Технология получения высококачественных кристаллов белков на МКС при выполнении совместных российско-японских экспериментов
Автор: Инака Кодзи, Ичимизу Саори, Йошизаки Изуми, Кихира Кийохито, Лавренко Елена Гавриловна, Прохорова Анастасия Викторовна, Прудкогляд Валерия Олеговна, Сорокин Игорь Викторович, Такахаши Сачико, Танака Хироаки, Фудзии Такахиро, Ямада Митсугу
Журнал: Космическая техника и технологии @ktt-energia
Рубрика: Инновационные технологии в аэрокосмической деятельности
Статья в выпуске: 2 (29), 2020 года.
Бесплатный доступ
Серия космических экспериментов, выполненная на борту Международной космической станции (МКС) и связанная с выращиванием высококачественных кристаллов белков (Protein Crystal Growth - PCG) в условиях микрогравитации, может рассматриваться как один из лучших примеров плодотворного сотрудничества между японскими и российскими учеными и инженерами в космосе, в обеспечении которого принимали участие и другие партнеры по Программе МКС. В статье детально описывается схема реализации эксперимента PCG, оригинальная и надежная технология его проведения, а также даются примеры использования его результатов.
Международная космическая станция, кристаллы белков, микрогравитация, международное сотрудничество
Короткий адрес: https://sciup.org/143177928
IDR: 143177928 | УДК: 629.78.001.891.55 | DOI: 10.33950/spacetech-2308-7625-2020-2-5-25
Текст научной статьи Технология получения высококачественных кристаллов белков на МКС при выполнении совместных российско-японских экспериментов
TEChNOlOgy OF hIgh qualITy prOTEIN CrySTalS’ OBTaININg aBOard ThE ISS aT EXECuTIONOF jOINT japaNESE-ruSSIaN EXpErImENTS
Inaka k.1, Ichimizu S.2, yoshizaki I.2, kihira k.2, lavrenko E.g.3, prokhorova a.v.4, prudkoglyad v.O.3, Sorokin I.v.4, Takahashi S.5, Tanaka h.5, Fujii T.6, yamada m.2
1MARUWA Foods and Biosciences, Inc.
170-1, Tsutsui-cho, Yamatokoriyama, Nara, 639-1123 Japan
2Japan Aerospace Exploration Agency (JAXA)
Tsukuba Space Center, JEM Utilization Center, 2-1-1, Sengen, Tsukuba-shi, Ibaraki-ken, 305-8505 Japan
3Central Research Institute for Machine Building (TsNIImash)
5Confocal Science Inc.
Hayakawa 2nd Bldg. 7F, 2-12-2 Iwamoto-cho, Chiyoda-ku, Tokyo, 101-0032 Japan
6JGC Corporation
MM Park Bldg., 3-6-3, Minato Mirai, Nishi-ku, Yokohama, 220-0012 Japan
A series of space experiments aboard the International Space Station (ISS) associated with high-quality Protein Crystal Growth (PCG) in microgravity conditions can be considered as a unique and one of the best examples of fruitful collaboration between Japanese and Russian scientists and engineers in space, which includes also other ISS International Partners.
X-ray diffraction is still the most powerful tool to determine the protein three dimensional structure necessary for Structure based drug design (SBDD). The major purpose of the experiment is to grow high quality protein crystals in microgravity for X-ray diffraction on Earth. Within one and a half decade, Japan and Russia have established an efficient process over PCG in space to support latest developments over drug design and structural biology.
One of the keys for success of the experiment lies in how precisely pre-launch preparations are made. Japanese party provides flight equipment for crystallization and ensures the required environment to support the experiment aboard of the ISS’s Kibo module, and also mainly takes part of the experiment ground support such as protein sample characterization, purification, crystallization screening, and solution optimization for microgravity experiment. Russian party is responsible for integration of the flight items equipped with proteins and precipitants on board Russian transportation space vehicles (Soyuz or Progress), for delivery them at the ISS, transfer to Kibo module, and returning the experiments’ results back on Earth aboard Soyuz manned capsule. Due to close cooperation of the parties and solid organizational structure, samples can be launched at the ISS every half a year if the ground preparation goes smoothly. The samples are crystallized using counter diffusion method at 20 degree C for 1–2.5 months. After samples return, the crystals are carefully taken out from the capillary, and frozen for X-ray diffraction at SPring8 facility in Japan. Extensive support of researchers from both countries is also a part of this process.
The paper analyses details of the PCG experiment scheme, unique and reliable technology of its execution, and contains examples of the application.
ФУДЗИИ Такахиро — менеджер проекта по кристаллизации белков корпорации JGC (на заслуженном отдыхе)
FUJII Takahiro — JGC Corporation, Manager of PCG Project (retired)
1. введение. цели и методы эксперимента
Рентгеноструктурный анализ в кристаллографии по-прежнему является наиболее мощным инструментом для определения трехмерной структуры белка, знание которой необходимо для создания лекарственных препаратов ( Structure-Based Drug Design — SBDD ). Главной целью рассматриваемого космического эксперимента является выращивание высококачественных кристаллов белков в условиях микрогравитации для проведения их рентгеноструктурного анализа на Земле. Эти исследования в настоящее время сосредоточены на решении двух разных задач, в основе постановки которых лежат разные научные точки зрения, методы и способы получения результата. С одной стороны, ряд биохимических институтов и лабораторий проводят космические эксперименты по выращиванию кристаллов биологически значимых макромолекул с целью уточнения исходных данных именно для их структурного анализа. С другой стороны, многие научные коллективы являются постановщиками экспериментов, в ходе которых исследуются физические и химические аспекты кристаллизации в условиях микрогравитации, а также влияние микрогравитации на качество кристаллов.
В конце 1990-х гг. в Лаборатории кристаллографии университета города Гранада, Испания (Laboratorio de Estudios Cristallograficos, University of Granada — LEC) совместно с Европейским космическим агентством (European Space Agency — ESA) было разработано новое оборудование для выращивания кристаллов макромолекул из раствора с лучшими (с точки зрения качества и/или размеров кристаллов) характеристиками и надежностью, чем ранее. В основе его конструкции — использование кристаллизационного метода «гель-акупунктуры» (Gel Acupuncture Method — GA), разработанного LEC для реализации условий встречной диффузии между растворами белка и осадителя [1]. Этот метод применяется в лабораториях по всему миру с использованием Блока ячеек кристаллизации «Гранада» (Granada Crystallization Box© — GCB) [2]. В настоящее время Японское агентство по аэрокосмическим исследованиям (JAXA) предоставляет возможность ученым проводить космические эксперименты с использованием модифицированного метода встречной диффузии, при реализации которого применяется оборудование, названное «Блок ячеек кристаллизации JAXA с гель-трубками в запечатанных емкостях» (JAXA Crystallization Box with Sealbag Gel Tubes — JCB–SGT). Поддержка всестороннего развития исследований в этой области и внедрения их результатов является неизменной позицией JAXA [3, 4].
Важной особенностью метода встречной диффузии является то, что в отличие от классического метода висячей или сидячей капли ( hanging or sitting drop method ), он дает возможность реализации широкого спектра условий кристаллизации в одном эксперименте. Такой эксперимент по результативности эквивалентен проведению большого числа экспериментов с висячей каплей по фазовой диаграмме. Кроме того, при его выполнении процесс кристаллизации все время направлен в сторону равновесия, и поэтому в ходе эксперимента с использованием встречной диффузии автоматически происходит поиск оптимального сценария кристаллизации. И, наконец, при его выполнении нет необходимости проверять кристаллы в разных каплях для поиска образцов с более высоким качеством. В рассматриваемом случае наилучшими кристаллами будут те, которые образовались в местах, далеких от точки раздела между белком и осаждающим раствором [5].
С целью реализации метода встречной диффузии оптимальным образом с точки зрения эффективности и простоты использования оборудования в космических условиях в ходе российских экспедиций посещения на МКС, LEC разработала установку для кристаллизации «Гранада» ( Granada Crystallization Facility — GCF ) для размещения блока ячеек кристаллизации GCB в условиях микрогравитации наиболее простым способом. Первый полет аппаратуры JAXA GCF при сотрудничестве с Роскосмосом был организован во время экспедиции посещения на МКС, осуществленной при финансовой поддержке Национального центра по космическим исследованиям Франции, CNES , в 2001 г. Второй полет этой аппаратуры на МКС был выполнен в ходе реализации программы
ЕSА в 2002 г. Работоспособность GCF была полностью подтверждена [6–11].
Проект GCF был реализован JAXA в сотрудничестве с Роскосмосом в период 2003–2008 гг. в качестве пилотного до перевода МКС из режима строительства в режим эксплуатации. Его основная цель — предоставить возможность исследовательским институтам и частным компаниям в Японии проводить на МКС космические эксперименты для накопления практического опыта в предметной области, а также обеспечить их участие в выполнении описываемого эксперимента с целью совершенствования методов и технических средств выращивания кристаллов белков в космосе.
Прежде всего, в JAXA был разработан метод кристаллизации с использованием гель-трубок GCB-GT ( Gel-Tube [12]) для модификации метода GCB-GA в обеспечение большей стабильности капилляров [13]. Затем была разработана новая аппаратура кристаллизации, именуемая «блок JCB » ( JAXA Crystallization Box — блок кристаллизации JAXA ), с целью расширения возможностей проведения космических экспериментов [13, 14]. Начиная с пятого полета аппаратуры JAXA GCF , блок JCB обеспечивал реализацию метода заправки образцов с высокой плотностью — High-Density sample filling method ( JCB–HD ), в дополнение к прежней его функции — реализации метода встречной диффузии с применением гель-трубок, в котором реализован принцип высокой плотности упаковки капилляров, и который использовался в предыдущих экспериментах. JAXA также обеспечило верификацию производительности GCF .
