и 357.5(6)° ( b ), the values of the individual angles differ from the theoretical 120° by not more than 7.1°. The axial angle OSbC ax is 177.9(4)° ( a ) and 168.1(6)° ( b ). The values of the angles С ax SbC eq and C eq SbO lie within the limits of 92.4(5)°–100.0(5)° ( a ), 93.5(6)°–97.0(6)° ( b ) and 81.1(4)°–85.5(4)° ( a ), 74.9(5)°–92.3(6)° ( b ), respectively, which differs from the theoretical value of 90° and is caused by the deviation of the Sb atom from the equatorial plane.
The variation intervals of the Sb–C eq bond lengths are 2.115(12)–2.128(13) Å ( a ), 2.103(18)– 2.122(14) Å ( b ). The axial bonds Sb–C (2.167(12) Å ( a ), 2.179(14) Å ( b )) are considerably longer than the equatorial bonds. The distances Sb–O (2.231(9) Å ( a ), 2.195(11) Å ( b )) significantly exceed the sum of the covalent radii of Sb and O atoms (2.07 Å [17]), as well as the distance between the Sb atom and the axial C atom, similarly, as in other compounds, where the O–N=C fragment is linked to electronaccepting functional groups [18–20]. Interestingly, the Sb–O distances (2.121(4)–2.179(1) Å) in previously studied tetraphenylantimony oximates, containing electron-donating substituents, are smaller than those in compound 1 and do not exceed the Sb–C ax distances (2.167(8)–2.207(1) Å) [8–10].
Артемьева Е.В., Шарутина О.К. Синтез и строение
β-изатоксимата тетрафенилсурьмы
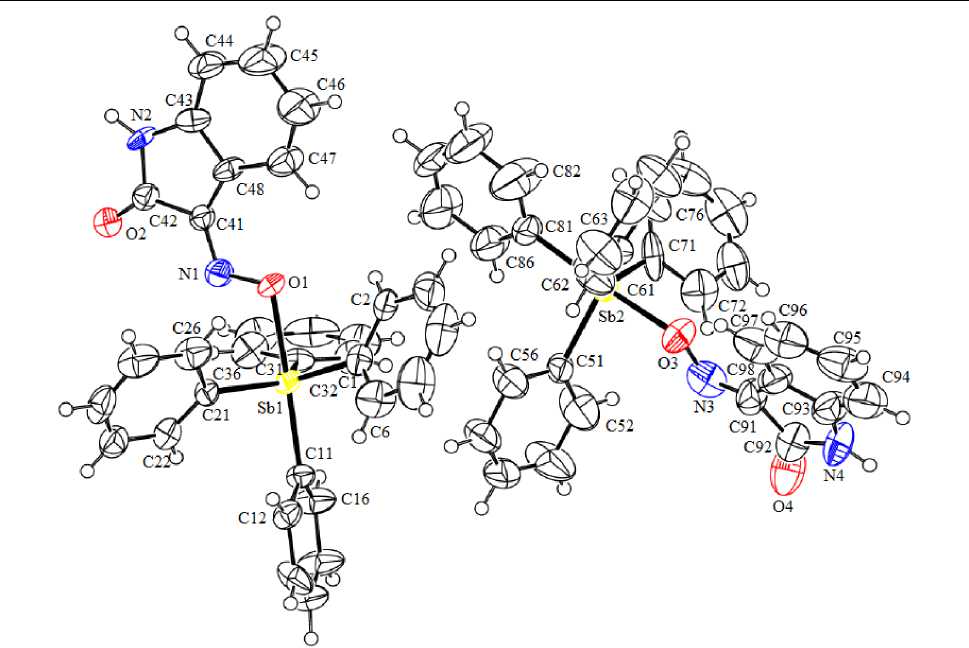
a) b)
Fig. 1. The structure of compound 1 (a, b)
In the molecules a and b , t he intramolecular contacts Sb···N are observed. Th e dis ta nce s betw e e n the Sb atom and the iminoxy- g roup N a tom S b· · · N [2. 94( 1 ) Å ( a ), 3.16(2) Å ( b )] are shorter than the s um of v a n de r W a als r a d ii of th e s e atoms (3.8 Å [17]). The reduction of the distanc e s Sb·· · N i s a cco mpanied by the decrease of the S bON a ng le v a lue s ( 108.5(7)° ( a ), 127.5(11)° ( b )), the SbON angle in molecule b i s a b nor ma lly la rg e , in c omparison with the corresponding value in a na log ous c ompound s (102.7(4)°–109.81(7)°) [8–10]. The v a lue s of the ONC angles in the molecules are close: 113(1)° ( a ), 111(1)° ( b ) . No de pende n ce in th e c ha ng e of the O –N and N–C bond lengths (1.331(14) Å, 1.330(16) Å ( a ) and 1.300(18) Å, 1.33(2) Å ( b )) is ob se r v e d.
