Таргетная терапия гормонорезистентного рака предстательной железы
Автор: Чернышев И.В., Жернов А.А., Перепечин Д.В.
Журнал: Экспериментальная и клиническая урология @ecuro
Рубрика: Онкоурология
Статья в выпуске: 1, 2012 года.
Бесплатный доступ
Гормонорезистентный рак предстательной железы, таргентная терапия, препараты, алгоритм лечения
Короткий адрес: https://sciup.org/142188289
IDR: 142188289
Текст статьи Таргетная терапия гормонорезистентного рака предстательной железы
ЭКСПЕРИМЕНТАЛЬНАЯ И КЛИНИЧЕСКАЯ УРОЛОГИЯ №1 2012
Таблица 1. Эффективность химиотерапии гормонорезистентного рака простаты
|
Исследование |
Тип исследования |
Число пациентов |
Препарат |
Частичный ответ (5): снижение ПСА > 50% |
Время до прогрессии (мес.) |
Общая выживаемость (мес.) |
|
Loriot et al., 2009 [4] |
Проспективное |
40 |
Carboplatin/etoposide |
23 |
2,1 |
19,0 |
|
Rosenberg et al., 2007 [5] |
Проспективное |
41 |
Mitoxantrone |
20 |
2,3 |
9,8 |
|
Rosenberg et al., 2007 [5] |
Проспективное |
41 |
Ixabepilone |
17 |
2,2 |
10,4 |
|
Ross et al., 2008 [6] |
Проспективное |
34 |
Docetaxel/carboplatine |
18 |
3 |
12,4 |
|
Sternberg et al.,2007 [7] |
Проспективное |
327 |
Satraplatine |
2,5 |
||
|
Eymard et al.,2007 [7] |
Ретроспективное |
31 |
Docetaxel |
59 |
н/д |
н/д |
|
Berthold et al., 2008 [9] |
Ретроспективное |
89 |
Mitoxantrone |
10 |
3,2 |
10 |
|
Nakabayashi et al., 2007 [10] |
Ретроспективное |
36 |
Vinorelbine |
14 |
6,9 |
|
|
Michels et al., 2006 [11] |
Ретроспективное |
35 |
Mitoxantrone |
12 |
2-3 |
12 |
|
Oh et al., 2006 [12] |
Ретроспективное |
35 |
Mitoxantrone |
6 |
1,5 |
Таблица 2. Таргетные препараты в лечении гормонорезистентного рака простаты [22]
Точка приложения Биологический эффект Препарат Стадия клинического внедрения
Новые надежды на улучшение результатов лечения ГР-РПЖ связываются с применением таргетной терапии [21]. В настоящее время предложены следующие препараты для лечения ГР-РПЖ (таблица 2).
Одним из направлений исследований по применению таргетных препаратов при лечении ГР-РПЖ являлась комбинация их с цитотоксичными препаратами. Было доказано, что потенцирование бе-вацизумабом эффективности химиотерапевтической схемы IFL при метастатическом колоректальном раке. Применительно к ГР-РПЖ в настоящее время в эксперименте также проверяются комбинации с доцетакселом. Доцетаксел оказывает противоопухолевое действие, стабилизируя микротрубочки, угнетает их распад, снижает концентрацию свободного тубулина, что приводит в конечном итоге к нарушению фазы митоза и межфазных процессов. Применение моноклональных антител к VEGF (сосудистому эндотелиальному фактору роста) бевацизумаб увеличивает антипролиферативное действие доцетаксела в эксперименте на клеточной линии рака простаты и молочной железы. Потенцирование достигается благодаря снижению васкуляризации опухоли. a
Таблица 3. Характеристика групп больных и результаты в исследовании Dror Michaelson M., 2009 [35]
|
Группы больных |
Группа больных без предшествующей ХТ |
Группа больных, резистентных к доцетакселу |
|
Число больных |
17 |
17 |
|
Возраст (медиана, разброс) |
71 (52-80) |
65 (45-84) |
|
Уровень ПСА (нг/мл) (медиана, разброс) |
51 (7-602) |
44 (8-752) |
|
Наличие костных метастазов |
12 |
15 |
|
Только повышение ПСА |
1 |
0 |
Предшествующее лечение
|
Гормонотерапия (1-3 курса) |
11 |
12 |
|
Гормонотерапия (4-6 курсов) |
