Taurine and/or camel milk mitigates haemolysis and malondialdehyde concentration in Alzheimer rats
Автор: Abdulkadir T.S., Ayo J.O., Isa A.Sh., Dawud F.A., Dimka-bashok L., Jafar F.Y.
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 4 т.19, 2023 года.
Бесплатный доступ
Alzheimer’s disease (AD) is a devastating neurodegenerative disease, affecting all age groups. Malondialdehyde (MDA) concentration and haemolysis are important oxidative stress biomarkers, implicated in the pathogenesis of AD. The study aimed to evaluate taurine, camel milk (CM) and their combined effects on MDA concentration and haemolysis in AD rats. The animal groupings were; Normal saline (0.2 mL/mg bw); AlCl3 (100 mg/kg); CM (33 mL/kg); Taurine (50 mg/kg); AlCl3 (100 mg/kg) + CM (33 mL/kg); AlCl3 (100 mg/kg) + Taurine (50 mg/kg) and AlCl3 (100 mg/kg) + CM (33 mL/kg) + Taurine (50 mg/kg). Concentration of MDA and percentage haemolysis were determined for all groups. At 0.5% NaCl, haemolysis in AD rats (64.00 ± 1.27 %) was higher ( p
Aluminum chloride, camel milk, malondialdehyde, taurine, oxidative stress
Короткий адрес: https://sciup.org/143180987
IDR: 143180987
Текст научной статьи Taurine and/or camel milk mitigates haemolysis and malondialdehyde concentration in Alzheimer rats
Alzheimer’s disease (AD) is a devastating and chronic disease in humans. It is characterized by loss of memory (Jahn, 2013; Borelli et al., 2020; Putcha et al., 2022), learning deficit (Bernaud et al., 2022), behavioural impairments (Tangen et al., 2014; Creese and Ismail, 2022) and ultimately reduced work output (Breijyeh and Karaman, 2020; Abdulkadir et al., 2021). A reduction in acetylcholinesterase (ACh) activity (Herholz, 2008; Hung et al., 2022), elevated amyloid-β peptide (Aβ) concentration (Hao et al., 2019; Gallego Villarejo et al., 2022) and concentrations of oxidative stress biomarkers (Cheignon et al., 2018; Fang et al., 2020) has been reportedly linked to AD. Elevated MDA concentration is an AD risk factor (López-Riquelme et al., 2016; Rao et al., 2021). Efforts to treat or prevent AD based on available prophylactic and therapeutic agents have not yielded the desirable beneficial results. Pathophysiological mechanisms underlying AD have been linked in part to redox imbalance and haematological disorders (Ma et al., 2010; Rahim et al., 2010; Issa et al., 2018; Emoto et al., 2021). Both camel milk (CM) and taurine have been shown to exhibit antioxidant activities (Jong et al., 2012; Homayouni-Tabrizi et al., 2017), and may be beneficial in AD intervention. There is need to shed more light on the impact of CM and taurine on the blood of aluminium chloride (AlCl3)-induced AD in rats. The CM represses the formation of MDA and inhibits oxidative stress (OS) levels (Al-Asmari et al. 2014; Badawy et al., 2018; Shaban et al., 2022). The increased generation of reactive oxygen species (ROS), occurring in AD (Mohsenzadegan and Mirshafiey, 2012; Leyane et al., 2022) may increase the haemolysis and lipid peroxidation of cytomembrane. Haemolysis, measured by percentage erythrocyte osmotic fragility (EOF), indicates the damage due to oxidative stress (Igbokwe, 2018; Vicente-Ferreira et al., 2021). It is of value in the assessment of degree of stabilization or resistance to membrane lipid peroxidative damage by ROS, generated in excess during oxidative stress (Gwozdzinski et al., 2021). One of the important byproducts of lipid peroxidation induced by oxidative stress is MDA, and its concentration in the body may indicate the level of redox imbalance and severity of AD (Greilberger et al., 2008). Therefore, variations in haemolysis and MDA concentration may be of diagnostic importance in AD cases.
The study aimed to evaluate changes in MDA concentration and haemolysis in AlCl 3 -induced AD rats.
MATERIALS AND METHODS
Aluminium chloride (AlCl3) (CAS: 7764-15-6) and taurine (CAS: 107-35-7) were bought from Sigma-Aldrich Company (Munich, Germany and St. Louis, U.S.A., respectively). Both rat AChE (CK-bio-14126) and Aβ 1-42 (CK-bio-14171) ELISA kits were bought from Shanghai Coon Koon Biotech Co., Ltd., China. At 09:00 h, fresh milk was obtained from normal camel reared in Chiranchi (12°40/N, 07°43/E) Nigeria. The CM until use was stored in an ice chest.