Кроме того, в JAXA было разработано новое устройство кристаллизации, названное «микрочип» ( Micro Chip — MC ). Конструктивно это устройство было выполнено в двух вариантах. В одном варианте MC ( MCA ) используется метод встречной диффузии в приложении к очень незначительному объему рабочего вещества белка. В другом варианте MC ( MCB ) также используется метод встречной диффузии, но в уже в большой ячейке, для выращивания кристаллов белков большой размерности. В ходе девятого полета JAXA GCF в дополнение к JCB–MCA использовалось устройство JCB–MCB .
В результате проведения последних девяти космических экспериментов GCF , JAXA удалось получить информацию о различных факторах, позитивно влияющих на качество кристаллизации белков, в т. ч. обеспечивающих подавление кластерообразования, низкий уровень мозаичности, получение информации о структуре белка с более высоким разрешением, преодоление двойникования кристаллов и нахождение различных пространственных групп кристаллов. Комплексный анализ результатов показал, что ключ к успеху эксперимента — в качестве его предполетной подготовки на Земле. Протокол предполетной подготовки детально описан в работе [3].
В контексте изложения материала следует еще раз подчеркнуть, что целью эксперимента, проводимого на Земле постановщиками (пользователями) из промышленных компаний и исследователями из академической среды в рамках национальных проектов, является получение кристаллов белков, предназначенных для изучения их структуры и функций.
«Эксперимент по выращиванию высококачественных кристаллов белков» ( High Quality Protein Crystal Growth Experiment — PCG ) на борту японского экспериментального модуля JEM ( Kibo ) был начат JAXA и Роскосмосом в 2009 г. [15] с привлечением российских ученых из Федерального научно-исследовательского центра «Кристаллография и фотоника» Российской академии наук (ФНИЦ КиФ РАН) с кооперацией, который предоставляет 30% образцов белков для эксперимента, а также с использованием российской транспортной космической инфраструктуры и специализированных лабораторных помещений в Москве и на космодроме Байконур в Казахстане. Координацию работ российских ученых обеспечивает ЦНИИмаш Госкорпорации «Роскосмос», а техническую реализацию проекта с российской стороны — РКК «Энергия». В период с июля 2009 по 2017 г. в рамках этого проекта была выполнена серия из двенадцати экспериментов, продолженная новой серией исследований, реализуемой до 2020 г. с частотой два эксперимента в год. Главная цель партнеров в этом проекте — с использованием результатов, полученных в космосе, создать продукты в области медицины, биологии и защиты окружающей среды для социальных нужд на Земле.
2. летное оборудование
Для выполнения проекта PCG в JAXA разработаны и применяются восемь типов кристаллизационных устройств, а именно: GCB–GT [12], JCB–HD [13], JCB–MC , JCB–SGT [3, 4], JCB–SLC , JCB–SGT (с нанопокрытием из золота), JCB–OT [4] и JCB–LCP . Устройства несколько отличаются друг от друга с точки зрения конструктивного исполнения, но идентичны в отношении реализации философии эксперимента, которая может быть ясно проиллюстрирована на примере использования устройств кристаллизации GCB–GT . Принцип работы устройства GCB-GT схематически показан на рис. 1.
Устройство GCB–GT состоит из следующих элементов: a — резервуар; b — направляющий держатель капилляров; c — крышка; d — капилляр; e — гель-трубка. Первые три элемента изготовлены из полистирола. Резервуар заполняется раствором осадителя до ~80% высоты (шаг 1 на рис. 1). Капилляры заполняются раствором белка и закрепляются в гель-трубках (шаг 2). Верхний конец капилляра запечатывается герметиком — силиконовым или глиной (шаг 3). Каждый капилляр устанавливается в отверстие направляющего держателя (шаг 4). Комплект капилляров в держателе размещается в резервуаре (шаг 5). Для удобства при реализации этого метода нижняя направляющая держателя не используется. В обеспечение герметичности бокса по газу, одна из щелей между направляющим держателем и резервуаром запечатывается силиконовым герметиком (шаг 6). В верхнюю часть резервуара помещается трехпроцентный раствор агарозы (шаг 7) и желируется. Раствор осадителя вливается через другую щель между направляющим держателем и резервуаром (шаг 8). Затем эта щель запечатывается силиконовым герметиком (шаг 9). 3%-ный раствор агарозы заливается на силиконовую смазку (шаг 10). Верхний конец капилляра запечатывается с помощью аральдита. Для поддержания капилляров в стабильном положении в верхней части резервуара размещается небольшой брусок из уретанового пеноматериала соответствующего размера (шаг 11). После этого резервуар закрывается крышкой с использованием силиконового герметика, а затем собранный контейнер герметично запечатывается тефлоновой лентой (шаг 12).
Момент времени, в который реально начинается эксперимент по кристаллизации, определяется длиной гель-трубки, через которую осадитель диффундирует до достижения им раствора белка. Этот промежуток времени может регулироваться вплоть до семи дней.
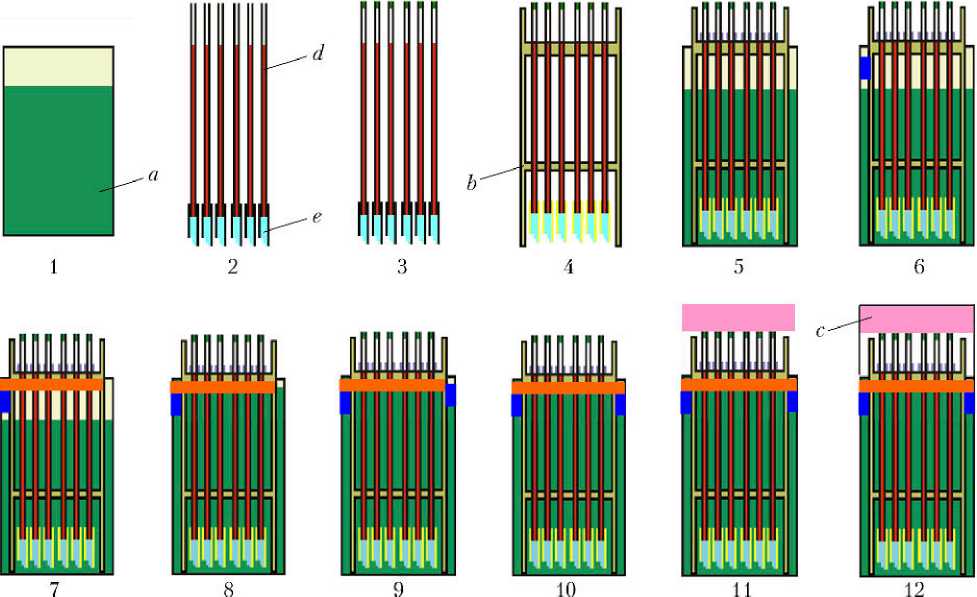
Рис. 1. Метод гель-трубок (описание — в тексте)
Устройство JCB–HD является модифицированной версией GCB–GT с уплотненной укладкой капилляров и использует блок кристаллизации HD [13]. Было бы уместным отметить, что устройство кристаллизации JCB–HD обеспечивает использование до двенадцати (в случае GCB–GT — шести) капилляров с максимальным диаметром 1 мм. Для активации оборудования не требуется использование каких-либо подвижных элементов в его конструкции или подачи электропитания.
JCB–MC является реализацией новой концепции устройства для кристаллизации белков [6]. Так называемый «чип» из материала PDMS (полидиметилсилоксан) заполняется гелем агарозы, раствором белка и раствором осадителя, с верхней стороны покрывается специальной пластиной, одно или два устройства кристаллизации MC размещаются в контейнере JCB ( JCB–MC , шаг 1 на рис. 2).
Два устройства кристаллизации HD комбинируются, фиксируются тефлоновой лентой и также размещаются в корпусе JCB ( JCB–HD , шаг 1 на рис. 2). Три или четыре сборки JCB–HD или JCB–MC и одна крышка JCB собираются вместе и скрепляются тефлоновой лентой (шаг 3 ).
Эта сборка помещается во внутренний защитный мешок (блок JCB , шаг 4 на рис. 2).
Для выполнения последних экспериментов PCG (после 2016 г.) блок JCB был существенно усовершенствован с целью повышения эффективности его использования на орбите, однако, все методы предполетной подготовки оборудования были сохранены (как это показано на рис. 2). Внутренний и внешний защитные мешки используются для упаковки любых типов кристаллизационных устройств в обеспечение надежного контроля загрязнений и удовлетворения требований по безопасности при транспортировке оборудования в составе транспортных грузовых и пилотируемых кораблей.
