Taurine supplementation modulates circadian rhythms of heat-associated cytokines in male broiler chickens reared under hot-dry conditions
Автор: Makeri H.K., Ayo J.O., Aluwong T., Minka N.S.
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 4 т.20, 2024 года.
Бесплатный доступ
An experiment was designed to study the modulatory effects of supplemental taurine on circadian rhythms of heat-associated cytokines in male broiler chickens of different ages under hot-dry conditions. Twenty, 21-day-old Arbor Acre male broiler chickens balanced for weight served as subjects and were assigned ten (10) each to control (Cgp) and treatment (Tgp) groups. The broiler chickens were fed on isonitrogenous and isocaloric commercial diets during the experimental period. Blood samples were collected from the wing veins from 10 chickens in each of the groups at four-hour intervals during a 24-h period on days 28, 35 and 42 of age. Harvested serum from blood samples were analysed for heat-shock protein 70 (HSP70), tumour necrosis factor-alpha (TNF-α) and interleukin 1-alpha (IL-1 α). Data obtained were fitted into the single cosinor application. The results showed that HSP70, TNF- α and IL-1α exhibited clear circadian rhythmicity in both groups. Taurine significantly (P function show_abstract() { $('#abstract1').hide(); $('#abstract2').show(); $('#abstract_expand').hide(); }
Broiler chickens, circadian rhythm, heat-associated cytokines, hot-dry conditions, taurine
Короткий адрес: https://sciup.org/143183446
IDR: 143183446
Текст научной статьи Taurine supplementation modulates circadian rhythms of heat-associated cytokines in male broiler chickens reared under hot-dry conditions
The impact of incremental weather factors is challenging for normal body functions and productivity in broiler chickens, that are genetically modified for faster growth. Thermal environmental factors may elicit heat stress and result in build-up of reactive oxygen species (ROS) in livestock. Heat stress decreases feed intake, body weight gain, carcass weight/quality and shelf -life of poultry products (Alagawany et al., 2017; Lu et al., 2017; Luo et al., 2018). It causes oxidative stress which negatively impacts poultry production (Akbarian et al., 2016), especially in hot-dry conditions (Egbuniwe et al., 2018; Jimoh et al., 2018). The hot-dry season in Northern Nigeria is characterised by high ambient temperature which induces heat stress, leading to high mortality and heavy economic losses (Dzenda et al., 2013; Minka et al., 2020; Ma et al., 2021). Hotta et al. (2007) reported that physiological parameters exhibit day and night rhythms in animals, which serve to separate incompatible temporal metabolic events. Ageing and disease modify ROS-dependent processes of physiological parameters, which exhibit circadian rhythmicity (Burioka et al., 2005). Circadian or daily variations in metabolism, including those related to locomotor and brain activities, result in corresponding temporal patterns of oxidant formation (Hardeland et al., 2003; Ayo et al., 2017). The ROS damage tissues, proteins and other macromolecules within the cell in poultry (Surai and Fisinin, 2016). At cellular level, heatstress alters the redox state (Keyse and Emslie, 1992), inducing transcription modulation (Sanchez et al., 1994), altered antioxidant enzyme activities (Hosseindoust et al., 2020). Cellular communication and immune responses during development are dependent on small extracellular signalling peptides called cytokines, which include: interleukin -1 (IL-1), interleukin-2 (IL-2), tumour necrosis factor- alpha (TNF-α) and heat-shock protein (HSP) (Saleh and Al-Zghoul, 2019). In avian species, HSPs are expressed constitutively and are highly conserved molecular chaperones that confer thermotolerance (Givisiez et al., 2001; Vinoth et al., 2018). The degree of stimulated heat tolerance is related to HSP expressions (Krebs and Bettencourt, 1999). The increase in inflammatory cytokines may be interactively associated with oxidative stress-induced HSP gene production in animals under heat stress (Anathan et al., 1986). The gene expression of different cytokines, including, IL-1β, IL-6 and TNF-α, are modulated by heat stress in broiler chickens (Ohtsu et al., 2015; Quinteiro-Filho et al., 2017). Takahashi et al. (1997) reported an association between the expression of HSP70 and those of IL-1β, IL-6 and TNF-α, respectively. Thus, HSP level results in increased production of inflammatory cytokines, such as IL-1β, TNF-α and IL-6 (Bouchama and Knochel, 2002; Chang et al., 2010). Taurine is a non-proteinogenic ß-aminosulphonic acid, found in the retina, neurons and muscles (Ripps and Shen, 2012; Seidel et al., 2018). It may prevent ROS overload and directly inhibit ROS in the respiratory chain (Grove and Karpowicz, 2017; Seidel et al., 2018). Taurine plays a neuromodulator role in cardiac and brain functions (Ito et al., 2012; Huang et al., 2014). Homeothermy is a complex physiologic process that is integrated in the hypothalamus (Baarendse et al., 2007). One of the regulatory and signaling pathways adopted by animals to optimize growth and well-being under such adverse conditions is the circadian clock, which accurately synchronises all internal processes depending on the external environment and day/night cycle. It has been proven that a better understanding of circadian rhythm in livestock may pave a new way in enhancing productivity, welfare, nutritional requirements, therapeutic interventions in livestock production. The aim of the current study was to determine the role of taurine in modulating the circadian rhythms of cytokines in the amelioration of adverse consequences of heat stress under natural hot-dry conditions.
MATERIALS AND METHODS
Experimental Site
The experiment was conducted in the experimental poultry pen of the Livestock Farm, College of Agriculture and Animal Science, Mando-Kaduna (11° 10/N, 07° 38/E), located in the Northern Guinea Savannah zone of Nigeria.
Thermal environmental parameters
Thermal environmental parameters of dry-bulb temperature (DBT) and wet-bulb temperature (WBT)
were recorded at four-hour intervals for a period of 24-h using a dry-wet-bulb thermometer (DTH 1; Clarke International Ltd., Essex, UK), placed in the middle of the pen and at a level just above the head of the birds (30 cm from the floor) (Silanikove, 2000) on days 28, 35 and 42 of experimental period. The RH values were calculated using Osmon’s hygrometric table (Narinda Scientific Industries®, Haryana, India), while the THI was calculated using the formula (Tao and Xin, 2003)
THI = 0.85 ( tdb ) + 0.15 ( twb )
Where THI = temperature -humidity index for broiler chickens, tdb = dry-bulb temperature and twb = wet-bulb temperature
Experimental birds
Twenty (20), 21-day-old clinically-healthy male Arbor Acres hybrid broiler chickens obtained from Nas Tech® Hatchery, Ibadan, Nigeria, at day-old and brooded in standard deep litter pen, served as subjects. They were given access to water and balanced broiler starter diets (Chikun Feeds®, Olam Feeds and Flour Mills, Kaduna, Nigeria) ad libitum during brooding and experimental phases .
Ethical approval for the experimental protocol was obtained from the Animal Care and Use Committee of Ahmadu Bello University, Zaria (ABUCAUC), Nigeria, with approval number ABUCAUC/2021/122. The handling and experimental procedures on the broiler chickens were performed in accordance to International Guideline for the Care and Use of Laboratory Animals (2011).