I n the c onde ns e d he t e r o c y c le in mole c ul e a , the iminoxy group is located in the heterocycle plane, while in molecule b t he O atom de v ia te s fr om the p la n e by 0. 308 Å a nd t he N atom deviates by 0.192 Å. The a ng le s be tw e e n the h e t e r oc y c l e pl a ne a nd t he p la n e of the b e nze ne ring a re 3.50° ( a ) and 6.02° ( b ).
Conclusions
Thus , r e g a r d less of the mol a r ratio of the reactants, as a result of the reaction of p e nta p he ny la n ti mony with β-isatoxime, (β-isa toxima to)tetraphenylantimony is formed. In spite of the f ac t that β-isatoxime contains se v e r al di f fe r ent func t ional g r oups (th e a mino - and the keto-group), it reacts with pentapheny l a n timony in a c onv e ntional ma nne r . The a nt imony a tom in the resulting compound has distorted trigon-al- b ipyra mida l c oor d inatio n with the iminoxy-group oxygen atom and the carbon atom of one of the phenyl groups.
Acknowledgments
We ar e g r ate f u l to V .V . S h a r ut in f or the X-ray diffraction analysis of complex 1 .
Список литературы Synthesis and structure of tetraphenylantimony β-isatoximate
- Schmidbaur, H. Hydrogendicarboxylat-Ionen als Wasserstoffbrückenverknüpfte Chelatsysteme in Organoantimonverbindungen/H. Schmidbaur, K. H. Mitschke//Angew. Chem. -1971. -V. 83, № 4. -P. 149-150.
- Синтез и строение аддуктов нитрата тетрафенилсурьмы с азотной кислотой и ацетата тетрафенилсурьмы с уксусной кислотой/В.В. Шарутин, В.С. Сенчурин, О.К. Шарутина и др.//Журн. неорг. химии. -2008. -Т. 53, № 7. -С. 1194-1198.
- Синтез и строение 3-гидрокси-4-ацетилфенокситетрафенилсурьмы/В.В. Шарутин, О.К. Шарутина, E.A. Бондарь и др.//Журн. общ. химии. -2003. -Т. 73, № 3. -С. 376-379.
- Синтез и строение кислых сульфатов тетрафенилсурьмы и -фосфора/В.В. Шарутин, А.П. Пакусина, И.В. Егорова и др.//Журн. общ. химии. -2003. -Т. 73, №. 4. -С. 569-572.
- Синтез и строение органосульфонатов тетра-и трифенилсурьмы/В.В. Шарутин, О.К. Шарутина, А.П. Пакусина и др.//Коорд. химия. -2004. -Т. 30, № 1. -С. 15-24.
