Терапевтически индуцированная нейроэндокринная дифференцировка рака предстательной железы
Автор: Ковченко Г.А., Сивков А.В., Ефремов Г.Д., Каприн А.Д.
Журнал: Экспериментальная и клиническая урология @ecuro
Рубрика: Онкоурология
Статья в выпуске: 3 т.17, 2024 года.
Бесплатный доступ
Введение. Применение селективных высокоэффективных антиандрогенных препаратов при кастрационно-резистентном раке предстательной железы (КРРПЖ) постепенно ведет к клеточной трансформации. Цель работы: изучить современные представления о распространенности нейроэндокринной дифференцировки (НЭД) у больных КРРПЖ, а также лечение данного агрессивного варианта РПЖ.
Терапевтически индуцированная нейроэндокринная дифференцировка рака предстательной железы, резистентность рецепторов андрогенов к терапии рпж, лечение нейроэндокринного рака предстательной железы
Короткий адрес: https://sciup.org/142243276
IDR: 142243276 | DOI: 10.29188/2222-8543-2024-17-3-62-67
Текст обзорной статьи Терапевтически индуцированная нейроэндокринная дифференцировка рака предстательной железы
онкоурология экспериментальная и клиническая урология № 3 2024
Традиционно первой линией терапии распространенной стадии рака предстательной железы (РПЖ) является гормональная или андроген-депривационная терапия (АДТ) с целью снижения уровня тестостерона до кастрационных значений (<20 нг/дл) и торможения дальнейшего роста и распространения опухоли [1, 2] АДТ с использованием агонистов или антагонистов го-надотропин-рилизинг-гормона (ГнРГ) и сегодня является основой лечения распространенного гормоночувствительного РПЖ. Однако способность опухолевых клеток ПЖ к адаптации неизбежно ведет к развитию резистентности к андрогенам и АДТ соответственно [3]. Опухолевые клетки предстательной железы (ПЖ) восстанавливают поступление к себе андрогенов из других источников и/или их внутриклеточную активность. Трансформация андрогензависимого РПЖ в кастрационно-резистентный (КРРПЖ), в среднем, наступает за 2-3 года [4]. В свою очередь, антиандрогены последнего поколения (абиратерон, энзалутамид, апа-лутамид, даролутамид) способны блокировать синтез «собственного» клеточного тестостерона, продлевая жизнь пациентам. Однако, и это лечение также обречено на постепенное развитие резистентности [1] Одним из механизмов возникновения резистентности к АДТ является нейроэндокринная дифференцировка (НЭД) опухоли [5]. В данном обзоре мы рассматриваем современные представления о распространенности НЭД РПЖ у больных КРРПЖ, а также лечение данного агрессивного варианта РПЖ.
МАТЕРИАЛЫ И МЕТОДЫ
Поиск научных исследований проводился в базах данных PubMed, Google Scholar и eLibrary по состоянию на сентябрь 2024 года. Поисковый запрос включал следующие термины: treatment-induced neuroendocrine prostate cancer, treatment-emergent neuroendocrine prostate cancer (терапевтически-индуцированный нейроэндокринный рак предстательной железы), androgen receptor resistance PCa therapy (резистентность рецепторов андрогенов к терапии РПЖ), neuroendocrine prostate cancer treatment (лечение нейроэндокринного рака предстательной железы). В обзор включены публикации, посвященные нейроэндокринной дифференцировке РПЖ и оценке новых данных по распространенности данного варианта рака у пациентов по мере наступления кастрационной резистентности. Публикации с первичным нейроэндокринным раком предстательной железы были исключены из обзора.