Experimental design
The 20, 21-day-old clinically-healthy Arbor Acres hybrid male broiler chicks were selected and balanced for weight served as subjects of the study. The birds were kept and managed under deep litter and natural photoperiod. The study was conducted between March and May 2020. The broiler chickens were randomly selected and assigned to two groups namely; control (Cgp) and treatment (Tgp) groups. The broiler chickens were kept in two partitions with dimensions 1,200 x 2,000 cm each under the same prevailing environmental conditions. The broiler chickens in the Tgp received daily supplementation of taurine purchased from Nutricost® (7 S 1550 W ♯ 200 Lindon, UT.84042. USA), with a purity of 1000 mg. g-1 and at a concentration of
0.1% in drinking water ad libitum (Belal et al., 2018). Taurine administration commenced from day 21 to 42 of the study period, while Cgp received a placebo at the same period as the Tgp. All broiler chickens were monitored on a 24 - h basis for mortality alongside all activities, as stipulated from day 28 below:
Activities on day 28 ( Blood sample collection for cytokine assay )
i Two millilitres (2 mL) of blood was drawn from wing vein (Samour, 2016) of each broiler chicken in Cgp and Tgps. At each blood sampling time, the 2 mL of drawn blood was dispensed into plain blood sample tubes for immediate serum collection.
ii The harvested serum was frozen and stored at -20°C for the determination of HSP, TNF-α and IL-1α. The blood assay sample collection carried out on day 28 were replicated on days 35 and 42 of the study period.
Determination of heat-shock protein70 and IL-1α
Quantitative detection of HSP and IL-1α in serum was determined according to the method described by Jeremy et al. (1990), with specific ELISA kits for quantification using Fine Test® Chicken HSP70 high sensitivity ELISA Kit (Wuhan Fine Biotech Co., Ltd., B9 Bld., High-Tech Medical Devices Park, Wuhan, China). The ELISA Kits had a range (0.156-10 ng/mL) and sensitivity (0.094 ng/mL) for HSP70; and range (7.813500 pg/mL) and sensitivity (4.688 pg/mL) for IL-1α. Briefly, using the sandwich principle, pre-coated wells were washed twice before dispensing 100 µL of set standard solution into standard wells, labelled A-G. Exactly 100 µL of the serum samples were dispensed into the test sample wells. The plates were covered with cover seal tape and incubated at 37°C for 1.30 h. The contents in the plates were discarded and washed twice using the wash buffer. Exactly 100 µL of Biotin-labelled antibody buffer was dispensed into standard, test sample and blank wells, and the plates were covered with cover seal tape and incubated at 37°C for 1.30 h. The plate covers were removed and the plates washed three times with wash buffer. Exactly 100 µL of SABC (HRP- Streptavidin Conjugate) was dispensed into each well and the plates covered with cover seal tape and incubated at 37° C for 30 minutes. The plate covers were removed and washed five times with the wash buffer. Exactly 90 µL of TMB (3,3’,5,5’-tetramethylbenzidine) substrate was added into each well and the plates were covered with cover seal tape and incubated at 37°C in dark for 10-20 minutes for optimization of colour. The reaction was stopped by the addition of 50 µL of Stop solution into each well with a resultant immediate colour change to yellow. The absorbance was immediately read at 450 nm in Microplate Reader (RT-2100C). A standard curve plot was made of the relative optical density of 450 nm of each standard solution (Y) versus the respective concentration of the standard solution (X). The target concentration from the standard curve was determined by interpolation.
Determination of tumour-necrosis factor-alpha
A TNF-α kit was used based on the principle of the double antibody sandwich technology ELISA (Jeremy et al., 1990). The randomly collected serum samples from each of the Cgp and Tgp of male broiler chickens were used for the quantification using Chicken TNF-α ELISA Kit (Shanghai Coon Koon Biotech Co., Ltd., Shanghai, China). The ELISA Kit had a standard curve range (20 pg/mL – 800 pg/mL) and a sensitivity of 10.0 pg/mL. Briefly, the plate method was used, and 50 µL of standard solution was added to the standard wells of the strip plate. Exactly 10 µL of the test serum sample was added to the sample wells; followed by 40 µL of sample diluent, which was added to the test sample wells. The chicken radish peroxidase-conjugate (HRP) reagent (100 µL) was added to each well (Standard and testing wells), and it was covered with a seal plate membrane. The contents were gently mixed by shaking and incubated for 60 minutes at 37°C. The plate-sealing membrane was carefully removed and the liquid drained. The wells were patted dry and filled with washing solution and allowed to stay for one minute. The procedure was repeated five times. To develop colour, 50 µL chromogen A was added to each well followed by 50 µL chromogen B. The contents of the plate were gently mixed by shaking and incubated for 15 minutes at 37°C for colour to develop. Exactly 50 µL of stop solution was added to each well to stop reaction with a resultant immediate colour change from blue to yellow. The absorbance of each well at 450 nm wavelength was measured. The concentration was determined using a standard curve from the concentration and the corresponding absorbance values recorded. The concentration of the corresponding samples was calculated according to the absorbance values of the samples.
Data Analyses
The data generated from the experiment are presented as mean ± SEM and evaluated for rhythmicity by the cosinor procedure (Cosinor. Online Software, Molcan, 2019). Three rhythmic parameters were characterised and determined: mesor (M) rhythm adjusted mean level, amplitude (A), acrophase (Ф) time at which peak of rhythm occurred at days 28, 35 and 42 of the study periods. For each broiler chicken, the mean level of the rhythm was computed as the arithmetic mean of all values in the data set. The amplitude of the rhythm was calculated as half the maximum – minimum range of the oscillation, computed as the difference between the peak and trough. The acrophase of a rhythm was determined by an iterative curve-fitting procedure, based on the single cosinor procedure. The difference between control, treatment groups and times of measurement were tested by two-way ANOVA. The results are presented as tables and chronograms. Values of P ˂ 0.05 were considered significant.
RESULTS
Thermal environmental parameters
During the experimental period, the values of DBT, RH, and THI recorded ranged between 28.1 – 28.7°C, 87.3 - 87.6% and 27.9 - 28.3° C , respectively. The overall mean values at days 28, 35 and 42 of experimental period for DBT, RH and THI were 28.3 ± 0.2 °C, 87.5 ± 0.1% and 28.1 ± 0.2 °C, respectively. The highest DBT value recorded was at 17.00 h and the lowest occurred at 05.00 h. The lowest THI (24.0°C) during the experimental period was obtained at 05.00 h, and the highest (33.7 °C) occurred at 17.00 h.