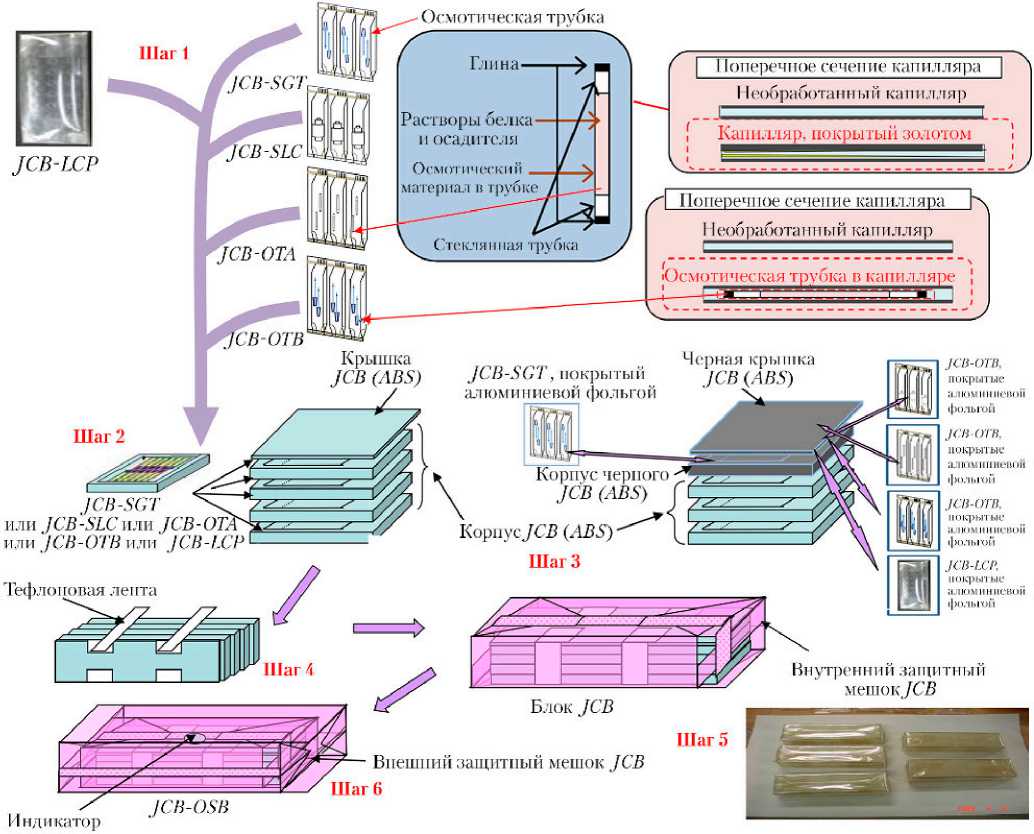
С самого начала реализации проекта PCG наряду с использованием устройства JCB–HD последовательно находило все более широкое применение кристаллизационное оборудование JCB–SGT [3, 4]. Это — простые, многофункциональные устройства, имеющие прозрачный контейнер (показан на рис. 3), изготовленный из листа полиэтилен-терефталата ( PET ) TECHBARRIER (корпорация Mitsubishi Chemical ). Поскольку он непроницаем для газа, раствор в контейнере может сохраняться годы.
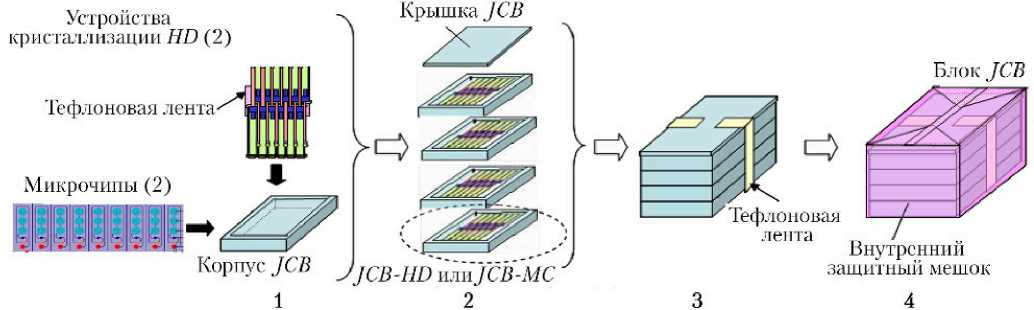
Рис. 2. Процесс предполетной подготовки блока JCB (описание представлено в тексте)

Рис. 3. Процесс предполетной подготовки устройства JCB–SGT: 1 — ячейки JCB–SGT заполняются раствором осадителя; 2 — в капилляр вводится раствор белка, капилляр помещается в ячейку JCB–SGT; 3 — JCB–SGT запечатывается с помощью термоустройства; 4 — запечатанная ячейка JCB–SGT; 5 — две ячейки JCB–SGT вместе укладываются в контейнер JCB; 6 — четыре контейнера JCB (содержащие восемь JCB–SGT) и одна крышка JCB соединяются тефлоновой лентой. Сборка помещается во внутренний защитный мешок
По состоянию на 2019 г. при проведении экспериментов на МКС используется, преимущественно, два типа устройств JCB–SGT : с тремя ячейками, обеспечивающее размещение двух капилляров в каждой ячейке; и с шестью ячейками, где в каждой ячейке размещается один капилляр. Эффективность использования объема такая же, как и в случае JCB–HD. В проекте PCG в устройстве JCB–SGT могут быть размещены различные типы капилляров, используемые для проведения кристаллизации различными методами (рис. 4): прямой капилляр для реализации классического метода встречной диффузии; осмос-трубка с использованием осмотического давления; капилляр большого диаметра ( Large-bore Capillary — LC ) для выращивания больших кристаллов методом встречной диффузии; капилляр большого диаметра с диализной мембраной ( Large-bore Capillary with Dialysis Membrane — LCDM ) для реализации диализного метода, а также диффузионная пара LC ( LC–LC ) для работы методом встречной диффузии с уменьшенным количеством кристаллизационного раствора.
I 'Komi 1аупд 1
Крышка АВС Капилляр Раствор белка Гель-трубка
а)
Силиконовая трубка Воздушная полость

Капилляр большого диаметра 1,95 мм в)
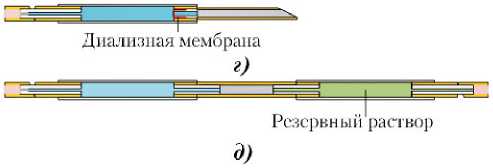
Рис. 4. Конфигурация различных типов капилляров, применяемых в JCB–SGT для исследований по проекту PCG: а — прямой капилляр; б — осмос-трубка; в — капилляр большого диаметра; г — капилляр большого диаметра с диализной мембраной; д — диффузионная пара LC–LC
Капилляры большого диаметра (LC, LCDM и LC–LC) были введены в состав оборудования из-за высокого спроса исследователей на кристаллы большой размерности, полученные в условиях микрогравитации. В особенности полезным для выращивания больших кристаллов белков оказался LCDM, поскольку диализная мембрана уменьшает потерю молекул белка в кристаллизационной ячейке. Диффузионная пара LC–LC применяется в том случае, когда объем кристаллизационного раствора должен быть уменьшен, поскольку содержит дорогостоящие реагенты.
Ряд исследователей желает выращивать кристаллы в условиях микрогравитации в бескислородной среде. Для этой цели используется устройство JCB–SGT DX , являющееся производным от JCB–SGT . Одна сторона JCB–SGT DX изготавливается из материала AGELESS OMAC (компании Mitsubishi Gas Chemical ), поглощающего кислород, так что уровень последнего в кристаллизационной ячейке эффективно уменьшается.
Полностью технологический цикл предполетной подготовки блока JCB , включающего различные типы устройств кристаллизации, представлен на рис. 5.
Как только блоки JCB заполняются образцами и запечатываются во внешние защитные мешки, они укладываются в алюминиевый контейнер, оснащенный регистратором температуры и показанный на рис. 6 и 7. Объем контейнера составляет 150×100×65 мм3. На контейнер надевается вставка термоизолятора, и эта сборка помещается в сумку контейнера — сумку из материала «Номекс» оранжевого цвета (в состав которой входят плоские листы теплоизоляционного материала, вставленного между слоями ткани «Номекс»). Сумка предназначена для хранения контейнера на МКС и его возврата на Землю в составе спускаемого аппарата транспортного корабля «Союз». Затем сумка контейнера помещается в пеноматериал размером 265×215×180 мм, и эта сборка упаковывается в полетную сумку, изготовленную из материала «Номекс» синего цвета. Она предназначена для доставки оборудования PCG на МКС в составе кораблей «Прогресс» или «Союз» (рис. 8). Полетная сумка, имеющая массу ~2,8 кг, передается от JAXA персоналу РКК «Энергия» для укладки на борт транспортного корабля за 10–12 ч до запуска.
Конструкция оборудования PCG обеспечивает три барьера безопасности и удовлетворяет требованиям по безопасности Программы МКС [16, 17].
влажности
Рис. 5. Процесс предполетной подготовки блока JCB
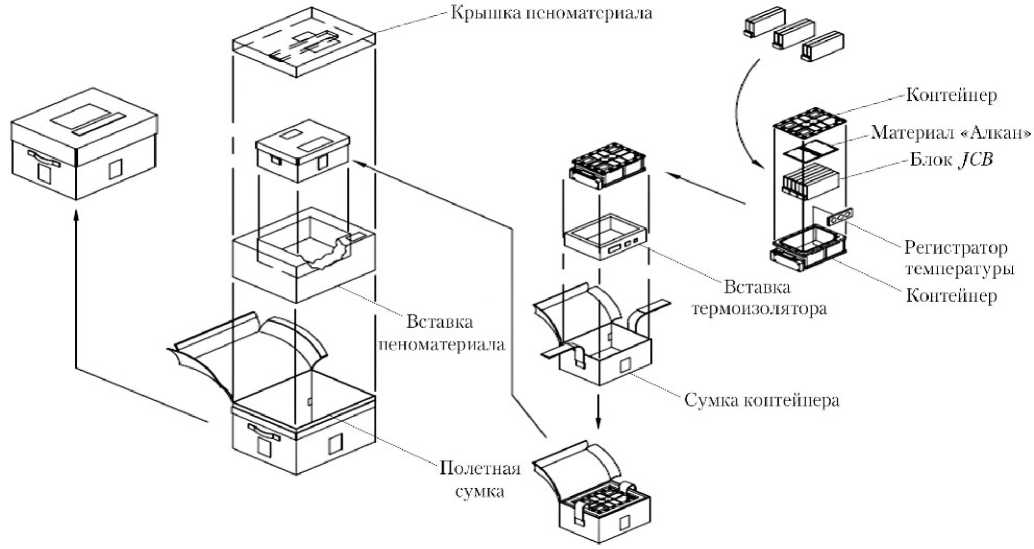
Рис. 6. Сборка JAXA PCG


б)
Рис. 7. Внутренний (a) и внешний (б) виды контейнера: образцы находятся внутри (фото JAXA)


a)
б)
в)

г) д)

е)
ж)
з)
Рис. 8. Сумка контейнера с контейнером и вставкой термоизолятора (a–г); полетная сумка и пеноматериалы (д); сумка контейнера со вставкой пеноматериала и крышкой пеноматериала в полетной сумке (е–ж); полетная конфигурация оборудования PCG (з)
3. работа на байконуре
Предполетная подготовка аппаратуры PCG на Байконуре проводится в специальной биотехнологической лаборатории в течение 3–5 дней перед запуском соответствующего корабля «Прогресс» или «Союз». Лаборатория удовлетворяет требуемому уровню чистоты помещения и оснащена всем необходимым оборудованием и инструментарием в обеспечение подготовки эксперимента, а именно: системой кондиционирования воздуха, холодильниками, специальными биотехнологическими инкубаторами, стандартным лабораторным оборудованием, ламинарными шкафами, низкотемпературными морозильниками и др.