РЕЗУЛЬТАТЫ
Терапевтически индуцированная НЭД РПЖ
Применение селективных высокоэффективных антиандрогенных препаратов при КРРПЖ постепенно снижает зависимость опухолевой клетки от андрогенных рецепторов (АР), что ведет к клеточной трансформации: потере первоначальной люминальной эпителиальной идентичности и приобретению нейроэндокринного (НЭ) фенотипа [6]. Такая фенотипическая трансформация является проявлением линейной пластичности, представляющей собой биологический процесс,посредством которого раковые клетки изменяются из одного морфологического и функционального типа клеток в другой под влиянием окружающей среды [7, 8]. В ряде публикаций линейная пластичность аденокарциномы ПЖ в НЭ опухоль получила обозначение терапевтически-индуцированной карциномы предстательной железы (t-NEPCs), поскольку НЭ фенотип опухоли был приобретен de novo и является причиной длительной АДТ [9]. В гистопатологической классификации опухолей Всемирной организации здравоохранения (пятое издание, 2022 г.) в главе «Опухоли предстательной железы» этому патологическому состоянию посвящен отдельный раздел: «Treatment-related neuroendocrine prostatic carcinoma» В нем t-NEPCs определяют как «опухоли, демонстрирующие полную или частичную нейроэндокринную дифференцировку аденокарциномы после андроген-депривационной терапии» (код ICD-O 8574/3) [10]. Во многих других работах то же состояние обозначают как нейроэндокринную дифференцировку РПЖ (НЭД РПЖ), связанную с лечением, чтобы разграничить его с первично нейроэндокринным мелкоклеточным РПЖ, встречающимся менее, чем в 1% случаев РПЖ тогда как НЭД РПЖ достигает 20% случаев КРРПЖ [11-14]. В своей практике мы, как и большинство авторов, используем именно этот термин.
Считают, что НЭД РПЖ исходит из базальных или НЭ клеток,которые присутствуют в ткани нормальной ПЖ в виде немногочисленных колоний и скоплений [15]. Гистологически опухоли с НЭД РПЖ обусловленные проводимой АДТ, могут демонстрировать как чистую мелкоклеточную морфологию, так и смешанную картину, включающую и мелкоклеточные участки, и клетки аденокарциномы [16-18]. Опухолевые клетки НЭД РПЖ характеризуются плохой дифференциацией и представлены спиралевидными или органоидными клетками (рис. 1) [10, 19, 20].
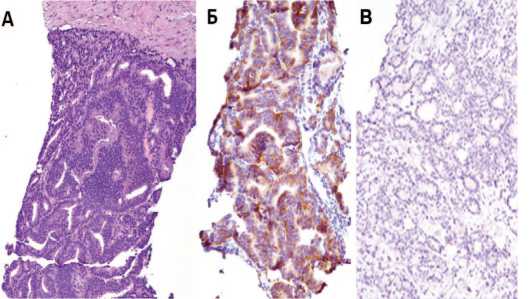
Рис 1. Больной М. НЭД РПЖ (гематоксилин и эозин) (А), тот же участок – иммуногисто-химическое исследованием (синаптофизин) (Б), хромогранин (В)
Fig. 1. Patient M. NED prostate cancer (hematoxylin and eosin) (A), the same area – immuno-histochemical study (synaptophysin) (Б), chromogranin (B)
Опухоли с НЭД РПЖ экспрессируют маркеры НЭ клеток,такие как нейронспецифическая енолаза (НСЕ), хромогранин A (ХгА), синаптофизин и ряд других, а также демонстрируют АР-независимое со-стояние,характеризующееся снижением или отсутствием экспрессии АР [18, 21, 22]. Такая особенность «новых» клеточных колоний подчеркивает необходимость поиска мишеней для лекарственной терапии этого летального заболевания. Тем не менее, доступные варианты ограничены, поскольку молекулярная основа, лежащая в основе образования НЭД РПЖ из клеток аденокарциномы, остается неясной [16, 23]. То есть механизмы и пути, лежащие в основе НЭД РПЖ, до сих пор плохо изучены,а эффективных методов лечения по-прежнему не существует [24].
Число наблюдений НЭД РПЖ растет, в том числе и в связи с широким использованием мощных ингибиторов АР [6]. По данным ряда исследований, НЭД РПЖ наблюдают у 10–20% пациентов с метастатическим КРРПЖ, выявляемым после АДТ и терапии анти-андрогенами нового поколения, а состояние характеризуется высокоагрессивным течением и сопровождается низкими показателями выживаемости [25]
Общие механизмы резистентности к дефициту андрогенов включают:изменения сигнального пути АР, обходные механизмы передачи сигналов АР, а также независимую от АР эволюцию клеточных клонов. Считают, что именно последний механизм, возникающий в ходе АДТ, у части больных вызывает НЭД РПЖ и летальную форму КРРПЖ [17].