Effect of supplemental taurine on serum heat-shock protein 70 concentrations in male broiler chickens at days 28, 35 and 42 during the hot-dry season
Cosinor analysis of HSP70 showed it exhibited circadian rhythmicity (Figure 1). The overall mesor of HSP70 concentration recorded in Cgp (4.20 ± 0.2
n g /mL) was significantly (P < 0.05) higher than that of Tgp (3.80 ± 0.2 n g /mL) (Table 1). At day 28, HSP70 mesor was not significantly (P > 0.05) different in both Cgp (3.66 ± 0.40 n g /mL) and Tgp (3.65 ± 0.44 ng/mL). However, the mesors for HSP70 concentration were significantly (P < 0.05) higher on days 35 and 42 for Cgp (4.5 ± 0.20 and 4.3 ± 0.26 ng/mL, respectively), compared to Tgp (3.9 ± 0.20 and 3.96 ± 0.29 ng/mL, respectively) (Table. 1). The overall mean amplitudes for Cgp and Tgp were similar for HSP70 concentration during the study period. The acrophase occurred during the early photophase (6.3 h) and late photophase (15.5 h) in the Cgp and Tgp on day 28. The acrophase for HSP70 concentration on days 35 for the Cgp and Tgp were recorded during the scotophase (21.3 h) and the photophase (13.2 h). On day 42, the acrophase of HSP70 concentration occurred at 15.5 h for both the Cgp and Tgp (Table 1).
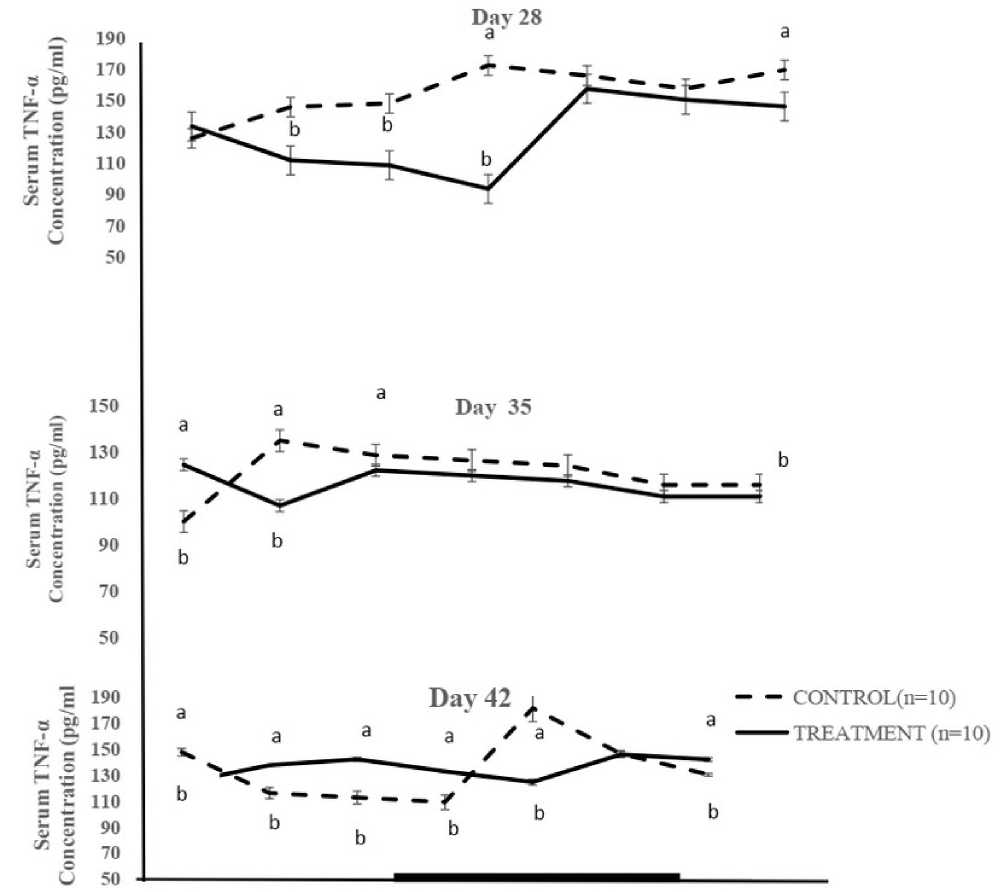
Effect of supplemental taurine on serum tumour necrosis factor-α concentration in male broiler chickens at days 28, 35 and 42 under hot-dry conditions .
The overall mean TNF-α concentration in male broiler chickens supplemented with taurine was significantly (P < 0.05) higher in Cgp (137.20 ± 9.0 pg/mL), compared to that recorded in Tgp (126.6 ± 4.6 pg/mL). Rhythmic expression of TNF-α concentrations in both Cgp and Tgp was recorded during both photophase and scotophase. Rhythmic characteristic of TNF-α (Table 2) showed that Cgp recorded a mesor in TNF-α of 156.1 ± 6.2 pg/mL compared to 126.1 ± 9.2 pg/mL for the Tgp on day 28 of the study period. However, the mesor value for TNF-α concentration on day 35 in Cgp had significantly (P < 0.05) higher value (121.8 ± 4.6 pg/mL) compared with that of the Tgp (116.3 ± 2.6 pg/mL). There was no significant (P > 0.05) difference in mesor for Cgp (133.8 ± 9.76 pg/mL) and Tgp (134.9 ±
-
3.3 6 pg/mL) on day 42. On day 28 the amplitude of TNF-α in Cgp was lower (12.9 pg/mL) than that of Tgp (29.1 pg/mL). The Tgp had a lower amplitude (3.0 pg/mL), compared with Cgp (10.8 pg/mL) on day 35. The amplitude of TNF-α was similar in both groups at day 42. The TNF-α acrophase for the Cgp was restricted to the late scotophase (23.8 h) as against (05.0 h) recorded during the early photophase for Tgp at day 28. The acrophases at day 35 of the study period for the Cgp and Tgp were restricted to the late photophase (18.4 h) and early scotophase (20.8 h). At day 42 of the study, the acrophases for TNF-α were obtained during the early photophase (03.6 h) in Cgp as opposed to 13.00 h recorded in Tgp, Fig. 2.
Effect of supplemental taurine on serum interleukin-1α concentrations in male broiler chickens at days 28, 35 and 42 under hot-dry conditions.
Figure 3 depicts the rhythmic expression of IL-1α in both Cgp and Tgp. The mesor of IL-1α obtained in Tgp (52.5 ± 3.2 p g /mL) was significantly (P < 0.05) higher, compared to that recorded for the Cgp (41.5 ± 1.3 p g /mL) on day 28 (Table 3). The amplitude for IL-1 was higher (6.9 p g /mL) in Tgp, compared to the value of 3.1 p g /mL, recorded in Cgp. The acrophases for IL-1α concentration were restricted to the early photophase (4.6 h) for the Cgp and mid-photophase (15.8 h) for the Tgp on day 28. The mesor of IL-1α in Cgp and Tgp were similar (35.7 ± 1.5 pg/mL and 35.7 ± 1.5 p g /mL, respectively), on day 35. However, the amplitudes differed, with the Cgp (4.3 p g /mL) and Tgp (4.0 p g /mL). The IL-1α acrophases on days 35 and 42 for Cgp and Tgp were restricted to the scotophase (21.7 h and 19.3 h, respectively), and early photophase (6.8 h and 8.4 h, respectively). The amplitudes of IL-1α were similar, with Cgp recording 6.1 p g /mL, while a value of 6.5 p g /mL was obtained for Tgp at day 42 (Table 3).