Процесс предполетной подготовки начинается с входного контроля летного оборудования, которое доставляется на Байконур РКК «Энергия» заблаговременно. При этом образцы белков и иные биологические и химические материалы, необходимые для заправки оборудования, доставляются на Байконур специалистами JAXA приблизительно за неделю до запуска транспортного корабля.
После загрузки блоков JCB с образцами протеинов в контейнер, которая выполняется персоналом JAXA , оборудование проходит испытание на герметичность с участием российских специалистов (рис. 9). После этого контейнер вместе со вставкой термоизолятора укладывается в сумку контейнера (оранжевая сумка из «Номекса»). Эта сумка, в свою очередь, упаковывается в пенома-териал (вставка с крышкой) и укладывается в полетную сумку (синяя сумка из «Номекса»), на которую наносится маркировка «Launch Bag (полетная сумка) / JAXA PCG Canister, Аппаратура JAXA PCG» (рис. 7, 8).
Представители РКК «Энергия» укладывают полетные сумки на борту транспортных кораблей «Прогресс» или «Союз» для доставки на МКС с соблюдением их требуемой ориентации (стрелки g маркировки на полетной сумке должны быть ориентированы по направлению силы тяжести), как это видно на рис. 10. Во время транспортировки оборудования кораблями «Прогресс» или «Союз» к МКС его технического обслуживания не требуется. Оборудование JAXA PCG — многократного использования.

a)

б)
Рис. 9. Специалисты JAXA производят сборку контейнера в лаборатории на Байконуре (a). Испытания контейнеров на герметичность (б) (фото РКК «Энергия»)

Рис. 10. Полетные сумки JAXA PCG внутри отсека срочных грузов транспортного грузового корабля «Прогресс» на стартовом комплексе космодрома Байконур
4. бортовые операции и оборудование на мкС. возврат образцов
После стыковки транспортного корабля с МКС российские члены экипажа станции извлекают из него оборудование PCG и передают полетные сумки членам экипажа Американского сегмента (АС). Они извлекают сумки контейнеров из полетных сумок, а затем извлекают контейнеры из сумок контейнеров и вставок термоизоляторов. После этого контейнеры устанавливаются в исследовательскую установку по кристаллизации белков (Protein Crystallization Research Facility — PCRF) на борту модуля Kibo под контролем экипажа АС (рис. 11, a). Контейнеры хранятся в установке PCRF без вмешательства экипажа в ход эксперимента. Работа российских космонавтов и экипажа АС тщательно синхронизируется из Центров управления полетом в Москве (ЦУП-M), Хьюстоне (ЦУП-Х) и Цукубе (ЦУП-Ц).
Эксперимент начинается по команде из ЦУП-Ц, как только контейнеры фиксируются внутри установки PCRF . В ходе него температура контейнеров поддерживается в диапазоне +20±2 ° C. Данные о температуре передаются на Землю в реальном времени и контролируются ЦУП-Ц.
Приблизительно за 15 ч до прекращения эксперимента вставки термоизоляторов из сумок контейнеров должны быть охлаждены до +15 °C (до отверждения находящегося в них термостабилизирующего вещества) в экспериментальной установке по клеточной биологии (Cell Biology Experiment Facility — CBEF), показанной на рис. 11, б. За проведение этой операции отвечает экипаж АС МКС.
После завершения эксперимента, за минимально возможное время до закрытия переходного люка в корабль «Союз» (за 6 ч, но не более чем в течение 32 ч), члены экипажа АС МКС извлекают контейнеры из установки PCRF . На них надеваются вставки термоизоляторов, а затем сборки помещаются в сумки контейнеров оранжевого цвета и передаются экипажу РС МКС. Российские члены экипажа инспектируют процесс упаковки оборудования. Затем участники работы с обеих сторон проводят проверку его готовности к возвращению в соответствии с бортовой документацией. Работа членов экипажа АС и РС МКС синхронизируется из ЦУП-М, ЦУП-Х и ЦУП-Ц.


a) б)
Рис. 11. Установка PCRF, входящая в состав стойки Ryutai на модуле Kibo МКС (a), а также установка CBEF, входящая в состав стойки Saibo модуля Kibo МКС (б) (фото JAXA)
После посадки спускаемого аппарата корабля «Союз» оборудование извлекается из него непосредственно на месте посадки специалистами РКК «Энергия» и в этот же день передается представителям JAXA в Москве для транспортировки в Японию. Российская часть возвращенных образцов передается JAXA российским коллегам в Москве во ФНИЦ КиФ РАН.
-
5. изучение возвращенных образцов и некоторые примеры полученных результатов
-
5.1. Примеры результатов, полученных группой исследователей из Японии. В ходе исследований получен ряд положительных результатов в части улучшения разрешающей способности при рентгеноструктурном анализе кристаллов белков [18–42]. Японскими учеными были получены кристаллы более 10 белков, которые до реализации проекта PCG либо вовсе не кристаллизовались, либо их структура ранее не была определена, но в ходе исследований по проекту качественные данные по их молекулярной структуре были получены. Более того, в результате исследований полученных в космосе кристаллов часто удавалось выявить связывающие моды ( the binding mode ) целевых белков и белков-кандидатов для разработки лекарственных препаратов.
Полученные кристаллы передаются исследователям в России и Японии. Так как кристаллы растут в капиллярах, они должны быть осторожно из этих капилляров извлечены и заморожены. После замораживания кристаллы подвергаются рентгеноструктурному анализу на синхротроне, таком как SPring -8 в Японии.
На рис. 12 в сравнении представлены фотографии кристаллов Alpha-amylase и Haematopoietic prostaglandin D synthase (HPGDS), выращенных в космосе и на Земле. Кристаллы Alpha-amylase, выращенные в космосе — красивые единичные кристаллы, дифрагирующие с разрешением лучше 0,9Å. В то же время, в земных условиях кристаллы этого белка формируются в кластеры, дающие при рентгеноструктурном анализе меньшее максимальное разрешение, чем кристаллы «космические». Выращенные в космосе кристаллы HPGDS также оказались больше, чем выращенные на Земле, и есть все основания считать, что данные, полученные при их рентгено- структурном анализе, внесут вклад в объяснение механизма молекулярного опознавания этого белка. HPGDS обеспечивает синтез Prostaglandin D2, который вовлечен в процессы, связанные с развитием некроза мышечной ткани при проявлениях мышечной дистрофии Дюшена (Duchenne muscular dystrophy — DMD). Поскольку HPGDS является ингибитором этих процессов и мог бы способствовать эффективной терапии DMD, в условиях микрогравитации были проведены эксперименты по кристал- лизации различных на основе HPGDS мация, полученная используется при типов ингибиторов [23–25], а инфороб их структуре, разработке новых
лекарственных препаратов.
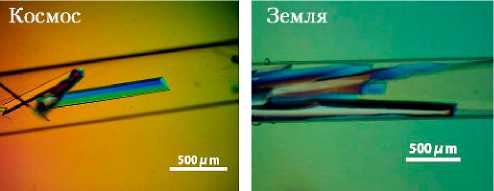
a)
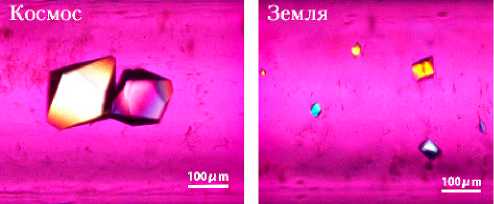
б)
Рис. 12. Кристаллы, выращенные в условиях микрогравитации: а — кристаллы Alpha-amylase, выращенные в космосе (слева) и в земных условиях (справа); б — кристаллы Haematopoietic prostaglandin D synthase, выращенные в космосе (слева) и в земных условиях (справа) (фото JAXA)
Еще один значимый результат экспериментов по получению высококачественных кристаллов белков в космосе относится к разработке лекарственных препаратов по лечению заболеваний периодонтальной области [30, 32, 42]. Белки DAP BII (DPP7) и DPP11 являются ферментами, которые принадлежат к семейству пептидазы S46, оказывающей существенное влияние на рост патогенов. Эти ферменты не найдены в человеческом организме, проявляя себя лишь в микроорганизмах, и в таком случае разработка лекарственных препаратов, которые обеспечивают подавление функций этих ферментов, дает возможность безопасно для человеческого организма лишь подавлять развитие бактерий. В обеспечение разработки эффективного антимикробиального препарата в условиях микрогравитации проводилась кристаллизация ряда веществ, которые ингибируют функции DPP. В синхронном наземном эксперименте были получены кристаллы DAP BII, которые дифрагировали с разрешением лишь 3,4 Å, в то время как кристаллы, выращенные в космосе, обеспечивали разрешение 1,95 Å, давая тем самым возможность детального исследования механизма распознавания пептидов [30, 42]. Кристаллы DPP11, полученные в наземном эксперименте, дифрагировали с разрешением 2,46 Å, а кристаллы этого белка, полученные в космосе, обеспечили разрешение 1,66 Å [32, 42]. Изучение их детальной структуры выявило центр связывания субстрата и то, что вблизи этого центра связывания расположена молекула воды. Это дает возможность исследователям поставить перед собой задачу разработки новых антибиотиков на основе SBDD, используя знания трехмерной структуры этих двух ферментов.