Имеющиеся данные свидетельствуют о том,что линейная пластичность, связанная с НЭД, обусловлена эпигенетическими изменениями,которые возникают в определенном геномном контексте [26, 27] Исследования показали,что у пациентов с подтвержденным НЭ фенотипом отмечают низкие уровни ПСА,быструю прогрессию с развитием костных и висцеральных метастазов. Это происходит на фоне частой потери генов ретинобластомы 1 ( Rb1 ) и супрессора опухоли p53 ( Tp53 ) [15, 18, 19, 28]. Появляется все больше свидетельств,подтверждающих идею о том, что эпигенетические события играют важнейшую роль в механизме трансдифференцировки РПЖ в АР-индифферентное состояние при определенных геномных условиях, включая потерю p53, Rb1 и Pten [27] Вызывают интерес данные, полученные на генно-инженерных мышиных моделях РПЖ с комбинированной потерей p 53 и Pten , демонстрирующие, что НЭ опухолевые клетки могут возникать непосредственно из ранее существовавших клеток ацинарной аденокар-циномы,а не только из параллельной,независимой популяции НЭ клеток [29].
Ранее X.Q. Zhang и соавт. в лабораторных условиях, имитируя клиническое состояние длительной андрогенной депривации, успешно создали НЭ-подоб-ные субклонированные клетки из популяции клеток чувствительной к андрогенам аденокарциномы ПЖ (LNCaP). Характеристика этих клеток показала не только то, что АДТ может индуцировать дифференцировку андроген-чувствительных клеток эпителия ПЖ человека в НЭ клетки, но также и то, что полученные НЭ клетки подавляют экспрессию АР и уровень простатспецифического антигена (ПСА). Данный факт обуславливает потенциальную уязвимость современного терапевтического подхода с использованием антиандрогенов нового поколения, который предполагает воздействие на «собственные» АР [30].



Кроме того, показано, что появление НЭ фенотипа при РПЖ, отчасти, опосредовано транскрипционным фактором плюрипотентности SOX2 и сопровождается клеточной пролиферацией,метастазированием и лекарственной устойчивостью, то есть ростом агрессивности опухоли и плохим прогнозом [9, 16].
H.T. Wang и соавт. проанализировали время развития НЭД РПЖ и выживаемость у 123 пациентов с КРРПЖ. Медианное время от первоначального диагноза РПЖ до развития НЭД РПЖ составило 20 месяцев, при этом высокий балл по шкале Gleason (≥ 8) при первичной диагностике являлся фактором риска раннего развития НЭД ( p =0,032). Медианная выживаемость после установки диагноза НЭД РПЖ составила 7 месяцев ( p =0,001) [7].
Позже анализ 87 клинических случаев показал что больные с первичным ( de novo ) нейроэндокринным РПЖ имеют худший прогноз,чем пациенты с НЭД РПЖ.Общая выживаемость была существенно ниже при НЭРПЖ выявленном de novo (8,9 месяцев) против 26,1 месяцев при НЭД РПЖ ( p <0,001). При этом медиана от диагностирования аденокарциномы до развития НЭД РПЖ составила 39,7 (24,5–93,8) месяцев. При этом, существенных молекулярных различий между первичным НЭРПЖ и НЭД РПЖ выявлено не было [18].
В другом более крупном исследовании 202 больных КРРПЖ, 148 пациентов ранее получали абирате-рон и/или энзалутамид. Частота выявления НЭД РПЖ по результатам проведенной биопсии из метастатических очагов и по данным генетического анализа составила 17%. Повышение уровня нейрогенспецифической энолазы (НСЕ) (> 6,05 нг/мл) и ХгА (> 3,1 нг/мл) в сыворотке крови наблюдали у 55% пациентов.Данные маркеры продемонстрировали: чувствительность – 95%; специфичность – 50%; отрицательную и положительную прогностическую значимость – 98% и 22% соответственно. Авторы отметили, что общая выживаемость пациентов с НЭД РПЖ была значительно ниже чем у пациентов с метастатическим КРРПЖ без НЭД что составило 36,6 и 44,5 месяцев соответственно (ОР – 2,02; 95% ДИ 1,07-3,82) [21].