6 |
4 |
|
Химиотерапия (медиана, разброс) |
0 |
8 (3-14) |
|
Эффективность лечения |
||
|
Динамика уровня ПСА |
||
|
Ответ: снижение уровня на ≥ 50% |
1 |
1 |
|
Стабилизация: нет признаков ответа или прогрессирования |
8 |
7 |
|
Прогрессирование: увеличение на ≥ 25% |
7 |
7 |
|
Результат невозможно оценить |
1 |
2 |
Оценка эффекта, основанная на радиологических данных
|
Частичный ответ |
0 |
1 |
|
Стабилизация |
10 |
8 |
|
Прогрессия |
5 |
5 |
|
Результат невозможно оценить |
1 |
3 |
|
Эффект, неприемлемый для оценки |
1 |
0 |
Описанный эффект открывает дополнительные возможности для клинических исследований как для бевацизумаба, так других таргет-ных препаратов, основанных на ингибировании ангиогенеза (суни-тиниб, сорафениб, гефитиниб) [23]. Потенциальную эффективность противоопухолевой терапии показали исследования эффекта ингибирования имидазола (1,2-а) пиразин диарилуреазой рецептора тирозин-киназы [24].
Влияние ангиогенеза, доказанное в преклинических и клинических исследованиях, на развитие РПЖ позволило рассматривать ингибиторы ангиогенеза (например, сунитиниб) в качестве перспективного направления в лечении ГР-РПЖ [25, 26, 27]. У мужчин, больных РПЖ, отмечается повышение VEGF (сосудистого эндотелиального фактора роста), при этом отмечается ассоциация его высокого уровня в плазме с большей смертностью [20, 28, 30].
Сунитиниб является хорошо зарекомендовавшим себя таргетным препаратом, наиболее изученным у больных генерализованным раком почки. Препарат представляет собой ингибитор рецептора киназы VEGF 1, 2 и 3 типа тромбоцитарного ростового фактора PDGF α- и β-, Kit and RET [31, 32]. Интересно, что данный препарат показал свою эффективность при лечении пациента с метастазом рака почки в предстательную железу через 10 лет после радикальной нефрэктомии [33].
Cumashi A. и соавторы в 2008 г. проводили лабораторным животным, которым прививались клетки человеческого ГР-РПЖ, химиотерапию сунитинибом в монорежиме в комбинации с низкодозной и высо-кодозной химиотерапией доцетакселом. Авторами сделаны выводы о перспективности применения суни-тиниба в комбинации с низкодозной ХТ доцетакселом либо в монорежиме в клинической практике [34].
Представляется наиболее актуальным вопрос о проведении химиотерапии сунитинибом в качестве второй линии химиотерапии после доцетаксела.
Dror Michaelson M. и соавторы в 2009 г. провели исследование в рамках II фазы исследования [35]. В работу было включено 34 больных ГР-РПЖ, из них 17 больных, не получавших химиотерапию, и 17 больных, перенесших в среднем 8 (3-14) курсов химиотерапии доцетакселом без эффекта (таблица 3).
Больным проводилось ХТ по схеме: 50 мг/день в течение 4 недель с последующим двухнедельным перерывом. При развитии осложнений уровень редукции составлял 37,5 или 25 мг. Стабилизация уровня ПСА отмечалось у 8 и 7 больных соответственно; повышение уровня ПСА – у 7 и 7 больных соответственно. Стабилизация процесса наблюдалась у 10 больных первой группы и у 8 – второй. Прогрессирование было у 5 пациентов в обеих группах. Действие сунитиниба было подтверждено маркерами ангиогенеза. С другой стороны, авторы отмечают периодическое расхождение показателей рентгенологических исследований и уровня ПСА. На основании этого были сделаны выводы о необходимости альтернативных методов оценки эффективности лечения, дополнительно к ПСА [35].