-
5.2. Примеры результатов, полученные российской группой исследователей. Для проведения исследований на борту МКС в период 2009–2017 гг. российскими учеными использованы 78 белков, из которых для 64 удалось получить кристаллы. В ходе послеполетных исследований решены пространственные структуры для 105 природных белков, их мутантных форм и их комплексов с функциональными лигандами. По результатам исследований в Международный банк белковых данных ( Protein Data Bank, https://www.rcsb.org ) депонированы координаты более 40 белковых структур [43–67].
Проведенные эксперименты показали, что используемая технология кристаллизации в невесомости методом встречной диффузии позволяет избежать двойникования кристаллов, приводит к увеличению размера кристаллов и повышает их дифракционное качество (таблица). Координаты, уточненные при более высоком разрешении, позволили построить более точные атомные модели, улучшить точность расчетов при их использовании в качестве стартовых для методов компьютерного моделирования, которые все шире применяются при исследовании функционирования макромолекул.
Большой цикл исследований проведен для таких ферментов и их комплексов с ключевыми лигандами, как фосфо-пантетеинаденилтрансфераза Mycobacterium tuberculosis ( PPAT Mt ), карбоксипептидазы Т и В , тимидинфосфорилаза [51].
Выяснение структурных основ селективности является одной из фундаментальных задач инженерной энзимологии, решение которой создаст предпосылки для рационального конструирования ферментов с заданными свойствами. Для белков семейства карбоксипептидаз (карбоксипептидазы В , Т и их мутантных форм) исследованы структурные детерминанты селективности и получены мутантные формы с измененной селективностью, имеющие на порядок большую избирательность к положительно заряженным субстратам, чем исходный фермент [44, 50, 54, 60–62, 69–71]. Найденные мутантные формы способны усовершенствовать и удешевить биотехнологические процессы, используемые, в частности, для производства инсулина.
С использованием координат белков-мишеней (фосфопантетеинаденилилтранс-феразы ( РРАТ ), имидазолглицеролфосфат-дегидратазы ( HisB ), пирофосфатазы ( PPase ) из M. tuberculosis, пуриннуклеозидфос-форилазы ( PNP )) методом молекулярной динамики были найдены селективные ингибиторы, пригодные в качестве лидерных соединений для разработки антитуберкулезных, антиопухолевых и антивирусных лекарственных средств [47, 58, 65, 68, 72–74].
Пуриннуклеозидфосфорилаза из E. сoli имеет большой медицинский потенциал для лечения рака. Трансфекция гена PNP в клетки опухоли позволяет активировать аналоги нуклеозидов до их цитотоксической формы. В условиях невесомости удалось получить кристаллы PNP из E. coli, дифрагирующие до 0,99 Å, что позволило детально изучить структуру фермента [58] (рис. 13, 1). Полученные с высокой точностью координаты модели, депонированные в банк данных (4RJ2), могут служить надежной структурной основой при конструировании ингибиторов фермента методом SBDD, а также пригодны для компьютерного моделирования катализируемой ферментом реакции.
Сводная статистика ряда характеристик кристаллов, использованных в космическом эксперименте «кристаллизатор» / JAXA PCG с 2009 по 2015 гг. [75–80]
|
Белок |
Максимальная размерность кристалла, мкм |
Лучшее разрешение, Е |
Мозаичность |
Двойникование |
|
Пуриннуклеозидфосфорилаза из E. coli |
300 (200) |
0,99 (1,50) |
0,1 (0,3) |
Нет (Да) |
|
Фосфопантетеинаденилилтрансфераза из M. tuberculosis |
400 (400) |
1,59 (2,10) |
0,8 (1,6) |
Нет (Нет) |
|
Тимидинфосфорилаза |
300 (300) |
1,50 (2,20) |
0,4 (0,6) |
Нет (Нет) |
|
Фосфорибозилпирофосфатсинтаза |
100 (100) |
2,71 (>4) |
1,8 (–) |
Нет (Нет) |
|
Карбоксипептидаза В |
200 (50) |
1,25 (1,60) |
0,2 (0,7) |
Нет (Да) |
|
Карбоксипептидаза Т |
300 (300) |
1,29 (2,10) |
0,3 (0,5) |
Нет (Нет) |
|
Уридинфосфорилаза из S. oneidensis |
500 (300) |
0,93 (1,6) |
0,3 (0,5) |
Да (Да)* |
|
BTB -домен белка CP 190 |
300 (200) |
1,4 (2,2) |
0,35 (1,6) |
Нет (Нет) |
|
Цитохром с нитритредуктаза из Tv. paradixus |
500 (500) |
1,6 (1,6) |
0,07 (0,1) |
Нет (Да) |
|
НАД-зависимая формиатдегидрогеназа из S. aureus |
100 (100) |
2,0 (2,2) |
0,4 (0,2) |
Нет (Нет) |
|
Гистоноподобный HU -белок из M. gallicepticum |
500 (400) |
4,0 (>5,0) |
1,1 (н,д,) |
Нет (Нет) |
|
Альдегиддегидрогеназа из Pyrobacullum sp 1147 |
300 (300) |
1,9 (2,2) |
0,15 (0,5) |
Нет (Нет) |
|
Xaa-pro аминопептидаза из T. sibiricus 0821 |
100 (200) |
2,3 (2,6) |
0,4 (0,8) |
Нет (Нет) |
|
Белок DJ -1 из H. sapiens |
300 (400) |
1,2 (1,2) |
0,2 (0,3) |
Нет (Нет) |
|
Белок с неизвестной функцией 2 Q 02 из S. typhimurium |
100 (100) |
1,6 (1,7) |
0,4 (0,7) |
Нет (Нет) |
|
Бета-гликозидаза из A. sacharovoronas |
400 (300) |
1,80 (2,0) |
0,9 (1,7) |
Нет (Нет) |
|
Пирофосфатаза из M. tuberculosis |
300(200) |
1,4(1,6) |
0,25 (0,4) |
Нет (Нет) |
Примечание. Для краткости приведены данные только для тех белков, у которых имелось улучшение характеристик кристаллов. В скобках указаны данные для параллельного наземного эксперимента. * — для кристаллов данного фермента степень двойникования в условиях микрогравитации была ниже по сравнению с наземными экспериментами.
Для белка алкогольдегидрогеназы 1998 из термофильной археи Thermococcus sibiricus кристаллы удалось вырастить только в космосе, и их структуры получены впервые. Этот белок интересен тем, что обладает высокой термостабильностью (время потери половины активности фермента составляет один час при инкубировании при температуре 100 ° С), имеет широкую субстратную специфичность, что представляет интерес для использования в биотехнологической промышленности для органического синтеза биологически активных веществ.
Впервые была установлена пространственная структура неорганической пирофосфатазы M. tuberculosis с ионами металлов (Mg) с высоким разрешением (1,65 Å), и определена структура апофор-мы пирофосфатазы E. coli, выращенной в присутствии цитрата (1,4 Å). Несмотря на то, что структура этого фермента известна, не было никакой структурной информации о каком-либо способе связывания ингибитора. Было показано, что структура пирофосфатазы M. tuberculosis с цитратом может служить основой для разработки ингибиторов этого фермента, являясь мишенью для разработки тагетных противотуберкулезных препаратов [68].
Для белка цитохром с нитритре-дуктазы из гипертермофильной бактерии Thioalkalivibrio paradoxus в рамках космического эксперимента впервые удалось получить кристаллы без двойникования, что положительным образом сказалось на качестве полученной структуры.
Для уридинфосфорилазы из Shewanella oneidensi получены данные с ультравы-соким разрешением — 0,93 Å (рис. 13, 2). Ранее, в земных экспериментах, были получены кристаллы, дифрагирующие не выше 1,7 Å [46, 67]. Полученные в космическом эксперименте данные позволяют получить новые знания в части организации тонкой структуры активного центра фермента, так как делают возможным определение положений отдельных атомов водорода, что невозможно при разрешении выше 1 Å. Это перспективный фермент для создания биокатализаторов биотехнологического синтеза нуклеозидов — потенциальных противовирусных и противораковых лекарств.