Table 1 : Rhythm characteristics of serum heat-shock protein 70 concentrations in taurine-supplemented male broiler chickens at days 28, 35 and 42 of study period under hot-dry conditions.
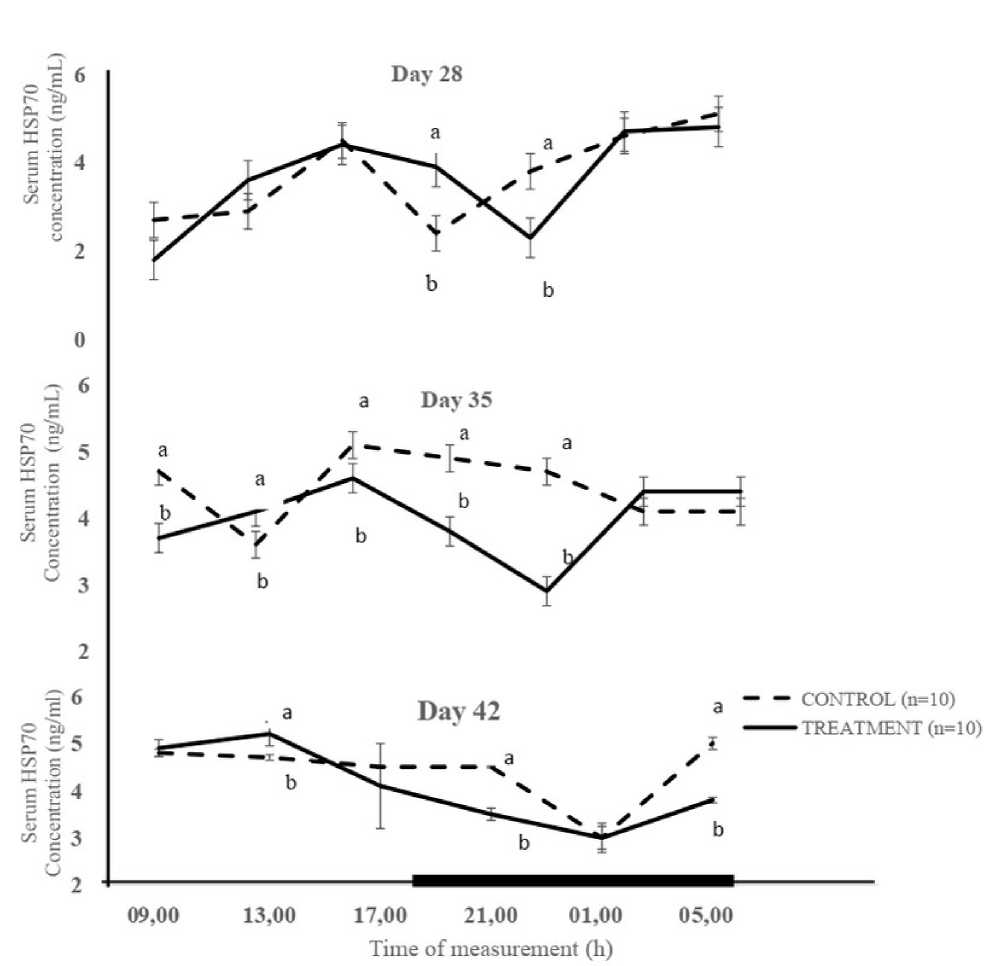
Figure 1: Diurnal variations in serum heat-shock protein 70 concentration in taurine-supplemented male broiler chickens at days 28, 35 and 42 of experiment under hot-dry conditions.
Note: Each data point represents the Mean ± SEM of 10 broiler chickens at each period of measurement. The black horizontal bar denotes the dark phase of the prevailing light/dark cycle a, b = Means with different letters are significantly (P < 0.05) different HSP 70 = Heat-shock protein 70
Table 2: Rhythm characteristics of serum tumour necrosis factor-α concentration in taurine-supplemented male broiler chickens at days 28, 35 and 42 of study period under hot-dry conditions.
09,00 13,00 17,00 21,00 01,00 05,00 09,00
Figure 2. Diurnal variations in tumour necrosis factor concentration in taurine-supplemented male broiler chickens at days 28, 35 and 42 of study period under hot-dry conditions.
Note: Each data point represents the Mean ± SEM of 10 broiler chickens at each period of measurement. The black horizontal bar denotes the dark phase of the prevailing light/dark cycle
-
a, b = Means with different letters are significantly (P < 0.05) different TNF -α = Tumour necrosis factor
Table 3: Rhythm characteristics of serum interleukin-1α concentration in taurine-supplemented male broiler chickens at days 28, 35 and 42 of experiment during hot-dry conditions.
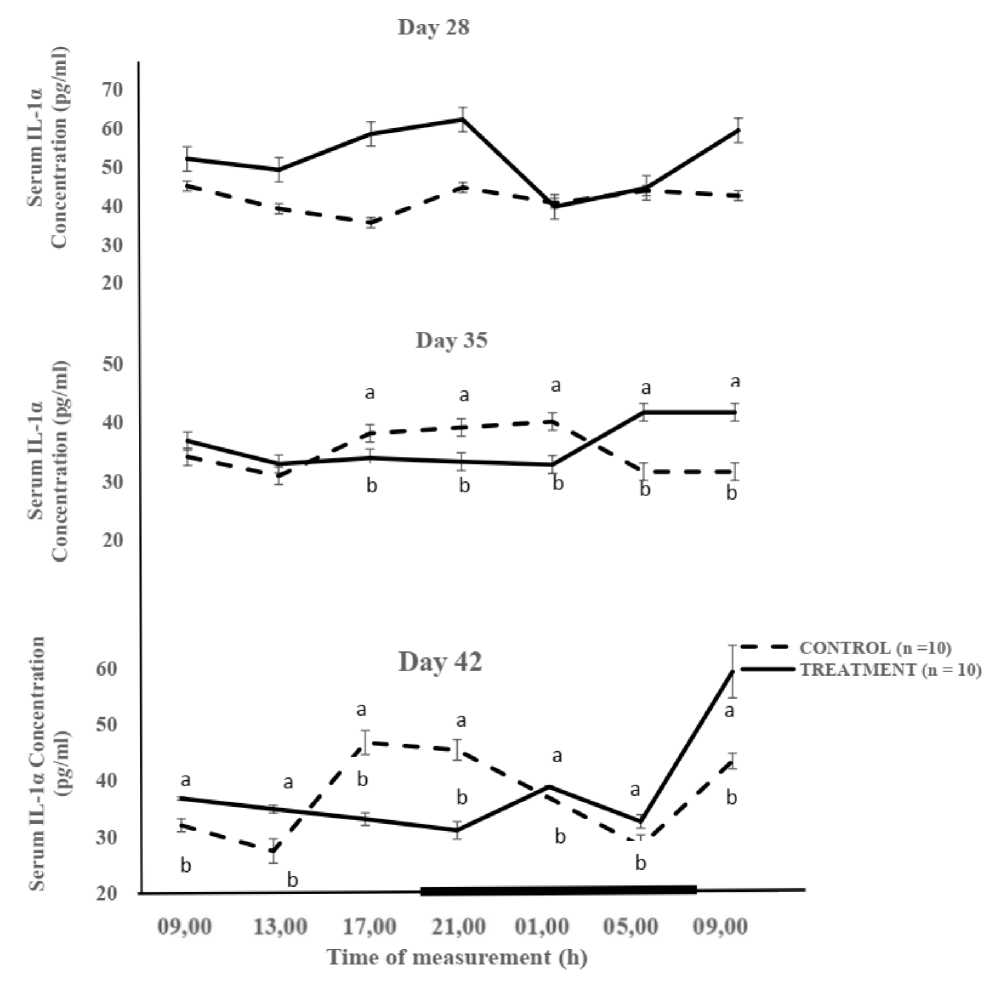
Figure 3: Diurnal variations in serum interleukin-1α in taurine-supplemented male broiler chickens at days 28, 35 and 42 of study period under hot-dry conditions
Note: Each data point represents the Mean ± SEM of 10 broiler chickens at each period of measurement. The black horizontal bar denotes the dark phase of the prevailing light/dark cycle a, b = Means with different letters are significantly (P < 0.05) different IL-α = Interleukin 1 alpha
DISCUSSION
In the current study, the result of the thermal conditions suggest that the experimental period was thermally stressful to the broiler chickens. The highest DBT (36.0 °C), and THI (35.6 °C), were outside the thermoneutral zone of 18 – 24.5°C for poultry in the tropics (Donkoh, 1989). In addition, the result showed that the RH was high and therefore, not favourable for evaporative cooling in broiler chickens under high thermal load, and this may adversely affect poultry welfare and performance (Minka and Ayo, 2011). The peak AT recorded during the study period was at 17.0 h, and the corresponding RH was 85%. The minimum AT recorded during the study period (24.3°C), was obtained in the early photophase (09.0 – 13.0 h) and scotophase (21.0 – 09.0 h). This shows that the early and late periods of the day were not thermally stressful to the broiler chickens.
The circadian rhythmicity of cytokines measured in the current study suggests that the circadian clocks were able to maintain a balance between sensitivity and resistance to changes in high AT and prolonged daylight cycle occurring during the hot-dry period at the molecular level. However, the mechanism(s) underlying the maintenance is yet to be elucidated. In addition, the result suggests that the transcriptional response of the circadian clock-gene in broiler chickens to heat stress depends on the time of the day as evident by the higher values during peak AT. The result in taurine-supplemented group suggests that the transcriptional response was downregulated, perhaps due to its neuromodulatory or the ROS-scavenging properties of taurine (Baliou et al., 2021).
The result shows that increase in AT resulted in activation of HSP response, which led to the increased synthesis of HSPs. The HSP concentration shows timing of peak-expression during the day and night, with rhythmic expression occurring during the photophase and scotophase and with different peak concentrations. The variation of cytokine concentrations on days 28, 35 and 42 suggests the effect of stage of development and the processes in broiler chickens on transcriptional regulation of the clock genes, which require further studies. However, the observed increase in mesor of HSP70 and TNF-α/IL-1α (endogenous pyrogenes) during the early photophase in Tgp on day 28, could be due to the thermogenic effect of taurine; increasing the body temperature during the early hours of the morning when the AT was low, thereby, enhancing thermoregulation at that age.