ЭКСПЕРИМЕНТАЛЬНАЯ И КЛИНИЧЕСКАЯ УРОЛОГИЯ
№1 2012
Sonpavde G. и соавторы в 2009 г. доложили результаты лечения 36 больных (медиана возраста 69,5 лет) в рамках II фазы исследования препарата у больных после предшествующей неэффективной химиотерапии доцетакселом. Больным перорально давалось 50 мг/день препарата в течение 4 недель с последующим двухнедельным перерывом. Параллельно проводилась гормональная терапия – до 8 курсов. Критерием исключения было наличие признаков прогрессирования или выраженные токсические эффекты. Проводилась оценка среднего времени без прогрессии.
Среднее время без прогрессии составило 19,4 недель, причем отсутствие прогрессии в течение 12 недель отмечалась у 75,8% больных. У 52,8% больных вследствие токсических эффектов потребовалось провести перерыв в химиотерапии [36].
Есть сведения о планирующихся и проводящихся в настоящее время клинических исследованиях пазо-паниба при раке предстательной железы.
В настоящее время в США проводится набор пациентов в исследование фазы II пациентов с метастатическим раком предстательной железы, устойчивым к полной андрогенной блокаде. Пациенты, которые будут включаться в исследование, ранее получали терапию агонистами рилизинг-фактора лю-теинезирующего гормона. Пазопа-ниб будет назначаться в дозировке 800 мг в день в течение 12 недель [37].
В США проводится рандомизированное исследование фазы II, в котором оценивается эффективность пазопаниба в лечении рецидивирующего рака предстательной железы у пациентов, ранее получавших гозелерин или лейпролид. В исследование включено 98 пациентов, они рандомизированы в две группы, в одной из которых они получают пазопаниб, а в другой – только активное наблюдение. Завершение исследования планируется в
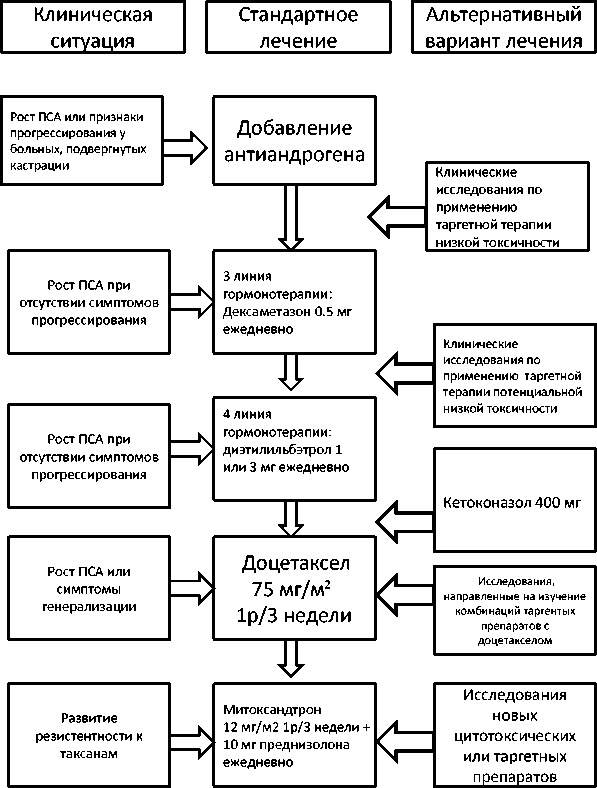
Рисунок 1. Алгоритм лечения больных ГР-РПЖ (22)
декабре 2011 г. [38].
В настоящее время проводится набор в исследование фазы I/II, в котором будет оцениваться эффективность и безопасность доцетак-села/преднизолона в комбинации с пазопанибом у пациентов с метастатическим раком предстательной железы. Завершение исследования планируется в декабре 2014 г. [39].
На основании представленных данных, алгоритм лечения ГР-РПЖ и место в нем таргетной терапии в современной онкоурологии можно представить следующим образом (рисунок 1).
Список литературы Таргетная терапия гормонорезистентного рака предстательной железы
- Yagoda A., Petrilak D. Cytotoxic chemotherapy for advanced hormone resistana prostate cancer//Cancer. 1993. Vol. 71. P. 1098-1109.