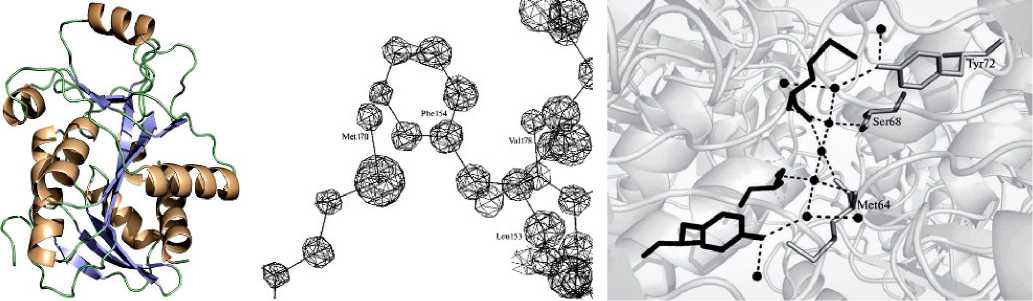
1а 1b 1с
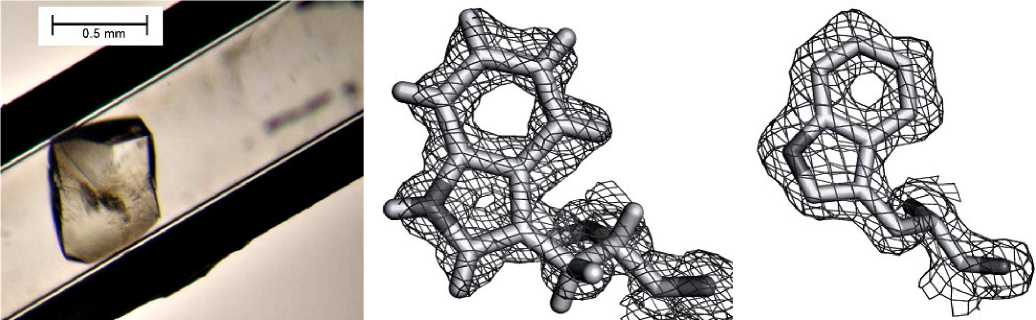
2а 2b 2с
Рис. 13. Примеры результатов исследования белковых структур: 1 — фрагменты пространственной структуры пуриннуклеозидфосфорилазы из E. coli [59]: а — одна из субъединиц гексамерной молекулы PNP; b — фрагмент карты электронной плотности; c — водный кластер между двумя субъединицами; 2 — кристаллы уридинфосфорилазы [46]: а — кристаллы из бактерии S. Oneidensis (0,5 мм), выращенные в космосе; b, c — вид картин электронной плотности для структуры атомного разрешения (слева — от кристаллов, выращенных в космосе, справа — на Земле)
6. заключение
Международное сотрудничество в ходе целевого использования МКС расширило доступ исследователей из различных стран к уникальным научным аппаратурным комплексам, размещенным на станции, а также обеспечило усиление значимости научных исследований, проводимых на МКС. Правительства и космические агентства-партнеры Программы МКС приняли решение о продлении полета международной станции до 2024 г., в то же время, гарантий дальнейшего его продления пока нет. В этих условиях продолжающееся расширение и углубление международного сотрудничества партнеров по Программе обеспечивает использование полученных на МКС научных результатов для блага людей на Земле [81]. Серия российско-японских экспериментов по высококачественной кристаллизации протеинов в условиях микрогравитации на борту МКС является составной частью этого важного процесса.
Список литературы Технология получения высококачественных кристаллов белков на МКС при выполнении совместных российско-японских экспериментов
- Garcia-Ruiz J.M., Moreno A. Investigations on protein crystal growth by the gel acupuncture method // Acta Cryst. 1994. V. D50. P. 484-490.
- Garcia-Ruiz J.M., Gonzalez-Ramirez L.A., Gavira J.A., Otalora F. Granada Crystallization Box: a new device for protein crystallization by counter-diffusion techniques // Acta Cryst. 2002. V. D58. P. 1638-1641.
- Yoshizaki I., Yamada M., Iwata M., Kato M, Kihira K., Ishida T., Wada Y, Nagao S. Recent Advance in High Quality Protein Crystal Growth Experiment on the International Space Station by JAXA // Int. J. Microgravity Sci. Appl. 2019. V. 36 (1). 360101.
- Takahashi S., Koga M., Yan B., Furubayashi N, Kamo M., Inaka K., Tanaka H. JCB-SGT crystallization devices applicable to PCG experiments and their crystallization conditions // Int. J. Microgravity Sci. Appl. 2019. V. 36 (1). 360107.
- Lopez-Jaramillo F.J., Otalora F, and Gavira J.A. Protein crystal quality in diffusive environments and its evaluation // J. Cryst. Growth. 2003. № 247. P. 177-184.
- Sorokin I., Yamada M., Kihira K., Nagao S, Fujii T., Takahashi S, Tanaka H., Inaka K., Yoshizaki I. Space support of drug design: Japanese-Russian collaboration over high-quality protein crystals growing aboard the ISS // 32nd International Symposium on Space Technology and Science (ISTS-32), 15-22 June 2019, Fukui City, Japan. 2019. P. 21.
- Garcia-Ruiz J.M., Otalora F, Novella M.L., Gavira J A, Sauter C, Vidal O. A supersaturation wave of protein crystallization // J. Cryst. Growth. 2001. № 232.P. 149-155.
- Vergara A., Lorber B., Zagari A., Giege R. Physical aspects of protein crystal growth investigated with the Advanced Protein Crystallization Facility in reduced-gravity environments // Acta Cryst. 2003. V. D59. P. 2-15.
- Sauter C, Otalora F, Gavira J.A., Vidal O, Giege R., Garcia-Ruiz J.M. Structure of tetragonal hen egg-white lysozyme at 0,94 A from crystals grown by the counter-diffusion method // Acta Cryst. 2001. V. D57. P. 1119-1126.
- Garcia-Ruiz J.M., Novella M.L., Moreno R., Gavira J.A. Agarose as crystallization media for proteins I: Transport processes // J. Cryst. Growth. 2001. № 232. P. 165-172.
- Gavira J.A., Garcia-Ruiz J.M. Agarose as crystallisation media for proteins II: trapping of gel fibres into the crystals // Acta Cryst. 2002. V. D58. P. 1653-1656.
- Tanaka H, Inaka K., Sugiyama S., Takahashi S., Sano S., Sato M., Yoshitomi S. A simplified counter diffusion method combined with a 1D simulation program for optimizing crystallization conditions // J. Synchrotron Rad. 2004. V. 11. P. 45.
- Sato M, Tanaka H, Inaka K, Shinozaki S, Yamanaka A., Takahashi S., Yamanaka M., Hirota E., Sugiyama S., Kato M., Saito C, Sano S., Motohara M., Nakamura T., Kobayashi T, Yoshitomi S, Tanaka T. JAXA-GCF project — High-quality protein crystals grown under microgravity environment for better understanding of protein structure // Microgravity sci. technol. 2006. V. XVIII-3/4. P. 5-10.
- Sorokin I., Nakamura T, Kato M, Saito C. Japanese-Russian cooperation on International Space Station now and in the future //IAC Paper IAC-05-B4.3.03, 2005.
- Takahashi S, Ohta K., Furubayashi N, Yan B., Koga M., Wada Y., Yamada M, Inaka K, Tanaka H., Miyoshi H, Kobayashi T., Kamigaichi S. JAXA protein crystallization in space: ongoing improvements for growing high-quality crystals // J. Synchrotron Rad. 2013. V. 20. P. 968-973.
- SSP 51700 «Payload safety policy and requirements for the International Space Station».
- SSP50094B «NASA/RSA joint specifications standards document for the ISS Russian Segment».
- Kinoshita T., Maruki R., Warizaya M., Nakajima H., Nishimura S. Structure of a high-resolution crystal form of human trisephosphate isomerase: improvement of crystals using the gel-tube method // Acta Cryst. 2005. V. F61. P. 346-349.
- Kitatani T., Nakamura Y, Wada K, Kinoshita T, Tamoi M., Shigeoka S., Tada T. Structure of apo-glyceraldehyde-3-phosphate dehydrogenase from Synechococcus PCC7942 // Acta Cryst. 2006. V. F62. P. 727-730.
- Tanaka H, Umehara T, Inaka K, Takahashi S., Shibata R., Bessho Y, Sato M., Sugiyama S, Fusatomi E, Terada T, Shirouzu M., Sano S., Motohara M., Kobayashi T., Tanaka T., Tanaka A., Yokoyama S. Crystallization of the archaeal transcription termination factor NusA: a significant decrease in twinning under microgravity condition // Acta Cryst. 2007. V. F63. P. 69-73.
- Meyer A., Rypniewski W, Szymanski M., Voelter W, Barciszewski J., Betzel C. Structure of mistletoe lectin I from Viscum album in complex with the phytohormone zeatin // Biophysica Biophysica Acta. 2008. V. 1784. P. 1590-1595.
- Oda K., Matoba Y., Noda M, Kumagai T., Sugiyama M. Catalytic Mechanism of bleomycin N-acetyltransferase proposed on the basis of its crystal structure // J. Biol. Chem. 2010. V. 285(2). P. 1446-1456.
- Takahashi S., Tsurumur, T., Aritake K., Furubayashi N, Sato M, Yamanaka M, Hirota E, Sano S., Kobayashi T, Tanaka T, Inaka K, Tanaka H,. Urade Y. High-quality crystals of human haematopoietic prostaglandin D synthase with novel inhibitors // Acta Crystallographica. 2010. Section F: Structural Biology and Crystallization Communications, V. 66 (Pt. 7). P. 846-850.
- Tanaka H., Tsurumura T, Aritake K., Furubayashi N., Takahashi S., Yamanaka M., Hirota E., Sano S., Sato M., Kobayashi T., Tanaka T., Inaka K., Urade Y. Improvement in the quality of hematopoietic prostaglandin D synthase crystals in a microgravity environment // J. Synchrotron Rad. 2011. V. 18. P. 88-91.
- Inaka K., Takahashi S., Aritake K., Tsurumura T., Furubayashi N, Yan B., Hirota E., Sano S., Sato M., Kobayashi T., Yoshimura Y., Tanaka H., Urade Y. High-quality protein crystal growth of mouse lipocalin-type prostaglandin D synthase in microgravity // Cryst. Growth Des. 2011. V. 11. P. 2107-2111.