Animals
Female Wistar rats (n =35) and weighing 120 – 150 g (135 ± g), served as subjects. They were kept in plastic cages in a well-ventilated animal pen under 12/12 h light/dark cycle. The animals had access freely to food and water. The study was performed based on the policy, ethics and regulations on care and use of research animals, approved by Ahmadu Bello University, Zaria, Nigeria (ABUCAUC/2021/004).
Experimental design and groupings (Table 1)
Alzheimer disease (AD) was induced by administering AlCl 3 that was dissolved in distilled water. The AlCl 3 was administered immediately after being dissolved via oral gavage and for eight weeks. Following 24 hours after the last administration, rats were adequately anaesthetised by ketamine/diazepam (75:25 mg/kg) injection. From each group (Table 1), blood sample was collected from each rat for biochemical analysis via cardiac puncture (Parasuraman et al., 2010).
Assessment of haemolysis and malondialdehyde concentration
Evaluation of percentage haemolysis
In vitro EOF was estimated to serve as indication of haemolysis in the rats in accordance with Faulkner and
King (1970) and based on sodium chloride (NaCl) concentrations (pH 7.4), ranging from 0.0 to 0.9 g/L of distilled water. Fresh blood samples obtained via cardiac puncture were transferred into NaCl-containing test tubes. The content was gently shaken and then incubated at 25 – 26°C (room temperature) for 30 minutes. Thereafter, the tubes were centrifuged at 800 x g , 10 minutes. The optical density of the supernatant was measured spectrophotometrically by a spectrophotometer (Spectrum 20, Philip Harris Limited, Shenstone, UK) at 540 nm.
Optical density of test solution
Percentage haemolysis (%) : ---------------------------× 100
Optical density of standard solution
Determination of malondialdehyde concentration
Hippocampus tissue was obtained from each rat as described by Ojha et al. (2021) for evaluation of MDA concentration. Hippocampus tissue MDA concentration was determined using MDA enzyme-linked immunosorbent assay (ELISA) kit on the basis of biotin double antibody sandwich technology in accordance with Janero (1990). The tissue sample (40 µL), 10 µL of rat MDA antibody and streptavidin-horseradish peroxidase (50 µL) were transferred to wells coated with MDA monoclonal antibody. A blank well without sample contained 10 µL of rat MDA, while 50 µL of standard and 5 µL streptavidin- horseradish peroxidase was poured into the standard well. Mixing of solution was performed by shaking the plate gently. The solution was incubated at 37°C for 60 minutes. Thereafter, the liquid was drained from the plate. A washing solution was poured into each well, which stood for 3 seconds and then washed off. The washing procedure was done for 5 cycles. Both Chromogen A and B (50 µL) were transferred into the blank, standard and sample wells, respectively. Mixing of the solution was performed by shaking the plate gently. The solution was incubated at 37°C for 10 minutes without access to light in order to prevent colour development. The removal of the plate was done after 10 minutes, and the reaction halted using stop solution (50 µL) as the colour was observed to change to yellow. The absorbance recorded from each well was obtained under 450 nm and 10 minutes after the addition of stop solution. The concentration in each sample was measured by determining the optical density using MyAssays Software (MyAssays, 2016).
Data analyses
Values recorded were presented as mean + standard error of the mean (Mean ± SEM). The analysis was performed using one-way analysis of variance. The percentage haemolysis and MDA concentration recorded in each group were evaluated using Tukey’s post-hoc test to determine the significance of the differences between the means. The statistical analysis was carried out by GraphPad Prism 5.03 for windows (GraphPad Software, San Diego, California, U.S.A.). Level of significance was set at p < 0.05.
RESULTS
Erythrocyte Osmotic Fragility
The percentage haemolysis (or EOF) at 0.7% NaCl concentration in AD rats was similar to that obtained in AD + Taurine + CM, although AD + CM and AD + Taurine rats showed a relatively higher percentage, when compared to AD rats (Figure 1). At 0.5% NaCl, the percentage EOF in AD (64.00 ± 1.27%) rats was higher ( p < 0.05) than the percentages obtained in AD + CM (51.50 ± 2.96%) and AD + CM + Taurine (52.00 ± 1.53%) rats. At 0.4% NaCl, percentage haemolysis was significantly higher ( p < 0.05) in AD (83.60 ± 1.94%) rats than in AD + CM (74.25 ± 3.40%). Taurine when administered either alone or to AD + Taurine rats did not significantly lower the percentage haemolysis, compared to AD rats. There was a significant negative relationship between the percentage haemolysis and NaCl concentration ( r = -0.984, p < 0.001) in AD rats (Table 2).