- Abrahamsson P.A. Intermittent androgen blockade. Too good to be true?//Scand J Urol Nephrol Suppl. 1999. Vol. 203. P. 45-49.
- Small E.J., Vogelzang N.J. Second-line hormone therapy for advanced prostate cancer: a shifting paradigm//J Clin Oncol. 1997. Vol. 15. P. 382-388.
- Loriot Y., Massard C., Gross-Goupil M., Di Palma M., Escudier B., Bossi A., Fizazi K. Combining carboplatin and etoposide in docetaxelpretreated patients with castration-resistant prostate cancer: a prospective study evaluating also neuroendocrine features//Annals of Oncology. 2009. Vol. 20. P. 703-708.
- Rosenberg J.E., Weinberg V., Kelly W.K., Michaelson D., Hussain M.H., Wilding G., Gross M., Hutcheon D., Small E.J. Activity of second-line chemotherapy in docetaxel-refractory hormone-refractory prostate cancer patients: randomized phase 2 study of ixabepilone or mitoxantrone and prednisone//Cancer. 2007. Vol. 110. № 3. P. 556563.
- Ross R.W., Beer T.M., Jacobus S., Bubley G.J., Taplin M.E, Ryan C.W., Huang J., Oh W.K.; Prostate Cancer Clinical Trials Consortium. A phase II trial of carboplatin plus docetaxel in hormone-refractory prostate cancer patients who have refractory after docetaxel chemotherapy.//Cancer. 2008. Vol. 112. № 3. P. 521-526.
- Sternberg C.N., Petrylak D., Witjes F., Ferrero J., Eymard J., Falcon S., Chatta K., Vaughn D., Berry W., Sartor O. Satraplatin demonstrates significant clinical benefits for the treatment of patients with HRPC: results of a randomized phase III trial//J Clin Oncology. 2007. ASCO Annual Meeting Proceedings Part I. Vol. 25. № 18S. Abstr. 5019.
- Eymard J., Oudard S., Gravis G. et al. Second-line chemotherapy with docetaxel (D) in men treated with docetaxel-based regimen for metastatic hormonerefractory prostate cancer (mHRPC).//ASCO Prostate Cancer Symp 2007. Abstr 249.
- Berthold D.R., Pond G., de Wit R., Eisenberger M., Tannock I.F.; TAX 327. Investigators. Survival and PSA response of patients in the TAX 327 study who crossed over to receive docetaxel after mitoxantrone or vice versa.//Ann Oncol. 2008. Vol. 19. № 10. P. 1749-1753.
- Nakabayashi M., Ling J., Xie W., Regan M.M., Oh W.K. Response to vinorelbine with or without estramustine as second-line chemotherapy in patients with hormone-refractory prostate cancer//Cancer J. 2007. Vol. 13. № 2. P. 125-129.
- Michels J., Montemurro T., Murray N., Kollmannsberger C., Nguyen Chi K. First and second-line chemotherapy with docetaxel or mitoxantrone in patients with hormone-refractory prostate cancer//Cancer. 2006. Vol. 106. № 5. P. 1041-1046.
- Oh W.K., Manola J., Babcic V., Harnam N., Kantoff P.W. Response to second-line chemotherapy in patients with hormone refractory prostate cancer receiving two sequences of mitoxantrone and taxanes.//Urology. 2006. Vol. 67. № 6. P. 1235-1240.
- Tannock I.F., Osoba D., Stockler M.R., Ernst D.S., Neville A.J., Moore M.J., Armitage G.R., Wilson J.J., Venner P.M., Coppin C.M., Murphy K.C. Chemotherapy with mitoxantrone plus prednisone or prednisone alone for symptomatic hormone-resistant prostate cancer: a Canadian randomized trial with palliative end points.//J Clin Oncol. 1996. Vol. 14. № 6. P. 1756-1764.
- Hartley-Asp B., Natale R.B., Dreicer R., Falcon S., Ricardez A., Redfern C. et al. Phase II study of weekly intravenous estramustine phosphate 2.000 mg/m2 in patients with hormone-refractory prostate cancer//Proc Am Soc Clin Oncol. 2001. 20. Abstr 183.