X.T. Weng и соавт. в случае неэффективности терапии КРРПЖ и клинической прогрессии рекомендуют повторную биопсию из новых быстро прогрессирующих метастатических очагов с последующим иммуногистохимическим анализом, а также применение рутинного использования НЭД маркеров для выявления НЭД РПЖ [31].
В том числе, именно поэтому в клинических рекомендациях Объединенной национальной онкологической сети США (National Comprehensive Cancer Network – NCCN) посчитали целесообразным рассматривать биопсию метастатического очага у всех пациентов с КРРПЖ для выявления НЭД РПЖ [32].
Продолжается дискуссия относительно эффективных методов лечения НЭД РПЖ,которому до сих пор уделяют недостаточно внимания. Так, в рекомендациях Европейской Ассоциации Урологов (ЕАУ 2024) нет раздела,посвященного лечению первичного НЭ РПЖ,равно как и данных о тактике лечения НЭД РПЖ [33]. В Российских Клинических Рекомендациях 2022 г. присутствует раздел о лечении первичного НЭ РПЖ, где в качестве рекомендованных режимов химиотерапии предложены: комбинации этопозида и цисплатина, карбоплатина и этопозида, доцетаксела и карбоплатина [34]. Согласно Национальному онкологическому руководству NCCN 2023 года (версии 4.0) основу терапии первичного НЭ РПЖ составляют препараты платины в различных комбинациях:циспла-тин/этопозид, карбоплатин/этопозид, доксорубицин/ карбоплатин и кабазитаксел/карбоплатин, несмотря на короткую медиану до возникновения резистентности [32]. Прогноз эффективности далек от удовлетворительного со средней общей выживаемостью с момента постановки диагноза нейроэндокринного рака 8,9 месяцев для мелкоклеточной карциномы и 26,1 месяца для опухоли смешанного типа [16]. И хотя в последнее время мы встречаем все больше доказательств того, что фенотипически КРРПЖ с НЭД не отличается от такового при первичном НЭ РПЖ,однако и сегодня в главных руководствах не описано единой установленной схемы первой линии терапии для КРРПЖ с НЭД [14, 35].
ОБСУЖДЕНИЕ
Недавно мы представили ретроспективные данные пилотного исследования,где была показана эффективность аналога соматостатина (октреотид-депо) у пациентов с выявленной НЭД при КРРПЖ. Целевая группа была стратифицирована в зависимости от сывороточного уровня ХгА. Примененная на том этапе схема комбинированной терапии (Октреотид-Депо 20 мг каждые 28 дней в сочетании с дексаметазоном и агонистами ГнРГ)позволила достичь наибольшей эффективности в группе с уровнем ХгА в крови 3-7 нг/мл то есть с умеренной выраженностью НЭД по уровню маркера. В данной группе положительного результата в виде стабилизации процесса и снижения ПСА удалось достичь у 56% больных, в том числе у 44,4% на 50% и более. При этом снижение медианы ПСА составило 73,6% ( р =0,004), а ХгА – 47,2% ( р =0,02). Что примечательно, общая доля больных КРРПЖ, ответивших на терапию, составила всего 28% [5].
Попытка применения аналогов соматостатина при НЭД КРРПЖ имеет патогенетическое обоснование и достаточно длительную историю. Известно, что предшествовавшие исследования не продемонстрировали значимой эффективности,однако ни в одном из них не проводили стратификацию больных по уровню маркеров НЭД (ХгА) [36, 37]. Появление антиандрогенов второго поколения переключило внимание клиницистов на новый объект. Однако проблема НЭД РПЖ никуда не исчезла,а накопление опыта применения подобных антиандрогенов привело к росту резистентности уже и на эту терапию, часть которой реализуется через механизмы НЭД [35, 38]. По нашему мнению, полученные в ходе исследования данные [26], как и информация о многообразии путей развития НЭД, подтверждают целесообразность дальнейшего изучения комплексного терапевтического подхода, включая использование аналогов соматоста- тина в комбинации с новыми методами антиандроген-ной и иной противоопухолевой терапии.