Malondialdehyde evaluation
The concentration of MDA was higher ( p < 0.05) in AD (55.32 ± 6.02 nmol/mL) rats than in either AD + Taurine (20.28 ± 1.37 nmol/mL) rats or AD + Taurine + CM (40.34 ± 5.54 nmol/mL) rats (Figure 3).
Table 3 below shows the correlation coefficients between MDA concentration and percentage haemolysis at 0.5% NaCl. The AD + CM and AD + Taurine + CM groups recorded higher values, when compared to AD group, respectively. The scattered plots showing the fluctuations in the relationships between MDA concentrations and percentage haemolysis, and their corresponding regression equations in individual AD rats, AD + Taurine and AD + CM rats, are presented in Figure 4 A - C, respectively. The relationship was negative in AD, and AD + Taurine rats, but positive in AD + CM rats.
Table 1 : The experimental animal groupings
|
S/No. |
Groups |
Treatment |
Duration |
|
1 |
Group I |
Normal saline at 0.2 mL/kg/body weight orally |
8 weeks |
|
2 |
Group II |
Aluminum chloride (AlCl 3 ) 100 mg/kg orally (Prakash et al., 2013) |
6 weeks |
|
3 |
Group III |
Camel milk (33 mL/kg) orally (Khatoon et al., 2016) |
8 weeks |
|
4 |
Group IV |
Taurine (50 mg/kg) orally (Cetiner et al., 2005) |
8 weeks |
|
5 |
Group V |
AlCl 3 (100 mg/kg) + Camel milk (33 mL/kg) orally |
8 weeks |
|
6 |
Group VI |
AlCl 3 (100 mg/kg) + Taurine (50 mg/kg) orally |
8 weeks |
|
7 |
Group VII |
AlCl 3 (100 mg/kg) + Taurine (50 mg/kg) + Camel milk (33 mL/kg) orally |
8 weeks |
N=5
-
■ Normal BAD eCM иТаи eAD + Tan eAD+CM eAD + Tau + CM