- Morote J., Lopez-Pacios M.A., Ahmad A., Vila J., De Torres J.A. Treatment of hormone-refractory prostate cancer with estramustine phosphate.//Actas Urol Esp. 1991. Vol. 15, N 5. P. 421-424.
- Hudes G., Einhorn L., Ross E., Balsham A., Loehrer P., Ramsey H., Sprandio J., Entmacher M., Dugan W., Ansari R., Monaco F., Hanna M., Roth B.L. Vinblastine versus vinblastine plus oral estramustine phosphate for patients with hormonorrefractory prostate cancer: a Hoosier Oncology Group and Fox Chase Network phase III trial//J Clin Oncol. 1999. Vol. 17. № 10. P. 3160-3166.
- Sweeny C.J., Monaco F.J., Jung S.H., Wasielewsky M.Y., Picus J., Ansar R.H. A phase II Hoosier Oncology Group study of vinorelbine and estramustine phosphate in the hormone-refractory prostate cancer.//Ann Oncol. 2002. Vol. 13. № 3. P. 435-440.
- Huguet Perez J., Maroto Rey P., Palou Redorta J., Villavicencio Mavrich H. Hormone-refractory prostate cancer. Changes in therapeutic strategies since the utility of chemotherapy.//Actas Urol Esp. 2006. Vol. 30. № 2. 123-133.
- Yap R., Veliceasa D., Emmenegger U., Kerbel R.S., McKay L.M., Henkin J., Volpert O.V. Metronomic low-dose chemotherapy boosts CD95-dependent antiangiogenic effect of the thrombospondin peptide ABT-510: a complementation antiangiogenic strategy//Clin Cancer Res. 2005. Vol. 11. № 18. P. 6678-6685.
- Lord R., Nair S., Schache A., Spicer J., Somaihah N., Khoo V., Pandha H. Low dose metronomic oral cyclophosphamide for hormone resistant prostate cancer: a phase II study//J Urol. 2007. Vol.177. № 6. P. 2136-2140.
- Aragon-Ching J.B., Dahut W.L. About tyrosine kinase inhibitors (TKIs) in prostate cancer: where do we go from here?//Ann Oncol. 2010. Vol. 21, № 1. P. 183-184.
- Attard G., Sarker D., Reid A., Molife R., Parker C., Bono de J.S. Improving the outcome of patients with castration-resistant prostate cancer through rational drug development//Br J Cancer. 2006. Vol. 95. № 7. P. 767-774.
- Ortholan C., Durivault J., Hannoun-Levi J.M., Guyot M., Bourcier C., Ambrosetti D., Safe S., Pagès G. Bevacizumab/docetaxel association is more efficient than docetaxel alone in reducing breast and prostate cancer cell growth: A new paradigm for understanding the therapeutic effect of combined treatment.//Eur J Cancer. 2010. Vol. 46. № 16. P. 3022-3036.
- Mitchell S.A., Danca M.D., Blomgren P.A., Darrow J.W., Currie K.S., Kropf J.E., Lee S.H., Gallion S.L., Xiong J.M., Pippin D.A., DeSimone R.W., Brittelli D.R., Eustice D.C., Bourret A., Hill-Drzewi M., Maciejewski P.M., Elkin L.L. Imidazo [1,2-a] pyrazine diaryl ureas: inhibitors of the receptor tyrosine kinase EphB4.//Bioorg Med Chem Lett. 2009. Volo. 19, N 24. P. 6991-6995.
- Singh R.P., Sharma G., Mallikarjuna G.U., Dhanalakshmi S., Agarwal C., Agarwal R. In vivo suppression of hormonerefractory prostate cancer growth by inositol hexaphosphate: induction of insulinlike growth factor binding protein-3 and inhibition of vascular endothelial growth factor.//Clin Cancer Res. 2004. Vol. 10. № 1. Pt 1. P. 244-250.
- Takei Y., Kadomatsu K., Yuzawa Y., Matsuo S., Muramatsu T. A small interfering RNA targeting vascular endothelial growth factor as cancer therapeutics.//Cancer Res. 2004. Vol. 64. № 10. P. 3365-3370.