ЗАКЛЮЧЕНИЕ
Высокая распространенность НЭД у больных КРРПЖ,как и отсутствие эффективных методов лече-ния,свидетельствуют о необходимости концентрации усилий по изучению этого клинически актуального, но недостаточно исследованного состояния. Поиск новых вариантов лечения НЭД РПЖ, основанных на патогенетическом комплексном подходе,является перспективным и востребованным.
ПТШТУРАШШПШ
Список литературы Терапевтически индуцированная нейроэндокринная дифференцировка рака предстательной железы
- Носов Д.А., Волкова М.И., Гладков О.А., Карабина Е.В., Крылов В.В., Матвеев В.Б., Митин Т., Попов А.М. Практические рекомендации по лечению рака предстательной железы. Злокачественные опухоли 2022;12(3S2-1):607-26. [Nosov D.A., Volkova M.I., Gladkov O.A., Karabina E.V., Krylov V.V., Matveev V.B., Mitin T., Popov A.M. Practical recommendations for the treatment of prostate cancer. Zlokachestvennyye opukholi = Malignant Tumoursis 2022;12(3S2-1):607-26. (In Russian)]. https://doi.org/10.18027/2224-5057-2022-12-3s2-607-626.
- Каприн А.Д., Алексеев Б.Я., Матвеев В.Б. и др. Клинические рекомендации: Рак предстательной железы 2021:1-175. [Kaprin A.D., Alekseev B.Ya., Matveev V.B. et al. Clinical guidelines: Prostate cancer 2021:1-175. (In Russian)].
- Vellky JE, Ricke WA. Development and prevalence of castration-resistant prostate cancer subtypes. Neoplasia 2020;22(11):566-75. https://doi.org/10.1016/j.neo.2020.09.002.
- Pienta KJ, Bradley D. Mechanisms underlying the development of androgen-independent prostate cancer. Clin Cancer Res 2006;12(6):1665-71. https://doi.org/10.1158/1078-0432.CCR-06-0067.
- Ковченко Г.А., Сивков А.В., Каприн А.Д. Роль определения хромогранина А в лечении больных кастрационно-резистентным раком предстательной железы. Экспериментальная и клиническая урология 2024;17(1):75-85. [Kovchenko G.A., Sivkov A.V., Kaprin A.D. The role of chromogranin A determination in the treatment of patients with cas-tration-resistant prostate cancer. Eksperimental'naya i klinicheskaya urologiya = Experimental and Clinical Urology 2024;17(1):75-85. (In Russian)]. https://doi.org/10.29188/2222-8543-2024-17-1-75-85.
- Niu Y, Guo C, Wen S, Tian J, Luo J, Wang K, et al. ADT with antiandrogens in prostate cancer induces adverse effect of increasing resistance, neuroendocrine differentiation and tumor metastasis. Cancer Lett 2018;439:47-55. https://doi.org/10.1016/j.canlet.2018.09.020.
- Wang HT, Yao YH, Li BG, Tang Y, Chang JW, Zhang J. Neuroendocrine Prostate Cancer (NEPC) progressing from conventional prostatic adenocarcinoma: factors associated with time to development of NEPC and survival from NEPC diagnosisa systematic review and pooled analysis. J Clin Oncol 2014;32(30):3383-90. https://doi.org/10.1200/JCO.2013.54.3553.
- Beltran H, et al. The role of lineage plasticity in prostate cancer therapy resistance. Clin Cancer Res 2019;25:6916-24. https://doi.org/10.1158/1078-0432.CCR-18-1912.
- Chen R, Dong X, Gleave M. Molecular model for neuroendocrine prostate cancer progression. BJU Int 2018;122:560-70. https://doi.org/10.1111/bju.14207.
- Kench JG, Amin MB, Berney DM, Compérat EM, Cree IA, Gill AJ, et al. WHO Classification of tumours fifth edition: evolving issues in the classification, diagnosis, and prognosti-cation of prostate cancer. Histopathology 2022;81(4):447-58. https://doi.org/10.1111/his.14711.
- Сивков А.В., Кешишев Н.Г., Ефремов Г.Д., Ковченко Г.А., Рабинович Э.З., Трудов А.А., и соавт. Нейроэндокринная дифференцировка рака предстательной железы, что нового? Экспериментальная и клиническая урология 2015;(3):22-30. [Sivkov A.V., Keshishev N.G., Efremov G.D., Kovchenko G.A., Rabinovich E.Z., Trudov A.A., et al. Neuroendocrine differentiation of prostate cancer, what's new? Eksperimental'naya i klinicheskaya urologiya = Experimental and Clinical Urology 2015;(3):22-30. (In Russian)].