Figure 1. Effect of taurine and camel milk on erythrocyte osmotic fragility in aluminum chloride-induced Alzheimer’s disease in rats
AD: Alzheimer’s disease, CM: Camel milk, Tau: Taurine, n = 5
a,b,c,d= means with different letters differ significantly (p < 0.05)
Table 2: The relationship between sodium chloride concentration and percentage erythrocyte osmotic fragility in AlCl 3 -induced AD rats administered with taurine and camel’s milk
|
Groups |
Correlation coefficient (r) |
|
Normal |
-0.990*** |
|
AD |
-0.971*** |
|
CM |
-0.974*** |
|
Taurine |
-0,984*** |
|
AD + Taurine |
-0.984*** |
|
AD + CM |
-0.987*** |
|
AD + Taurine + CM |
-0.980*** |
AD: Alzheimer’s disease, CM: Camel milk *** = p < 0.001
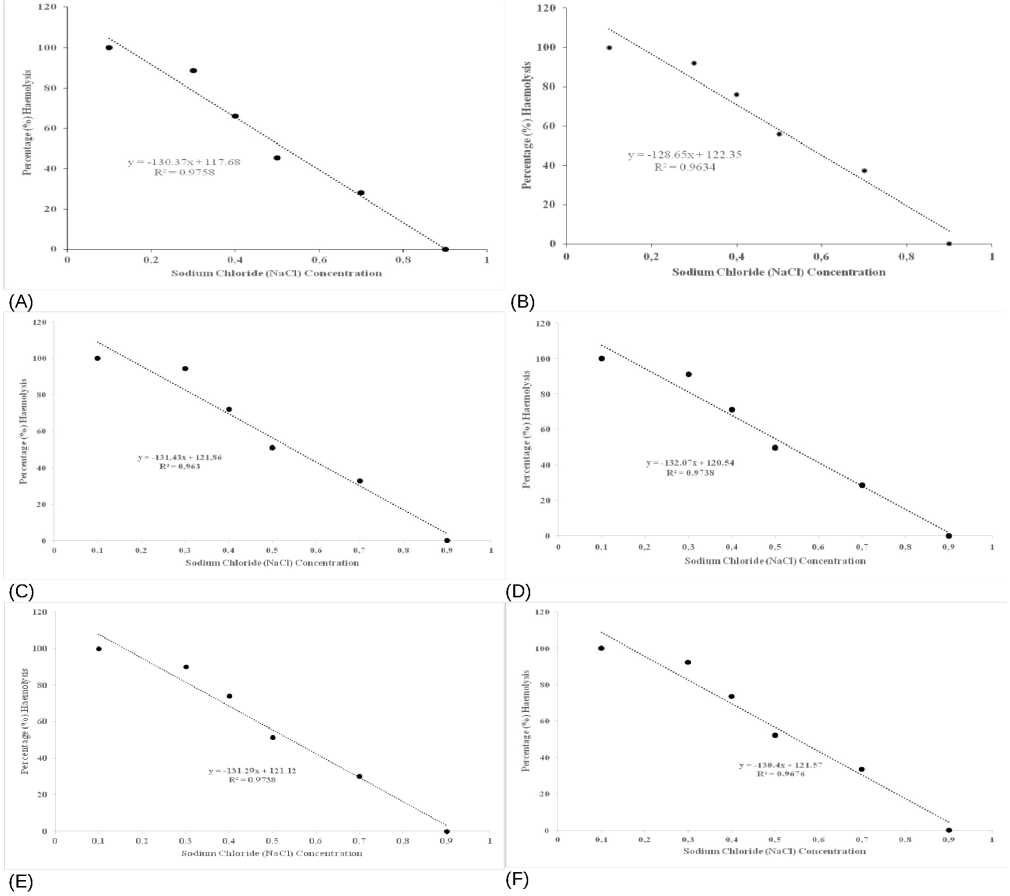
Figure 2: Scattered plot showing the relationship between percentage sodium chloride concentration and percentage erythrocyte osmotic fragility in (A) Alzheimer’s disease group, (B) in taurine group, (C) in camel milk group and (D) Scattered plot for percentage sodium chloride concentration against Alzheimer’s disease group treated with taurine group, (E) Alzheimer’s disease group treated with camel milk, (F) Alzheimer’s disease group treated with taurine and camel milk.
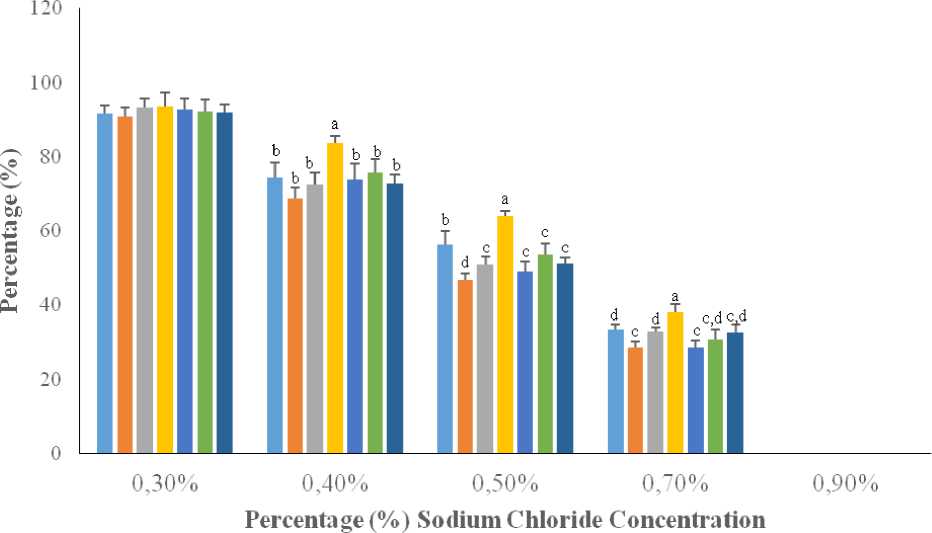
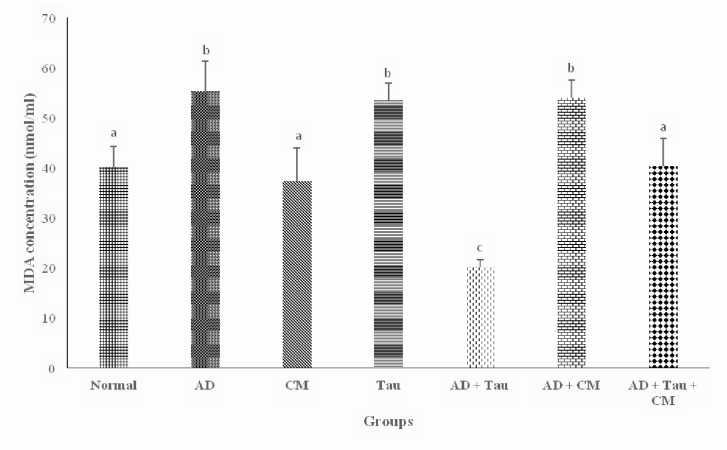
Figure 3 Effect of taurine and camel milk on malondialdehyde concentration in aluminum chloride-induced Alzheimer’s disease in rats. AD: Alzheimer’s disease, CM: Camel milk, Tau: Taurine, MDA: Malondialdehyde
Table 3 : The relationship between malondialdehyde concentration and percentage erythrocyte osmotic fragility in Alzheimer’s disease rats.