The result demonstrates that high thermal environmental conditions affect thermal gradient between the body and environment, thereby, suggesting interference of exchange of excessive metabolic heat out of the body or its influx at thermoneutral ambient temperatures (AT) (Williams and Tieleman, 2001). The result show that homeothermy is complex central integrated mechanism, mediated by the hypothalamus (Baarendse et al., 2007). It has been reported that poultry resort to hyperthermia to alleviate the high cost of evaporative cooling and dehydration (Nilsson et al., 2016). Hyperthermia is associated with heat stress due to high AT, which induces a cyclic diurnal variation in patterns of oxidant formation (Harderland et al., 2003; Ayo et al., 2017). Therefore, the broiler chickens develop thermotolerance by HSP production. The increase in HSP produced initiates a cascade of pro-inflammatory cytokines, which aggravate the situation. The current study show that the cytokines exhibited circadian rhythm, with acrophase occurring during the photophase in both groups. Lenin et al., (2019) in their review, reported that taurine exhibits a mechanism that inhibits GABA A receptors, therefore acting as an inhibitory neurotransmitter.
The current study supports previous reports that taurine is a potent scavenger of ROS, which are important in cell signaling. Oxidative stress has been reported to mediate HSP induction and biosynthesis (Mahmoud et al., 2003). Therefore, scavenging of ROS by ascorbic acid was reported to decrease the need to stimulate HSP gene expression, hence, a lowered HSP70 and a less stress response was recorded in ascorbic acid-fed chickens (Mahmoud et al., 2003). Taurine chloramine, an intermediate product generated within the cell during chloride burst, sequesters NFKB, a transcription factor, leading to the downregulation of pro-inflammatory cytokines during heat stress. In avian species, HSPs are expressed constitutively (Givisiez et al., 2001; Vinoth et al., 2018), and the degree of heat stimulation and tolerance are related to HSP expressions (Krebs and Bettencourt, 1999). The concentration of HSP70 recorded in the current study in both groups was similar during the early phase of growth. The circadian rhythmicity of HSP70 in both groups at day 28 of the study was similar. Al-Aquil and Zulkifli (2009), showed that cells protect themselves by increased HSP production through gene transcription, is mediated by oxidative stress. However, in the current study, there was a decrease in HSP 70 concentration in the taurine-supplemented group at the peak AT. This finding could be due to a general response of cells to defend themselves against damage and apoptosis (Khan et al., 2012). This observation was similar with that of Belal et al., (2018), who reported a downregulation in HSP70 in broiler chickens administered taurine and exposed to chronic heat. Besides, it has been shown that pathophysiological changes induced by heat stress in the body may elevate the demand of amino acid for energy supplementation and HSP synthesis (Hoskin et al., 2016). The HSP concentrations remained significantly lower on days 35 and 42 during the study period in the current study, probably due to its down-regulation by taurine.
The poorly developed thermoregulatory mechanisms at the early stage of growth in the broiler chickens resulted in the heightened HSP production, observed on day 28 in both groups to protect against heat stress. The decrease in HSP concentration recorded in the taurine group at peak AT suggests that those broiler chickens were not as thermally stressed at that period of the day (05.00 – 17.00 h). The decrease in concentration of HSP may be induced by ROS scavenging property of taurine (Grove and Karpowicz, 2017), and its capacity to directly inhibit ROS generation in the respiratory chain (Seidel et al., 2018), thereby reducing oxidative stress during that hour of day.