- Xie Y, Ning S, Hu J. Molecular mechanisms of neuroendocrine differentiation in prostate cancer progression. J Cancer Res Clin Oncol 2022;148(7):1813-23. https://doi.org/10.1007/s00432-022-04061-7.
- Puca L, Vlachostergios PJ, Beltran H. Neuroendocrine Differentiation in Prostate Cancer: Emerging Biology, Models, and Therapies. Cold Spring Harb Perspect Med 2019;9(2):a030593. https://doi.org/10.1101/cshperspect.a030593.
- Zamora I, Freeman MR, Encío IJ, Rotinen M. Targeting Key Players of Neuroendocrine Differentiation in Prostate Cancer. Int J Mol Sci 2023;24(18):13673. https://doi.org/10.3390/ijms241813673.
- Wishahi M. Treatment-induced neuroendocrine prostate cancer and de novo neuroendocrine prostate cancer: Identification, prognosis and survival, genetic and epigenetic factors. World J Clin Cases 2024;12(13):2143-6. https://doi.org/10.12998/wjcc.v12.i13.2143.
- Long Z, Deng L, Li C, He Q, He Y, Hu X, et al. Loss of EHF facilitates the development of treatment-induced neuroendocrine prostate cancer. Cell Death Dis 2021;12(1):46. https://doi.org/10.1038/s41419-020-03326-8.
- Dhavale M, Abdelaal MK, Alam ABMN, Blazin T, Mohammed LM, Prajapati D, et al. Androgen receptor signaling and the emergence of lethal neuroendocrine prostate cancer with the treatment-induced suppression of the androgen receptor: a literature review. Cureus 2021;13(2):e13402. https://doi.org/10.7759/cureus.13402.
- Conteduca V, Oromendia C, Eng KW, Bareja R, Sigouros M, et al. Clinical features of neu-roendocrine prostate cancer. Eur J Cancer 2019;121:7-18. https://doi.org/10.1016/j.ejca.2019.08.011.
- Akoto T, Bhagirath D, Saini S. MicroRNAs in treatment-induced neuroendocrine differentiation in prostate cancer. Cancer Drug Resist 2020;3(4):804-18. https://doi.org/10.20517/cdr.2020.30.
- Patel GK, Chugh N, Tripathi M. Neuroendocrine differentiation of prostate canceran intriguing example of tumor evolution at play. Cancers (Basel) 2019;11. https://doi.org/10.3390/cancers11101405.
- Aggarwal R, Huang J, Alumkal JJ, Zhang L, Feng FY, Thomas GV, et al. Clinical and genomic characterization of treatment-emergent small-cell neuroendocrine prostate cancer: A multi-institutional prospective study. J Clin Oncol 2018;36(24):2492-503. https://doi.org/10.1200/JCO.2017.77.6880.
- Fine SW. Neuroendocrine tumors of the prostate. Mod Pathol 2018;31(S1):S122S132. https://doi.org/10.1038/modpathol.2017.164.
- Beer TM, Armstrong AJ, Rathkopf DE, Loriot Y, Sternberg CN, Higano CS, et al. Enzalu-tamide in metastatic prostate cancer before chemotherapy. N Engl J Med 2014;371(5):424-33. https://doi.org/10.1056/NEJMoa1405095.
- Kaarijärvi R, Kaljunen H, Ketola K. Molecular and Functional Links between Neurodevel-opmental Processes and Treatment-Induced Neuroendocrine Plasticity in Prostate Cancer Progression. Cancers (Basel) 2021;13(4):692. https://doi.org/10.3390/cancers13040692.
- Abida W, Cyrta J, Heller G, Prandi D, Armenia J, Coleman I, et al. Genomic correlates of clinical outcome in advanced prostate cancer. Proc Natl Acad Sci USA 2019;116(23):11428-36. https://doi.org/10.1073/pnas.1902651116.