|
Groups |
Correlation |
|
Normal |
-0.993*** |
|
AD |
-0.973*** |
|
CM |
-0.975*** |
|
Taurine |
-0.996*** |
|
AD + Taurine |
-0.980*** |
|
AD + CM |
-0.990*** |
|
AD + Taurine + CM |
-0.979*** |
Key: AD: Alzheimer’s disease, CM: camel milk, *** p < 0.001
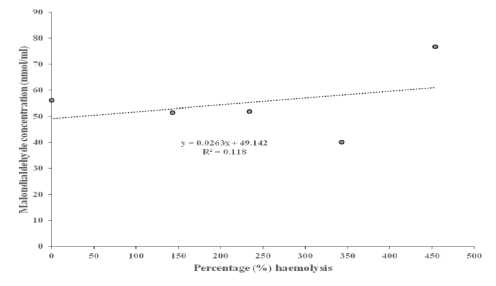
(A)
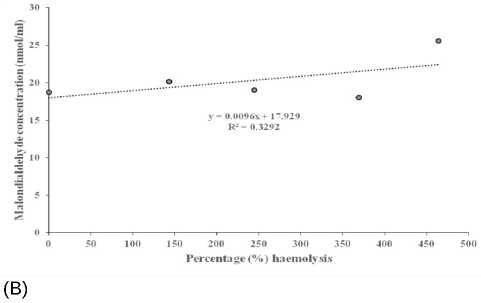
Figure 4 Scattered plot of malondialdehyde concentration against percentage (%) haemolysis in Alzheimer’s disease group (A), haemolysis in Alzheimer’s disease group rats treated with taurine (B) and in Alzheimer’s disease group rats treated with camel milk (C).
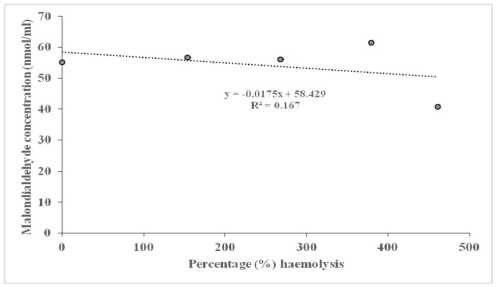
(C)
DISCUSSION
Effect on haemolysis
The result of haemolysis demonstrates that CM and/or taurine significantly decreased haemolysis in AD rats; indicating that the two agents exhibited antioxidant activity, apparently by increasing the cytomembrane integrity of the erythrocytes. The EOF as an index of haemolysis has been demonstrated to be also an index of lipoperoxidation in rats exposed to increased oxidative stress, induced by poisoning (Ambali et al., 2010; Uchendu et al., 2014; Yusuf et al., 2018), heat stress (Alhassan et al., 2010), and transportation stress (Yaqub et al., 2014). The current study has established, for the first time, that AlCl 3 -induced oxidative stress resulted in increased haemolysis, which was alleviated by the administration of taurine and CM.
The findings show that CM exerted a more potent inhibitory effect on haemolysis than taurine. The mechanism by which CM exerted its inhibitory effect on haemolysis was not elucidated in this study. However, the results suggest that the antioxidant, taurine, also known to be a neurotransmitter exerting inhibitory effect on the nervous system, exerted its antioxidant effect via a pathway that differs from that of CM. The CM which is also an antioxidant contains both electrolytes and other compounds (Rasheed, 2017; Rahmeh et al., 2019) that, apparently, enhanced the anti-haemolytic activity of CM at the erythrocyte membrane level. This requires further investigations.
Effect on malondialdehyde
The result of MDA concentration shows that taurine significantly reduced MDA concentration in AD rats compared to any other group; thus, indicating that the degree of lipid peroxidation in AD + Taurine rats was the least. The MDA is established to be the product of damage to cell membranes. Therefore, its low concentration is an evidence of low ROS generation or high scavenging activity by taurine and/or CM in the treated rats. The result was similar to that of Rao et al. (2021), who showed that the administration of resveratrol and its combination with donepezil lower the levels of MDA in colchicine-induced AD. Akbari et al. (2016) reported that the probiotic (also an antioxidant)
supplementation significantly decreased MDA level in a randomised, double-blinded and controlled trial of AD patients.
Overall, CM and/or taurine, especially in combination, decreased percentage haemolysis and MDA concentration. The CM and taurine may exhibit protective mechanism in the rats partly by stabilizing cell membrane from ROS damage, induced with AD by AlCl 3 administration.