The results of TNF-α reflected a positive association with HSP during the 24-h period, similar to the report by Takahashi et al. (1997). The interactive increase was apparently associated with oxidative stress-induced HSP gene production (Anathan et al., 1986), which modulates different cytokines, including IL-1β, IL-6 and TNF-α (Takahashi et al., 1997; Ohtsu et al., 2015; Quinteiro-Filho et al., 2017). Thus, increased HSP level may increase the production of pro-inflammatory cytokines, such as IL-1β, TNF-α and IL-6 (Bouchama and Knochel 2002; Chang et al., 2010). The increase in cytokine concentrations induced by taurine, apparently, played a significant role in heat stress and the amelioration of immunosuppression induced by pro-inflammatory cytokines.
Taurine was observed to have advanced the acrophases of TNF- α and IL-1 α. Broiler chickens in the taurine-supplemented group attained acrophases earlier than the period of peak AT. The TNF-α and IL-1α are both endogenous pyrogenic cytokines, whose decreased concentration and advanced acrophase in Tgp may play a significant role in decreasing the cloacal temperature compared to the Cgp. The endogenous pyrogenic cytokines that are generated as a result of heat stress suggest that the cytokines play a significant role in the synthesis and release of prostaglandin E2 (PGE2) in the anterior hypothalamus, which re-adjusts the thermoregulatory set-point upwards (Soszynski, 2001). The result shows that taurine exerted an inverse effect on TNF-α concentration by decreasing in Tgp during the 24–h study period at every hour of the day. The reversal may be associated with the thermogenic effect of taurine, to ensure homeothermy. Taurine has been reported to regulate mitochondrial proteins, which control energy expenditure by uncoupling respiration from ATP synthesis (Chang et al. 2011). This infers that taurine additionally may increase energy expenditure by promoting fatty acid β-oxidation, while increasing adaptive thermogenesis (Song et al., 2021). High environmental temperature increases plasma levels of IL-1, IL-6 and TNF-α (He et al., 2018). Takahashi et al. (1997) reported the association between HSP and pro-inflammatory cytokines. The current result disagrees with the earlier observations by Takashi et al. (1997) in the Cgp with respect to IL-1α. However, the Tgp had significantly higher concentration of IL-1α at day 28. The decreased response with age in the current study with respect to the pro-inflammatory cytokines agrees with the finding of Moberg and Mench (2000), who reported that animals subjected to repeated heat stress only show greater response in the first few days after exposure, thereafter a decrease occurs. The observation in the current study may be associated with the independent nature of the clocks that regulate the production of the cytokines and the role exerted by the hypothalamic-pituitary-adrenal axis and glucocorticoids, resulting in the decrease in IL-1α concentration (Baccan et al., 2004). The current study has, for the first time, and to the best of our knowledge, reported that pro-inflammatory cytokines produced in response to heat stress exhibited circadian rhythmicity, with acrophases occurring during the photophase. The result has shed light on the mechanism of involvement of taurine in the regulation and amelioration of heat stress in male broiler chickens reared under hot-dry conditions.
CONCLUSION
It was concluded that HSP70, TNF- α and IL-1α in response to their gene expressions exhibited circadian rhythmicity. Taurine decreased the concentrations of HSP70, TNF- α and IL-1α, while it advanced the acrophases of TNF-α and IL-1α. To our knowledge, this is the first study on the circadian rhythms of HSP70, TNF-α and IL-1α in serum of male broiler chickens supplemented with taurine under natural hot-dry conditions.
ACKNOWLEDGMENT
We acknowledge the contributions of Mr. Abraham Mathias and Mr Adetiba Bamidele who assisted in sample collection and laboratory analyses.
CONFLICTS OF INTEREST
The authors declare that they have no potential conflicts of interest.
Список литературы Taurine supplementation modulates circadian rhythms of heat-associated cytokines in male broiler chickens reared under hot-dry conditions
- Akbarian, A., Michiels, J., Degroote, J., Majdeddin, M., Golian, A. and De smet, S. (2016). Association between heat stress and oxidative stress in poultry; mitochondrial dysfunction and dietary interventions with phytochemicals. Journal of Animal Science and Biotechnology. 7: 1 - 14.
- Alagawany, M., Farag, M. R., Abd El-Hack, M. E. and Patra, A. (2017). Heat stress: effects on productive and reproductive performance of quail. World's Poultry Science Journal, 73(4): 747 - 756.
- Al-Aqil, A. and Zulkifli, I. (2009). Changes in heat-shock protein 70 expression and blood characteristics in transported broiler chickens as affected by housing and early age feed restriction. Poultry Science, 88(7): 1358 - 1364.
- Anathan, J., Goldberg, A. L. and Voellmy, R. (1986). Abnormal proteins serve as eukaryotic stress signals and trigger the activation of heat shock genes. Science, 4749: 522 - 524.
- Ayo, J. O., Makeri, H. K., Minka, N. S. and Aluwong, T. (2017). Circadian rhythms of biomarkers of oxidative stress and their characteristics in broiler chickens reared under natural light/dark cycle. Biological Rhythm Research, 49(1): 119 - 127.
- Ayo, J.O., Owoyele, O.O. and Dzenda, T. (2007). Effects of ascorbic acid on diurnal variations in rectal temperature of Bovan Nera pullets during the harmattan season. International Journal of Poultry Science, 6: 612 - 616.
- Baarendse, P, J. J., Debonne, M., Decuypere, M., Kemp, B. and VanDenBrand, H. (2007). Ontogenyof avian thermoregulation from neural point of view. World Poultry Science, 63(2): 267 -276.
- Baccan, G. C., Oliveira, R. D. R. and Mantovani, B, (2004). Stress and immunological phagocytosis: possible nongenomic action of corticorsterone. Life Sciences, 75(11): 1357 - 1368.
- Baliou, S., Adamaki, M., loannou, P., Pappa, A., Panayiotidis, M. I., Spandidos, D. A., Christodoulou, I., Kyriakopoulos, A. M. and Zoumpourlis, V. (2021). Protective role of taurine against oxidative stress (Review). Molecular Medicine Reports, 24: 605. DOI: 10.3892/mmr.2021.12242
- Belal, S. A., Kang, D. R., Cho, E. S. R.. , Park, G. H. and Shim, K. S. (2018). Taurine reduces heat stress by regulating the expression of heat-shock proteins in broilers exposed to chronic heat. Brazilian Journal of Poultry Science, 20(3): 479 - 486.
- Bouchama, A. and Knochel, J. P. (2002). Heat stroke. New England Journal of Medicine, 346(25): 1978 -1988.
- Burioka, N., Miyata, M., Endo, M., Fukuoka, Y., Suyama, H., Nakazaki, H., Igawa, K. and Shimizu, E. (2005). Alteration of the circadian rhythm in peak expiratory flow of nocturnal asthma following nighttime transdermal beta 2-adrenoceptor agonist tulobuterol chronotherapy. Chronobiology International, 22: 383 - 390.