- Fox W.D., Higgins B., Maiese K.M., Drobnjak M., Cordon-Cardo C., Scher H.I., Agus D.B. Antibody to vascular endothelial growth factor slows growth of an androgen-independent xenograft model of prostate cancer.//Clin Cancer Res. 2002. Vol. 8, № 10. P. 3226-3231.
- Kohli M., Kaushal V., Spencer H.J., Mehta P. Prospective study of circulating angiogenic markers in prostate-specific antigen (PSA)-stable and PSAprogressive hormone-sensitive advanced prostate cancer.//Urology. 2003. Vol. 61. P. 765-769.
- Shariat S.F., Anwuri V.A., Lamb D.J., Shah N.V., Wheeler T.M., Slawin K.M. Association of preoperative plasma levels of vascular endothelial growth factor and soluble vascular cell adhesion molecule-1 with lymph node status and biochemical progression after radical prostatectomy//J Clin Oncol. 2004. Vol. 22, № 9. P. 1655-1663.
- George D.J., Regan M.M., Oh W.K., Tay M.H., Manola J., Decalo N., Duggan S., Dewolf W.C., Kantoff P.W., Bubley G.J. Radical prostatectomy lowers plasma vascular endothelial growth factor levels in in patients with prostate cancer//Urology. 2004. Vol. 63. № 2. P. 327-332.
- Mendel D.B., Laird, A.D., Xin X., Louie S.G., Christensen J.G., Li G., Schreck R.E., Abrams T.J., Ngai T.J., Lee L.B., Murray L.J., Carver J., Chan E., Moss K.G., Haznedar J.O., Sukbuntherng J., Blake R.A., Sun L., Tang C., Miller T., Shirazian S., McMahon G., Cherrington J.M. In vivo antitumor activity of SU11248, a novel tyrosine kinase inhibitor targeting vascular endothelial growth factor and platelet-derived growth factor receptors: determination of a pharmacokinetic/pharmacodynamic relationship.//Clin Cancer Res. 2003. Vol. 9. № 1. P. 327-337.
- O'Farrell A.M., Abrams T.J., Yuen H.A., Ngai T.J., Louie S.G., Yee K.W., Wong L.M., Hong W., Lee L.B., Town A., Smolich B.D., Manning W.C., Murray L.J., Heinrich M.C., Cherrington J.M. SU11248 is a novel FLT3 tyrosine kinase inhibitor with potent activity in vitro and in vivo//Blood. 2003. Vol. 101. № 9. P. 3597-3605.
- Fokt R.M., Templeton A., Gillessen S., Ohlschlegel C., Schmid H.P. Prostatic metastasis of renal cell carcinoma successfully treated with sunitinib.//Urol Int. 2009. Vol. 83. № 1. P. 122-124.
- Cumashi A., Tinari N., Rossi C., Lattanzio R., Natoli C., Piantelli M., Iacobelli S. Cancer Lett. Sunitinib malate (SU-11248) alone or in combination with low-dose docetaxel inhibits the growth of DU-145 prostate cancer xenografts//Cancer Lett. 2008. Vol. 270. № 2. P. 229-233.
- Dror Michaelson M., Regan M.M., Oh W.K., Kaufman D.S., Olivier K., Michaelson S.Z., Spicer B., Gurski C., Kantoff P.W., Smith M.R. Phase II study of sunitinib in men with advanced prostate cancer.//Ann Oncol. 2009. Vol. 20, № 5. P. 913-920.
- Sonpavde G., Periman P.O., Bernold D., Weckstein D., Fleming M.T., Galsky M.D., Berry W.R., Zhan F., Boehm K.A., Asmar L., Hutson T.E. Sunitinib malate for metastatic castration-resistant prostate cancer following docetaxel-based chemotherapy.//Ann Oncol. 2010. Vol. 21. № 2. P. 319-324.
- http://www.clinicaltrials.gov/ct2/show/NCT00945477?term=pazopanib&rank=21
- http://www.clinicaltrials.gov/ct2/show/NCT00454571?term=pazopanib&rank=86
- http://www.clinicaltrials.gov/ct2/show/NCT01385228?term=pazopanib&rank=3