- Nakano H., Hoshokawa A., Tagawa R., Inaka K., Ohta K., Nakatsu T., Kato H., Watanabe K. Crystallization and preliminary X-ray crystallographic analysis of Pz peptidase B from Geobacillus collagenovorans MO-1 // Acta Cryst. 2012. V. F68. P. 757-759.
- Malecki P.H., Rypniewski W., Szymanski M., Barciszewski J., Meyer A. Binding of the plant hormone kinetin in the active site of Mistletoe Lectin I from Viscum album // Biochimica et Biophysica Acta. 2012. V. 1824. P. 334-338.
- Yoshikawa S., Kukimoto-Niino M., Parker L., Handa N, Terada T, Fujimoto T., Terazawa Y., Wakiyama M., Sato M., Sano S., Kobayashi T., Tanaka T., Chen L, Liu Z-J., Wang B-C, Shirouzu M., Kawa S, Semba K., Yamamoto T., Yokoyama S. Structural basis for the altered drug sensitivities of non-small cell lung cancer-associated mutants of human epidermal growth factor receptor // Oncogene. 2013. V. 32. P. 27-38.
- Aris S.N.A.M., Chor A.L.T., Ali M.S.M., Basri M., Salleh A.B., Rahman R.N.Z.R.A. Crystallographic analysis of ground and space thermostable T1 lipase crystal obtained via counter diffusion method approach // BioMed Research International. 2014. 904381.
- Sakamoto Y, Suzuki Y, Iizuka I., Tateoka C., Roppongi S, Fujimoto M., Inaka K, Tanaka H, Masaki M, Ohta K., Okada H, Nonaka T, Morikawa Y., Nakamura K.T., Ogasawara W., Tanaka N. S46 peptidases are the first exopeptidases to be members of Clan PA // Scientific Reports. 2014. V. 4. P. 4977.
- Nakamura A., Ishida T, Kusaka K, Yamada T, Fushinobu S., Tanaka I., Kaneko S., Ohta K, Tanaka H, Inaka K, Higuchi Y, Niimura N, Samejima M, Igarashi K. «Newton's cradle» proton relay with amide-imidic acid tauromerization in inverting cellulose visualized by neutron crystallography // Sci. Adv. 2015. Article number 1:e1500263.
- Sakamoto Y., Suzuki Y., Iizuka I., Tateoka C, Roppongi S., Fujimoto M, Inaka K., Tanaka H, Yamada M, Ohta K, Gouda H, Ogasawara W, Tanaka N. Structural and mutational analyses of dipeptidyl peptidase 11 from Porphyromonas gingivalis reveal the molecular basis for strict substrate specificity // Scientific Reports. 2015. V. 5. Article № 11151.
- Yoshida H, Yoshihara A., Ishii T, Izumori K, ad Kamitori S. X-ray structures of the Pseudomonas cichorii D-tagatose 3-epimerase mutant form C66S recognizing deoxy sugars as substrates // Appl. Microbiol. Biotechnol. 2016. № 100(24). P. 10403-10415.
- Itoh T, Hibi T, Suzuki F, Sugimoto I., Fujiwara A., Inaka K, Tanaka H., Ohta K., Fujii Y., Taketo A., Kimoto H. Crystal structure of chitinase ChiW from paenibacillus sp. str. FPU-7 reveals a novel type of bacterial cell-surface-expressed multi-modular enzyme machinery // PLOS ONE. 2016: https://doi. org/10.1371/journal.pone.0167310.
- Kinoshita T, Hashimoto T, Sogabe Y, Fukada H, Matsumoto T, Sawa M. High resolution structure discloses the potential for allosteric regulation of mitogen-activated protein kinase kinase 7 // Biochemical and Biophysical Research Communications. 2017. V. 493. P. 313-317.
- Yokomaku K, Akiyama M, Morita Y, Kihira K., Komatsu T. Core-shell protein cluster comprising haemoglobin and recom binant feline serum albumin as an artificial O2 carrier for cats // Journal of Materials Chemistry B. 2018. № 6. P. 2417-2425.
- Negoro S, Shibata N, Lee Y, Takehara I., Kinugasa R, Nagai K, Tanaka Y., Kato D., Takeo M., Goto Y., Higuchi Y. Structural basis of the correct subunit assembly, aggregation and intracellular degradation of nylon hydrolase // Scientific Reports. 2018. V. 8. P. 9725.
- Morita Y, Yamada T, Kureishi M, Kihira K, Komatsu T. Quaternary structure analysis of a hemoglobin core in hemoglobin-albumin cluster // J. Phys. Chem. B. 2018. V. 122. № 50. P. 12031-12039.
- Hatae H., Inaka K, Okamura R., Furubayashi N, Kamo M, Kobayashi T, Abe Y, Iwata S, Hamasaki N. Crystallization of human erythrocyte band 3, the anion exchanger, at the International Space Station «KIBO» // Analytical Biochemistry. 2018. V. 559. P. 91-93.
- Nakae S, Shionyu M, Ogawa T, Shirai T. Structures of jacalin-related lectin PPL3 regulating pearl shell // Proteins. 2018. № 86(6). P. 1-10.
- Nakamura T, Hirata K, Fujimiya K., Chirifu M, Arimori T, Tamada T, Ikemizu S, Yamagata Y. X-ray structure analysis of human oxidized nucleotide hydrolase MTH1 using crystals obtained under microgravity // Int. J. Microgravity Sci. Appl. 2019. № 36(1). Article № 360103.
- Sakamoto Y, Roppongi S, Suzuki Y, Ishihara T, Hidaka K, Nakamura A., Honma N, Ogasawara W, Tanaka N. The crystal structure of peptidase toward drug discovery // Int. J. Microgravity Sci. Appl. 2019. № 36(1). Article № 360106 (in Japanese).
- Tikhonova T., Tikhonov A., Trofimov A., Polyakov K., Boyko K., Cherkashin E., Rakitina T., Sorokin D., Popov V. Comparative structural and functional analysis of two octaheme nitrite reductases from closely related Thioalkalivibrio species // FEBS Journal. 2012. V. 279. P. 4052-4061.
- Akparov V.K., Timofeev V.I., Kuranova I.P. Three-dimensional structure of recombinant carboxypeptidase T from Thermoactinomyces vulgaris without calcium ions // Crystallography Reports. 2011. V. 56. № 4. P. 596-602.
- Petrova T, Bezsudnova E.Y, Boyko K.M., Mardanov A.V., Polyakov K.M., Volkov V.V., Kozin M., Ravin N.V., Shabalin I.G., Skryabin K.G., Stekhanova T.N., Kovalchuk M.V., Popov V.O. ATP-dependent DNA ligase from Thermococcus sp. 1519 displays a new arrangement of the OB-fold domain // Acta Crystallogr. Section F, Struct. Biol. Cryst. Commun. 2012. V. 68. (Pt. 12). P. 1440-1447.
- Safonova T.N., Mordkovich N.N., Polyakov K.M., Manuvera V.A., Veiko V.P., Popov V.O. Crystallization of uridine phosphorylase from Shewanellaoneidensis MR-1 in the laboratory and under microgravity and preliminary X-ray diffraction analysis // Acta Crystallogr. Section F, Struct. Biol. Cryst. Commun. 2012. V. 68. (Pt. 11). P. 1387-1389.
- Timofeev V.I., Smirnova E.A, Chupova L.A., Esipov R.S., Kuranova I.P. X-ray study of the conformational changes in the molecule of phosphopantetheineadenyl-yltransferase from Mycobacterium tuberculosis during the catalyzed reaction // Acta Crystallographica. 2012. Section D68. Р. 1660-1670.
- Trofimov A.A., Polyakov K.M., Tikhonova T.V., Tikhonov A.V., Safonova T.N., Boyko K.M., Dorovatovskii P.V., Popov V.O. Covalent modifications of the catalytic tyrosine in octahaem cytochrome c nitrite reductase and their effect on the enzyme activity // Acta Crystallogr. 2012. Section D68. P. 144-153.
- Горбачева М.А., Ярош А.Г., Дорова-товский П.В., Ракитина Т.В., Бойко К.М., Корженевский Д. А., Липкин А.В., Попов В.О., Шумилин И.А. Новый подход к исследованию структурно-функциональных свойств белков с неизвестными функциями / / Биорганическая химия. 2012. Т. 38. № 1. С. 99.
- Timofeev V.l., Kuznetsov S.A., Akparov V.Kh., Chestukhina G.G., Kuranova l.P. Three-dimensional structure of carboxypeptidase T from thermoactinomyces vulgaris in complex with N-BOC-L-leucine // Biochemistry (Moscow). 2013. V. 78. № 3. P. 252-259.
- Timofeev V.l., Zhukhlistova N.E., Kuranova l.P., AbramchikYu.A, Fateev I.V., Murav'eva T.l., Esipov R.S. Three-dimensional structure of thymidine phosphorylase from E. coli in complex with 3'-Azido-2'-Fluoro-2',3'- Dideoxyuridine // Crystallography Reports. 2013. V. 58. № 6. P. 842-853.
- Boyko K.M., Gorbacheva M.A., Rakitina T.V., Korzhenevsky D.A., Dorovatovsky P.V., Lipkin A.V., Popov V.O. Identification of a ligand in a protein structure with an unknown STM4435 function from Salmonella typhimurium // Reports of the Academy of Sciences. 2014. V. 457. № 1. P. 107-110. (in Russian).
- Strelov V.l., Kuranova l.P., Zakharov B.G., Voloshin A.E. Crystallization in space: Results and prospects // Crystallography Reports. 2014. V. 59. № 6. P. 781-806.