- Шарутин, В.В. Синтез и строение ароксидов тетрафенилсурьмы Ph4SbOAr (Ar = C6H4C6H7, C6H2(Br2-2,6)(tert-Bu-4), C6H3(NO2)2-2,4, C6H2(Br2-2,6)(NO2-4))/В.В. Шарутин, О.К. Шарутина, В.С. Сенчурин//Журн. неорг. химии. -2017. -Т. 62, № 3. -С. 290-295 DOI: 10.7868/S0044457X17030151
- Синтез и строение оксиматов тетра-и триарилсурьмы/В.В. Шарутин, О.К. Шарутина, О.В. Молокова и др.//Коорд. химия. -2002. -Т. 28, № 8. -С. 581-590.
- Синтез и строение оксиматов тетрафенилсурьмы Ph4SbON=CRR′(CRR′ = C6H9-C6H9 -2 и R = Ph, R′ = C(O)Ph)/В.В. Шарутин, О.К. Шарутина, О.В. Молокова и др.//Коорд. химия. -2004. -Т. 30, № 8. -С. 596-602.
- Шарутин, В.В. Синтез и строение салицилальдоксиматов тетра-и трифенилсурьмы/В.В. Шарутин, О.К. Шарутина, О.В. Молокова//Журн. неорг. химии. -2012. -Т. 57, № 6. -С. 902-907.
- Шарутин В.В., Шарутина О.К. Синтез и строение оксиматов тетрафенилсурьмы: Ph4SbON=CHR (R=C6H4Br-2, C6H4NO2-2, C4H3S)/V.V. Sharutin, O.K. Sharutina//Коорд. химия. -2017. -Т. 43, № 4. -С. 54-59.
- Synthesis, Crystal Structures and in vitro Antitumor Activities of Some Arylantimony Derivatives of Analogues of Demethylcantharimide/G.C, Wang, J. Xiao, L. Yu et al.//J. Organomet. Chem. -2004. -V. 689, N 9. -P. 1631-1638.
- Synthesis, Crystal Structures and in vitro Antitumor Activities of Some Organoantimony Arylhydroxamates/G.C. Wang, Y.-N. Lu, J. Xiao et al.//J. Organomet. Chem. -2005. -V. 690, № 1. -P. 151-156.
- Синтез и строение оксиматов тетра-и триарилсурьмы/В.В. Шарутин, O.K. Шарутина, O.В. Молокова и др.//Журн. общ. химии. -2001. -Т. 71, № 8. -C. 1317-1321.
- Bruker (2000) SMART. Bruker Molecular Analysis Research Tool, Versions 5.625 Bruker AXS, Madison, Wisconsin, USA.
- Bruker (2000) SAINTPlus Data Reduction and Correction Program Versions 6.02a, Bruker AXS, Madison, Wisconsin, USA.
- Doak, G.O. The Infrared Spectra of Some Phenylsubstituted Pentavalent Antimony Compounds/G.O. Doak, G.G. Long, L.D. Freedman//J. Organomet. Chem. -1965. -V. 4. -№ 1. -P. 82-91.
- Бацанов, С.С. Атомные радиусы элементов/С.С. Бацанов//Журн. неорг. хим. -1991. -Т. 36, № 12. -С. 3015-3037.
- Kopf, J. Nitrosolate und Nitrosolatokomplexe. III. Darstellung und Struktur von Nitrosolsäurederivaten organosubstituierter Antimon (V)-, Tellur (IV)-und Jod (III)-Verbindungen/J. Kopf, G. Vetter, G. Klar//Ztschr. Anorg. und Allg. Chem. -1974. -V. 409, № 3. -P. 285-298.
- Домасевич, К.В. Синтез, кристаллическая и молекулярная структура комплекса тетрафенилсурьмы (V) с 2-изонитрозо-2-(4-метилтиазолил-2)-ацетамидом/К.В. Домасевич, В.В. Скопенко, Р. Кемпе и др.//Журн. неорг. хим. -1998. -Т. 43, № 2. -С. 246-249.
- Domasevitch, K.V. Organoantimony (V) Cyanoximates: Synthesis, Spectra and Crystal Structures/K.V. Domasevitch, N.N. Gerasimchuk, A. Mokhir//Inorg. Chem. -2000. -V. 39, № 6. -P. 1227-1237.