CONCLUSION
Taurine either alone or in combination with CM alleviated AlCl 3 oxidative stress changes in AD rats, induced with AlCl3 by decreasing haemolysis and MDA concentration. The administration of taurine and, especially, CM prophylactically mitigated AD-induced oxidative stress changes, and may be utilised in drug discovery.
DECLARATION
All applicable international, national, and/or institutional guidelines for the care and use of laboratory animals were adhered to in the study.
CONFLICTS OF INTEREST
The authors declare that they have no potential conflicts of interest.
Список литературы Taurine and/or camel milk mitigates haemolysis and malondialdehyde concentration in Alzheimer rats
- Abdulkadir, T. S., Dawud, F. A., Isa, A. S., Ayo, J. O. (2021). Taurine and camel milk modulate neurobehavioral and biochemical changes in aluminium chloride-induced Alzheimer's disease in rats. Journal of Alzheimer's Disease, 84: 291-302.
- Al-Asmari, A. K., Abbasmanthiri, R., Al-Elewi, A. M., Al-Omani, S., Al-Asmary, S. and Al-Asmari, S. A. (2014). Camel milk beneficial effects on treating gentamicin-induced alternation in rats. Journal of Toxicology, 917608.
- Akbari, E., Asemi, Z., Kakhaki, R. D., Bahmani, F., Kouchaki, E., Tamtaji, O. R., Hamid, G. A. and Salami, M. (2016). Effect of probiotics supplementation on cognitive function and metabolic status in Alzheimer's disease: A randomised, double-blind and controlled trail. Frontiers in Ageing Neuroscience, 8(256): 1-8.
- Alhassan, A. W., Adenkola, A. Y., Yusuf, A., Bauchi, Z. M., Saleh, M. I. and Ochigbo, V. I. (2010). Erythrocyte osmotic fragility of Wistar rats administered ascorbic acid during the hot-dry season. Journal of Cell and Animal Biology, 4(2): 29-33.
- Ambali, S. F., Idris, S. B., Onukak, C., Shittu, M. and Ayo, J. O. (2010). Ameliorative effects of vitamin C on short-term sensorimotor and cognitive changes induced by acute chlorpyrifos exposure in Wistar rats. Toxicology and Industrial Health, 26(9): 54758.
- Badawy, A. A., El-Magd, M. A. and AlSadrah, S. A. (2018). Therapeutic effect of camel milk and its exosomes on MCF7 cells in vitro and in vivo. Integrative Cancer Therapies, 17(4): 1235-1246.
- Bernaud, V. E., Bulen, H. L., Pena, V. L., Koebele, S. V., Northup-Smith, S. N., Manzo, A. A., Sanchez, M. V., Opachich, Z., Ruhland, A. M. and Bimonte-Nelson, H. A. (2022). Task-dependent learning and memory deficits in the TgF-344-AD rat model of Alzheimer's disease: three key time-points from young adulthood to middle-age in females. Scientific Reports, 12: 14596.
- Borelli, C. M., Grennan, D. and Muth, C. C. (2020). Causes of memory loss in elderly persons. JAMA, 323(5): 486.
- Breijyeh, Z. and Karaman, R. (2020). Comprehensive review on Alzheimer's disease: causes and treatment. Molecules, 25(24): 5789.
- Cetiner, M., Sener, G., Sehirli, A. O., Eksiogiu-Demiralp, E., Ercan, F., Sirvanci, S., Gedik, N., Akpulat, S., Tecimer, T. and Yegan, B. C. (2005). Taurine protects against methotrexate-induced toxicity and inhibits leucocyte death. Toxicology and Applied Pharmacology, 209 (1): 39-50.
- Cheignon, C., Tomas, M., Bonnefont-Rousselot, D., Faller, P., Hureau, C., and Collin, F. (2018). Oxidative stress and the amyloid-beta peptide in Alzheimer's disease. Redox Biology, 14: 450-464.
- Creese, B. and Ismail, Z. (2022). Mild behavioural impairment: measurement and clinical correlates of a novel marker of preclinical Alzheimer's disease. Alzheimer's Research and Therapy, 14(2).
- Emoto, M. C., Sato-Akaba, H., Hamaue, N., Kawanishi, K., Koshino, H., Shimohama, S. and Fujiii, H. G. (2021). Early detection of redox imbalance in the APPswe/PS1dE9 mouse model of Alzheimer's disease by in vivo electron paramagnetic resonance imaging. Free Radical Biology and Medicine, 172: 9-18.