- Mu P, Zhang Z, Benelli M, Karthaus WR, Hoover E, Chen CC, et al. SOX2 promotes line-age plasticity and antiandrogen resistance in TP53and RB1-deficient prostate cancer. Science 2017;355(6320):84-8. https://doi.org/10.1126/science.aah4307.
- Zehir A, Benayed R, Shah RH, Syed A, Middha S, Kim HR, et al. Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nat Med 2017;23(8):1004. https://doi.org/10.1038/nm0817-1004c.
- Beltran H, Rickman DS, Park K, Chae SS, Sboner A, MacDonald TY, et al. Molecular characterization of neuroendocrine prostate cancer and identification of new drug targets. Cancer Discov 2011;1:487. https://doi.org/10.1158/2159-8290.
- Zou M, Toivanen R, Mitrofanova A, Floch N, Hayati S, Sun Y, et al. Transdifferentiation as a mechanism of treatment resistance in a mouse model of castration-resistant prostate cancer. Cancer Discov 2017;7(7):736-49. https://doi.org/10.1158/2159-8290.CD-16-1174.
- Zhang XQ, Kondrikov D, Yuan TC, Lin FF, Hansen J, Lin MF. Receptor protein tyrosine phosphatase alpha signaling is involved in androgen depletion-induced neuroendocrine differentiation of androgen-sensitive LNCaP human prostate cancer cells. Oncogene 2003;22(43):6704-16. https://doi.org/10.1038/sj.onc.1206764.
- Weng XT, Lin WL, Pan QM, Chen TF, Li SY, Gu CM. Aggressive variant prostate cancer: A case report and literature review. World J Clin Cases 2023;11(26):6213-22. https://doi.org/10.12998/wjcc.v11.i26.6213.
- Schaeffer EM, Srinivas S, Adra N, Barocas D, Bitting R, Bryce A, et al. Prostate Cancer, Version 4.2023, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2023;21(10):1067-96. https://doi.org/10.6004/jnccn.2023.0050.
- Tilki D, van den Bergh RCN, Briers E, Van den Broeck T, Brunckhorst O, Darraugh J, et al. EAU-EANM-ESTRO-ESUR-ISUP-SIOG Guidelines on prostate cancer. Part II-2024 update: treatment of relapsing and metastatic prostate cancer. Eur Urol 2024;86(2):164-82. https://doi.org/10.1016/j.eururo.2024.04.010.
- Носов Д.А., Волкова М.И., Гладков О.А., Карабина Е.В., Крылов В.В., Матвеев В.Б. и соавт. Практические рекомендации по лечению рака предстательной железы. Злокачественные опухоли 2022;12(3S2):607-26. [Nosov D.A., Volkova M.I., Gladkov O.A., Karabina E.V., Krylov V.V., Matveev V.B. et al. Practical recommendations for the treatment of prostate cancer. Zlokachestvennyye opukholi = Malignant Tumors 2022;12(3S2):607-26. (In Russian)]. https://doi.org/10.18027/2224-5057-2022-12-3s2-607-626.
- Yamada Y, Beltran H. Clinical and Biological Features of Neuroendocrine Prostate Cancer. Curr Oncol Rep 2021;23(2):15. https://doi.org/10.1007/s11912-020-01003-9.
- Алексеев Б.Я., Каприн А.Д., Нюшко К.М. Роль аналогов соматостатина в лечении больных гормонорефрактерным раком предстательной железы. Онкоурология 2011;(2):84-8. [Alekseev B.Y., Kaprin A.D., Nyushko K.M. The role of somatostatin analogues in the treatment of patients with hormone-refractory prostate cancer. Onkourologiya = Cancer Urology 2011;(2):84-8. (In Russian)].
- Mitsiades CS, Bogdanos J, Karamanolakis D, Milathianakis C, Dimopoulos T, Koutsilieris M. Randomized controlled clinical trial of a combination of somatostatin analog and dexa-methasone plus zoledronate vs. zoledronate in patients with androgen ablation-refractory prostate cancer. Anticancer Res 2006;26(5B):3693-700.
- Ge R, Wang Z, Montironi R, Jiang Z, Cheng M, Santoni M, et al. Epigenetic modulations and lineage plasticity in advanced prostate cancer. Ann Oncol 2020;31(4):470-9. https://doi.org/10.1016/j.annonc.2020.02.002.