- Chang, L. T., Yuen, C. M., Liou, C. W., Lu, C. H., Chang, W. N., Youssef, A. A. and Yip, H. K. (2010). Link between interleukin-10 level and outcome after ischemic stroke. Neuroimmunomodulation, 17: 223 - 228.
- Denbow, D. M. and Edens, F. W. (1980). Effects of intraventricular injections of sodium and calcium on Mathias and Mr Adetiba Bamidele who assisted in body temperature in the chicken. American Journal of Physiology, 239: 62 - 65.
- Donkoh, A. (1989). AT: a factor affecting performance and physiological response of broiler chickens. International Journal of Biometeorology, 33: 259 -265.
- Dzenda, T., Ayo, J. O., Lakpini, C, A. M. and Adelaiye, A. B. (2013). Seasonal, sex and liveweight variations in feed and water consumptions of adult captive African Giant rat (Cricetomys gambianus, Waterhouse - 1840) kept individually in cages. Journal of Animal Physiology and Animal Nutrition, 97: 465 -474.
- Egbuniwe, I. C., Ayo, J. O., Kawu, M. U. and Mohammed, A. (2018). Ameliorative effect of betaine and ascorbic acid on erythrocyte osmotic fragility and malondialdehyde concentrations in broiler chickens during the hot- dry season. Journal of Applied Animal Research, 46(1): 380 -385.
- Gamal El-Din, M. M., El-Gamal, M. I., Abdel-Maksoud, M. S., Lee, H., Choi, J., Kim, T. W., Shin, J. S., Lee, H. H., Kim, H. K., Lee, K. T. and Baek, D. (2020). Inhibitory effects of triarylpyrazole derivatives on LPS-induced nitric oxide and pge2 productions in murine raw 264.7 macrophages. Bioorganic and Medicinal Chemistry Letters, 30(4): 126884.
- Givisiez, P. E. N., da Silva, M. M., Mazzi, C. M., Ferro, M. I. T., Ferro, J. A.,Gonzales, E. and Macari, M. (2001). Heat or cold chronic stress affects organ weights and Hsp70 level in chicken embryos. Canadian Journal of Animal Science, 81: 83 - 87.
- Grove, R. Q. and Karpowicz, S. J. (2017). Reaction of hypotaurine or taurine with superoxide produces the organic peroxysulfonic acid peroxytaurine. Free Radical Biology and Medicine, 108: 575 - 584.
- Guide for the Care and Use of Laboratory Animals. (2011). Committee for the Update of the Guide for National Research Council of the National Academies, 8th Ed., Washington (DC): The National Academies Press, 45(1): 1 - 13.
- Hardeland, R., Coto-Montes, A. and Poeggeler, B. (2003). Circadian rhythms, oxidative stress, and antioxidative defence mechanisms. Chronobiology International, 20: 921 - 962.
- He, X. F., Lu, Z., Ma, B., Zhang, L., Li, J. L., Jiang, Y., Zhou, G. H., and Gao, F. (2018). Effects of chronic heat exposure on growth performance, intestinal epithelial histology, appetite-related hormones and genes expression in broilers. Journal of the Science of Food and Agriculture, 98(12): 44714478.
- Hoskin, S. O., D. M. Bremner, G. Holtrop, and G. E. Lobley. (2016). Responses in whole-body amino acid kinetics to an acute, subclinical endotoxin challenge in lambs. British Journal of Nutrition, 115: 576 - 584.
- Hosseindoust, A., Oh, S. M., Ko, H. S., Jeon, S. M., Ha, S. H., Jang, A., Son, J. S., Kim, G. Y., Kang, H. K. And Kim, J. S. (2020). Muscle antioxidant activity and meat quality are altered by supplementation of astaxanthin in broilers exposed to high temperature. Antioxidants, 9(11): 1032. Doi: 103390/antiox9111032.
- Hotta, C. T., Gardner, M. J., Hubbard, K. E., Baek, S. J., Dontamala suhita, N. D., Dodd, A. N. and Webb, A. A. R. (2007). Modulation of environmental responses of plants by circadian clocks. Plant Cell and Environment, 30(3): 333 - 349.
- Huang, C., Guo, Y. and Yuan J. (2014). Dietary taurine impairs intestinal growth and mucosal structure of broiler chickens by increasing toxic bile acid concentrations in the intestine. Poultry Science, 93(6): 1475 - 1483.
- Ito, T., Schaffer, S.W. and Azuma, J. (2012). The potential usefulness of taurine on diabetes mellitus and its complications. Amino Acids, 42: 1529 - 153.
- Jakaria, M., Azam, S., Haque, M. E., Jo, S. H., Uddin, M. S., Kim, I. S. and Choi, D. K. (2019). Taurine and its analogues in neurological disorders: focus on therapeutic potential and molecular mechanisms. Redox Biology, 24: 101223.
- Jeremy M. P., Elwood, N. J., Ramadi, L. T., Pica, M. R. and McKenzie, I. F. C. (1990). Improvement in sensitivity of enzyme-linked immunosorbent assay for tumour necrosis factor. Immunology and Cell Biology, 68: 51 - 55.
- Jimoh, A. A., Ayedun, E. S., Oyelade, W. A., Oloruntola, O. D., Daramola, O. T., Ayodele, S. O. and Omoniyi, I. S. (2018). Protective effect of soursop (Annona muricata Linn.) juice on oxidative stress in heat stressed rabbits. Journal of Animal Science Technology, 60(28) https://doi.org/10.1186/s40781-018-0186-4.
- Keyse, S. M., and Emslie, E. A. (1992). Oxidative stress and heat shock induce a human gene encoding a protein-tyrosine phosphatase. Nature, 359: 644 -647.
- Khan, R. U., Naz, S., Nikousefat, Z., Selvaggi, M., Laudadio, V. and Tufarelli, V. (2012). Effect of ascorbic acid in heat stressed poultry. World's Poultry Science Journal, 68(3): 477-490.
- Krebs, R. A. and Bettencourt, B.R. (1999). Heat-shock protein variation and the evolution of thermotolerance in Drosophila. American Zoologist, 39(6): 910 - 919.
- Lenin, O., Edgar, Z., Rosario, G. and Hugo, Q. (2019). Taurine and GABA neurotransmitter receptors, a relationship with therapeutic potential? Expert Review of Neurotherapeutics, 19(4): 289 - 291.
- Lu, Z., X. He, B. Ma, L. Zhang, J. Li, Y. Jiang, G. Zhou, and F. Gao. (2017). Chronic heat stress impairs the quality of breast-muscle meat in broilers by affecting redox status and energy-substance metabolism. Journal of Agriculture and Food Chemistry, 65: 11251 - 11258.
- Luo, J., J. Song, L. Liu, B. Xue, G. Tian, and Y. Yang. (2018). Effect of epigallocatechin gallate on growth performance and serum biochemical metabolites in heat-stressed broilers. Poultry Science, 97: 599-606.
- Ma, B., Zhang, L., Li, J., Xing, T., Jiang, Y. and Gao, F. (2021). Dietary taurine supplementation ameliorates muscle loss in chronic heat stressed broilers via suppressing the perk signalling and reverse endoplasmic reticulum-stress-induced apoptosis. Journal of the Science of Food and Agriculture, 101(5): 2125 - 2134.