- Akparov V., Sokolenko N., Timofeev V, Kuranova l. Structure of the complex of carboxypeptidase B and N-sulfamoyl-L-arginine // Acta Crystallographica, Section F, Structural Biology Communications. 2015. V. 71 P. 1335-1340. DOl: 10.1107/ S2053230X15016799.
- Timofeev V.l., Slutskaya E.A., Korzhenevskiy D.A., Gorbacheva M.A., Boyko K.M., Rakitina T.V., Lipkin A.V., Popov V.O. Crystal structure of recombinant prolidase from Thermococcus sibiricus at P21221 spacegroup // Acta Cryst. 2015. Section F. V. 71(8). P. 951-957.
- Petrova T., Slutskaya E., Boyko K., Sokolova O, Rakitina T., Korzhenevskiy D., Gorbacheva M, Bezsudnova E., Popov V. The localization of Trp residues on the surface of the dodecamer of the aminopeptidase APDkam598 from the archaeon Desulfurococcuskamchatkensis // Acta Cryst. 2015. Section F. V. 71(3). P. 277-285.
- Timofeev V.I., Abramchik Yu.A.; Zhukhlistova N.E.; et al. Threedimensional structure of phosphoribosyl pyrophosphate synthetase from E-coli at 2.71 angstrom resolution // Crystallography Reports. 2016. V. 61. № 1. P. 44-54.
- Timofeev V.I., Abramchik Yu.A. Zhukhlistova N.E.; et al. Threedimensional structure of E-Coli purine nucleoside phosphorylase at 0.99 resolution // Crystallography Reports. 2016. V. 61. № 2. P. 249-257.
- Boyko K.M., Gorbacheva M.A., Korzhenevskiy D.A., Alekseeva M.G., Mavletova D.A., Zakharevich N.V., Elizarov S.M., Rudakova N.N., Danilenko V.N., Popov V.O. Structural characterization of the novel aminoglycoside phosphotransferase AphVIII from Streptomyces rimosus with enzymatic activity modulated by phosphorylation // Biochem. Biophys. Res. Commun. 2016. № 477. P. 595-601.
- Akparov V.Kh, Timofeev V.I., Maghsoudi N.N., Kuranova I.P. Threedimensional structure of porcine pancreatic carboxypeptidase B with an acetate ion and two zinc atoms in the active site // Crystallography Reports. 2017. V. 62. № 2. P. 249-253.
- Akparov V., Timofeev V., Khaliullin I., Svedas V., and Kuranova I. Structure of the carboxy peptidase B complex with N-sulfamoyl-L-phenylalanine — a transition state analog of non-specific substrate. 2017. http://dx.doi.org/10.1080/07391102.201 7.1304242. P. 1-10.
- Akparova V.Kh., Timofeev V.I., Khaliullin I.G., Svedase V., Kuranova I.P., Rakitina T.V. Crystal structures of carbo-xypeptidase T complexes with transition-state analogs // Journal of Biomolecular Structure and Dynamics. 2017. DOI: http://doi.org/ 10.1080/07391102.2017.1404932.
- Timofeev V.I., Sinitsyna E.V., Kostromina M.A., Muravieva T.I., Makarov D.A., Mikheeva O.O., Kuranova I.P., Esipov R.S. Crystal structure of recombinant phosphoribosylpyrophosphate synthetase 2 from Thermus thermophilus HB27 complexed with ADP and sulfate ions // Acta Cryst. 2017. Section F. V. 73. Part 6. P. 369-375.
- Boyko K.M., Nikolaeva A.Y., Kachalova G.S., Bonchuk A.N., Popov V.O. Purification, isolation, crystallization, and preliminary X-ray diffraction study of the BTB domain of the centrosomal protein 190 from Drosophila Melanogaster // Crystallography Reports. 2017. V. 62. № 6. P. 909-911.
- Timofeev V.l., Zhukhlistova N.E., Abramchik Y.A., Muravieva T.l., Esipov R.S., Kuranova l.P. Crystal structure of Escherichia coli purine nucleoside phosphorylase complexed with acyclovir // Acta cryst.2018. Section F. V. 74. Issue 7. P. 402-409. DOl: https://doi.org/10.1107/S2053230X18008087.
- Timofeev V.l., Zhukhlistova N.E., Abramchik Y.A., Fateev l.l, Kostromina M.A., Muravieva T.l., Esipov R.S., Kuranova l.P. Crystal structure of E. Coli purine nucleoside phosphorylase with 7-deaza-hypoxanthine // Acta cryst. 2018. Section F. V. 74. lssue 6. P. 355-362. DOl: https:// doi.org/10.1107/S2053230X18006337.
- Safonova T.N., Mikhailov S.N., Veiko V.P., Mordkovich N.N., Manuvera V.A., Alekseev C.S., Kovalchuk M.V., Popova V.O., Polyakov K.M. High-syn conformation of uridine and asymmetry of the hexameric molecule revealed in the highresolution structures of Shewanellaoneidensis MR-1 uridine phosphorylase in the free form and in complex with uridine // Acta Crystallogr. 2014. Section D. V. 70. lssue 12. P. 3310.
- Samygina V.R. lnorganic pyro-phosphatases: structural diversity serving the function // Russian Chemical Reviews. 2016. V. 85. P. 464-474.
- Akparov V.Kh., Timofeev V.l., Khaliullin l.G., Svedas V., Chestukhina G.G., Kuranova l.P. The new mechanism of substrate recognition by etallocarboxypeptidases // J. Med. Res. Dev. 2014. V. 3. № 4. P. 200.
- Akparov V.Kh., Timofeev V.l., Kuranova l.P. Crystallization and preliminary X-ray diffraction study of porcine carboxy peptidase B // Crystallography Reports. 2015. V. 60. lssue 3. P. 367-369. DOl: 10.1134/S1063774515030025.
- Akparov V.Kh, Timofeev V.l., Khaliullin l.G., Svedas V., Chestukhina G.G., Kuranova l.P. Structural insights into the broad substrate specificity of carboxypeptidase T from Thermoactinomyces vulgaris // Febs Journal. 2015. V. 282. Issue 7. P. 1214-1224. DOI: 10.1111/febs.13210.
- Timofeev V.I., Chupova L.A., Esipov R.S., Kuranova I.P. Crystallization and preliminary X-ray diffraction study of phosphopantetheine adeny lyltransferase from M. tuberculosis crystallizing in Space Group P32 // Crystallography Reports. 2015. V. 60. Issue 5. P. 682-684. DOI: 10.1134/S10 6377451505017X.
- Podshivalov D.D., Timofeev V.I., Sidorov-Biryukov D.D., Kuranova I.P. Virtual Screening of Selective Inhibitors of Phosphopantetheine Adenylyltransferase from Mycobacterium Tuberculosis // Crystallography Reports. 2017. V. 62. № 3. P. 405.
- Куранова И.П. Фосфопантетеинаде-нилилтрансфераза Mycobacterium tuberculosis и тимидинфосфорилаза E. coli-белки-мишени для действия лекарств: пространственная структура и механизм действия // Вестник РФФИ. 2014. № 2(82). С. 45-55.
- Timofeev V.I., Abramchik Yu.A, Zhukhlistova N.E., Kuranova I.P. Crystallization and preliminary X-ray diffraction study of phosphoribosyl pyrophosphate synthetase from E. Coli // Crystallography Reports. 2015. V. 60. Issue 5. P. 685-688. DOI: 10.1134/S1063774515050181.
- Nikolaeva A.Yu, Timofeev V.I., Boiko K.M., Korzhenevskii D.A., Rakitina T.V., Dorovatovskii P.V., Lipkin A.V. Isolation, purification, crystallization, and preliminary X-ray diffraction study of the crystals of HU protein from M. gallisepticum // Crystallography Reports. 2015. V. 60. № 6. P. 880-883.
- Esipov R.S., Abramchik Yu.A., Fateev I.V., Muravyova T.I., Artemova K.G., Konstantinova l.D, Kuranova l.P., Miroshnikov A.l. Recombinant phosphoribosyl pyrophosphate synthetases from Thermus thermophilus HB27: lsolation and properties // Russian journal of bioorganic chemistry. 2016. V. 42. № 5. P. 512-521.
- Abramchik Y.A., Muravieva T.l., Sinitsyna E.V., Esipov R.S., Timofeev V.l., Kuranova l.P. Crystallization and preliminary X-ray diffraction analysis of recombinant phosphoribosylpyrophosphate synthetase from the Thermophilic thermus thermophilus strain HB27 // Crystallography Reports. 2017. V. 62. № 1. P. 78-81.
- Sinitsyna E.V., Timofeev V.l., Tuzova E.S., Kostromina M.A., Murav'eva T.l., Esipov R.S., Kuranova l.P. Crystallization and preliminary X-ray diffraction study of recombinant adenine phosphoribosyltransferase from the thermophilic bacterium Thermus thermophilus strain HB27 // Crystallography Reports. 2017. V. 62. № 4. P. 580-583.
- Timofeev V.l., Sinitsyna E.V., Kostromina M.A., Muravieva T.l., Makarov D.A., Mikheeva O.O., Kuranova l.P., Esipov R.S. Crystal structure of recombinant phosphoribosyl-pyrophosphate synthetase 2 from Thermus thermophilus HB27 complexed with ADP and sulfate ions // Acta Crystallographica. Section F-Structural Biology Communications. 2017. V. 73. P. 369-375. DOl: 10.1107/ S2053230X17007488.
- Rai A., Robinson J.A., Tate-Brown J., Buckley N, Zell M., Tasaki K, Karabadzhak G., Sorokin l.V, Pignataro S. Expanded Benefits for Humanity from the lnternational Space Station // Acta Astronautica. 2016. V. 126. P. 463-474. Статья поступила в редакцию 14.10.2019 г. Окончательный вариант — 12.12.2019 г.