- Fang, Y., Ou, S., Wu, T., Zhou, L., Tang, H., Jiang, M., Xi, J. and Guo, K. (2020). Lycopene alleviates oxidative stress via the P13K/Akt/Nrf2 pathway in a cell model of Alzheimer's diease. Peer Journal, 8: e9308. Doi: 10.7717/peerj.9308.
- Faulkner, W. R. and King, J. W. (1970). Manual of Clinical Laboratory Procedure. Chemical Rubber Company, Cleaveland, Ohio, U.S.A., 354 pp.
- Gallego Villarejo, L., Bachmann, L., Marks, D., Brachthäuser, M., Geidies, A. and Müller, T. (2022). Role of intracellular amyloid ß as pathway modulator biomarker, and therapy target. International Journal of Molecular Sciences, 23: 4656.
- Greilberger, J., Koidl, C., Greilberger, M., Lamprecht, M., Schroecksnadel, K., Leblhuber, F., Fuchs, D. and Oettl, K. (2008). Malondialdehyde, carbobny-proteins and albumin-disulphide as useful oxidative markers in mild cognitive impairment and Alzheimer's disease. Free Radical Research, 42(7): 633-638.
- Gwozdzinski, K., Pieniazek, A. and Gwozdzinski, L. (2021). Reactive oxygen species and their involvement in red blood cell damage in chronic kidney disease. Oxidative Medicine and Cellular Longevity, Article ID 6639199: 1-19.
- Hao, S., Li, X., Han, A., Yang, Y., Fang, G., Liu, J. and Wang, S. (2019). CLVFFA-functionalized gold nanoclusters inhibit Aß40 fibrillation, fibrils' prolongation, and mature fibrils' disaggregation. ACS Chemical Neuroscience, 10(11): 4633-44642.
- Herholz, K. (2008). Acetylcholinesterase activity in mild cognitive impairment and Alzheimer's disease. European Journal of Nuclear Medicine Molecular Imaging, 35: S25-S29
- Homayouni-Tabrizi, M., Asoodeh, A. and Soltani, M. (2017). Cytotoxic and antioxidant capacity of camel milk peptides: Effects of isolated peptides on superoxide dismutase and catalase gene expression. Journal of Food and Drug Analysis, 25(3): 567-575.
- Hung, N. H., Quan, P. M., Satyal, P., Dai, D. N., Hoa, V. V., Huy, N. G., Giang, L. D., Ha, N. T., Huong, L. T., Hien, V. T. and Setzer, W. N. (2022). Acetylcholinestrase inhibitory activities of essential oils from Vietnamese traditional medicinal plants. Molecules, 27(7092).
- Igbokwe, N. A. (2018). A review of the factors that influence erythrocyte osmotic fragility. Sokoto Journal of Veterinary Sciences, 16(4): 1-23.
- Issa, A. M. A., Mahmoud, A. O., Ullah, K. S., Ilyas, N. M., Bin, S. N., Zubaidi, L.A., Amin, B. A. and Mohammed, A. T. (2018). Effect of oxidative stress on Alzheimer's disease, Haematological perspective. Research Journal of Pharmacy and Technology, 11(9): 3881-3886.
- Jahn, H. (2013). Memory loss in Alzheimer's disease. Dialogues Clinical Neuroscience, 15(4): 445-454.
- Janero, D. R. (1990). Malondialdehyde and thiobarbituric acid-reactivity as diagnostic indices of lipid peroxidation and peroxidative tissue injury. Free Radical Biology and Medicine, 9: 515-540.
- Jong, C. J., Azuma, J. and Schaffer, S. (2012). Mechanism underlying the antioxidant activity of taurine: prevention of mitochondrial oxidant production. Amino Acids, 42(6): 2223-2232.
- Khatoon, H., Najam, R., Mirza, T. and Sikandar, B. (2016). Beneficial anti-Parkinson effects of camel milk in chlorpromazine induced animal model: Behavioural and histopathological study. Pakistan Journal of Pharmaceutical Sciences, 29(5): 15251529.
- Leyane, T. S., Jere, S. W. and Houreld, N. N. (2022). Oxidative stress in ageing and chronic degenerative pathologies: molecular mechanisms involved in counteracting oxidative stress and chronic inflammation. International Journal of Molecular Sciences, 23: 7273. Doi: 10.3390/ijms23137273.
- López-Riquelme, N., Alom-Poveda, J., Viciano-Morote, N., Llinares-Ibor, I. and Tormo-Díaz, C. (2016). Apolipoprotein E £4 allele and malondialdehyde level are independent risk factors for Alzheimer's disease. SAGE Open Medicine, 4. Doi:10.1177/2050312115626731.