- Mahmoud, K. Z., Edens, F. W., Eisen, E. J. and Havenstein, G. B. (2003). Effect of ascorbic acid and acute heat exposure on heat shock protein expression by young white leghorn chickens, Comparative Biochemistry and Physiology Part C: Toxicology and Pharmacology, 136(4): 329 - 335.
- Minka N.S. and Ayo J.O. (2011). Modulating role of vitamins C and E against transport induced stress in pullets during the hot-dry conditions. ISRN Veterinary Science, ID 497138, doi:10.5402/2011/497138.
- Minka, N.S. and Ayo, J.O. (2014). Influence of cold-dry (harmattan) season on colonic temperature and the development of pulmonary hypertension in broiler chickens, and the modulating effect of ascorbic acid. Open Access Animal Physiology, 6: 1 - 11.
- Minka, N. S., Ayo, J. O., Hassan, F. B. and Makeri. H. K. (2020). Seasonal patterns of circadian rhythmicity of colonic and body surface temperatures in adult Rouen ducks (Anas platyrhynchos domesticus) under natural light/day cycles in a tropical savannah, Biological Rhythm Research, DOI: 10.1080/09291016.2020.1736414.
- Moberg, G. P. and Mench, J. A. (2000). Biological response to stress: Basic principles and implications for animal welfare. Biology of Animal Stress, CABI Publishing International, Wallingford, United Kingdom. Pp 1-21.
- Molcan, L. (2019). Time distribution data analysis by Cosinor. Online application. bioRxiv; 2019. Doi.org/10.1101/805960.
- Nilsson, J. A., Molokwu, M. N. and Olsson, O. (2016). Body temperature regulation in hot environments. PLoS ONE, 11: e0161481. doi: 10.1371/journal.pone.0161481.
- Ohtsu, H., Yamazaki, M., Abe, H., Murakami, H. and Toyomizu, M. (2015). Heat stress modulates cytokine gene expression in the spleen of broiler chickens. Journal of Poultry Science, 52: 282-287.
- Quinteiro-Filho, W. M., Calefi, A. S., Cruz, D. S. G., Aloia, T. P. A., Zager, A., Astolfi-Ferreira, C. S., Piantino Ferreirab, J.A., Sharifc, V. and Palermo-Netoa, J.(2017). Heat stress decreases expression of the cytokines, avian beta-defensins 4 and 6 and Toll-like receptor 2 in broiler chickens infected with Salmonella enteritidis. Veterinary Immunology and Immunopathology, 186: 19 - 28.
- Ripps, H. and Shen, W. (2012). Taurine: A "very essential" amino acid. Molecular vision, 18: 2673 -2686.
- Ruan, L., Zhou, C., Jin, E., Kucharavy, A., Zhang, Y., Wen, Z., Florens, L., and Li, R. (2017). Cytosolic proteostasis through importing of misfolded proteins into mitochondria. Nature, 543: 443-446.
- Saleh, K. M. M. and Al-Zghoul, M. B. (2019). Effect of acute heat stress on the mRNA levels of cytokines in broiler chickens subjected to embryonic thermal manipulation. Animals, 9: 499.
- Samour, J. (2016). Diagnostic value of haematology. In: Harrison, G. and Lightfoot, T. (Ed.). Clinical Avian Medicine, Harrison's Bird Foods, Brenthwood, Tennessee, USA, Pp. 588 - 597.
- Sanchez, I., Hughes, R. T., Mayer, B. J., Yee, K., Woodgett, J. R., Avruch, J., Kariakis, J. M. and Zion, L. I. (1994). Role of SAPK/ERK kinase-1 in the stress-activated pathway regulating transcription factor c-Jun. Nature, 372: 794 -798.
- Seidel, U., Huebbe, P. and Rimbach, G. (2018). Taurine: A regulator of cellular redox and homeostasis skeletal muscle function. Molecular Nutrition and Food Research, 63(16), e1800569. https://doi.org/10.1002/mnfr.201800569.
- Shini, S., Huff, G. R, Shini, A. and Kaiser, P. (2010). Understanding stress-induced immunosuppression: Exploration of cytokine and chemokine gene profiles in chicken peripheral leukocytes. Poultry Science, 89: 841 - 851.
- Silanikove, N. (2000). Effect of heat stress on welfare of extensively managed domestic ruminants. Livestock Production Science,67(1): 1 - 18.
- Song, Q., Guo, J., Zhang, Y. and Chen, W. (2021). The beneficial effects of taurine in alleviating fatty liver disease. Journal of Functional Foods, 77: 104351.
- Soszynski, D. (2001). The inhibition of nitric oxide synthase suppresses LPS-and psychological stress-induced fever in rats. Physiology and Behaviour, 72(1-2): 65 - 72.
- Surai, P. F. and Fisinin, V. I. (2016a). Vitagenes in poultry production. Part 1. Technological and environmental stresses. World's Poultry Science Journal, 72: 721 -734.
- Takahashi, K., Kubo, T., Goomert, R. S., Amiel~ Kappei Kobayashi, D., Imanishi, J., Teshima, R. and Hirasawa, Y. (1997). Analysis of heat-shock proteins and cytokines expressed during early stages of osteoarthritis in a mouse model. Osteoarthritis and Cartilage, 5: 321-329
- Tao, X. and Xin, H. (2003). Acute synergistic effects of air temperature, humidity and velocity on homoestasis of market-size broiler. Transactions of the American Society of Agricultural and Biological Engineers,46: 491-497.
- Trotter, E. W., Berenfeld, L., Krause, S. A., Petsko, G. A., and Gray, J. V. (2001). Protein misfolding and temperature up-shift cause G1 arrest via a common mechanism dependent on heat-shock factor in Saccharomyces cerevisiae. Proceedings of the National Academy of Sciences of the United States of America, 98: 7313 - 7318.
- Vinoth, A., Thirunalasundari, T., Shanmugam, M., Uthrakumar, A., Suji, S. and Rajkumar, U. (2018). Evaluation of DNA methylation and MRNA expression of heat-shock proteins in thermal manipulated chicken. Cell Stress and Chaperones, 23(2): 235 -252.
- William, J. B. and Tieleman, B. I. (2001). Physiological ecology and behavior of desert birds. Current Ornithology. 16: 299 -p 353.
- Wu, Q. J., Liu, N., Wu, X. H., Wang, G. Y. and Lin, L. (2018). Glutamine alleviates heat stress-induced impairment of intestinal morphology, intestinal inflammatory response, and barrier integrity in broilers. Poultry Science, 97: 2675 - 2683.
- Yang, M., Tan, H., Yang, Q. L., Wang, F., Yao, H. L., Wei, Q., Tanquay, R. M. and Wu, T. (2006). Association of HSP70 polymorphisms with risk of noise-induced hearing loss in Chinese automobile workers. Cell Stress Chaperones, 11(3): 233- 239