- Ma, W., Yuan, L., Yu, H., Ding, B., Xi, Y., Feng, J. and Xiao, R. (2010). Genistein as a neuroprotective antioxidant attenuates redox imbalance induced by ß-amyloid peptides 25-35 in PC12 cells. International Journal of Developmenal Neuroscience, 28(4): 289-295.
- Mohsenzadegan, M. and Mirshafiey, A. (2012). The immunopathogenic role of reactive oxygen species in Alzheimer's disease. Iranian Journal of Allergy, Asthma and Immunology, 11(3): 203-216.
- MyAssays, My assay software, (Retrieved on 17/03/2018 from: http://www.myassays.com/linear-regression.assay).
- Ojha, P. S., Biradar, P. R., Tubachi, S. and Patil, V. S. (2021). Evaluation of neuroprotective effects of Canna indica L against aluminium chloride memory impairment in rats. Advances in Traditional Medicine, 1-18. Doi: 10.1007/s13596-021-00627-x.
- Parasuraman, S., Raveendran, R. and Kesavan, R. (2010). Blood sample collection in small laboratory animals. Journal of Pharmacology and Pharmacotherapeutics, 1(2): 87-93.
- Prakash, A., Shur, B. and Kumar, A. (2013). Naringin protects memory impairment and mitochondrial oxidative damage against aluminium-induced neurotoxicity in rats. International Journal of Neuroscience, 123(9): 636-645.
- Putcha, D., Carvalho, N., Dev, S., McGinnis, S. M., Dickerson, B. C. and Wong, B. (2022). Verbal encoding deficits impact recognition memory in atypical "non-amnestic" Alzheimer's disease. Brain Sciences, 12: 843-857.
- Rahmeh, R., Alomirah, H., Akbar, A. and Sidhu, J. (2019). Composition and properties of camel milk. Milk production, processing and marketing. Doi:10.5772/intechopen.82592. Rahim, E., Keikhaei, B., Sakaki, A. and Doulah, A. H. (2010). Ibotenic acid-induced hematological disorders in rat model of Alzheimer's disease. Asian Journal of Animal and Veterinary Advances, 5(1): 13-23.
- Rao, Y. L., Ganaraja, B., Marathe, A., Manjrekar, P. A., Joy, T., Ullal, S., Pai, M. M. and Murlimanju, B. V. (2021). Comparison of malondialdehyde levels and superoxide dismutase activity in resveratrol and resveratrol/donepezil combination treatment groups in Alzheimer's disease induced rat model. Biotechnology, 11(7): 329. Doi: 10.1007/s13205-021-02879-5.
- Rasheed, Z. (2017). Medicinal values of bioactive constituents of camel milk: A concise report. International Journal of Health Sciences, 11(5): 1-2
- Shaban, A. M., Raslan, M., Qahl, S. H., Elsayed, K., Abdelhameed, M. S., Oyouni, A. A. A., Al-Amer, O. M., Hammouda, O. and El-Magd, M. A. (2022). Ameliorative effects of camel milk and its exosomes on diabetic nephropathy in rats. Membranes, 12: 1060
- Tangen, G. G., Engedal, K., Bergland, A., Moger, T. A, Marit, A. and Mengshoel, A. M. (2014). Relationships between balance and cognition in patients with subjective cognitive impairment, mild cognitive impairment and Alzheimer's disease. Physical Therapy, 94(8): 1123-1134.
- Uchendu, C., Ambali, S. F., Ayo, J. O., Esievo, K. A. N. and Umosen, A. J. (2014). Erythrocyte osmotic fragility and peroxidation following chronic co-exposure of rats to chlorpyrifos and deltamethrin, and the beneficial effect of alpha-lipoic acid. Toxicology Reports, 1: 373-378.
- Vicente-Ferreira, G. S., Martins, G. S., Chaves, N. A., Silva, D. G. H. and Bonini-Domingos, C. R. (2021). Oxidative and osmotolerant effects in Salvator merianae (Suamata: teiidae) red blood cells during hibernation. Brazilian Journal of Biology, 84: e249617
- Yaqub, L. S., Mshelia, W. P. and Ayo, J. O. (2014). Erythrocyte osmotic fragility and haematological responses of horses administered ascorbic acid and exposed to road transportation. Journal of Equine Veterinary Science, 34: 1324-1328.
- Yusuf, I. L., Akefe, I. O., Tijjani, M. B., Yusuf, H., Salihu, S. I. and Muhammed, Z. (2018). Lead-induced increase in erythrocyte osmotic fragility and malondialdehyde concentration in Wistar rats: chemo-protective effects of flavonoid mixture. Biochemical and Biotechnology Research, 6(1): 914.


