The acute effect of running exercise on liver ABCA1 gene expression in male Wistar rats
Автор: Seyed Morteza Tayebi , Mitra Khademosharie , Tayebe Amiri Parsa , Mehdi Abaszadegan , Ayoub Saeidi , Nenasheva A.V.
Журнал: Человек. Спорт. Медицина @hsm-susu
Рубрика: Физиология
Статья в выпуске: S1 т.19, 2019 года.
Бесплатный доступ
Objectives. ATP-binding cassette transporters transfer a variety of substrates across the lipid bilayers in an energy-dependent manner. ABCA1 is a member of this family, which plays a crucial role in plasma HDL-C metabolism. On the other hand, the short-term effects of exercise training are less studied. The aim of this study was the effects of a single bout of exercise on liver ABCA1 gene expression in the male Wistar rats. Methods. Twenty four male Wistar rats were housed in a standard environment and randomly divided into two Control (n = 12) and Experimental (n = 12) groups. The exercise included running on a treadmill for 120 min (18 m/min). Immediately, 2 and 24 hours after exercise, rats were anesthetized, and samples were taken from the adipose tissue and liver. Liver ABCA1 was assessed by RT-PCR. Results. The results showed that liver ABCA1 gene expression had significant elevation immediately, 2 and 24 hours after exercise (p
Abca1, gene expression, exercise, liver, adipose tissue, wistar rats, крысы породы wistar
Короткий адрес: https://sciup.org/147233707
IDR: 147233707 | УДК: 612.01 | DOI: 10.14529/hsm19s106
Текст научной статьи The acute effect of running exercise on liver ABCA1 gene expression in male Wistar rats
Cardiovascular diseases are the leading cause of mortality throughout the world and have gained the first rank among chronic diseases (disease burden in the world). In Iran, after mental illness, most statistics are related to cardiovascular disease [12]. Blood lipid profile is one of the health markers and especially of cardiovascular system health. So, high level of cholesterol, LDL, triglyceride, and low level of HDL is associated with increased CVD risk factors [19, 20]. Many efforts have been made to identify the protective mechanisms of HDL in cardiovascular complications [9]. It has been proposed that reverse cholesterol transport is a possible mechanism. It includes the process of collecting excess cholesterol from peripheral tissues such as arteries macrophages and returning them to the liver, which accompanies with HDL deformation [18]. The first phase of reverse cholesterol transport is related to extracellular receptors, namely Apo A-I that mediated by ABCA1 transporter and cause to form the pre-beta HDL particles. ABCA1 is expressed in different tissues, but its role in these tissues is uncertain. Given that the liver is the major source of HDL [16], the study of ABCA1 in this tissue can provide valuable information [2].
Various factors can affect ABCA1 gene expression, and one of them is physical activities and exercise. Also, many studies have surveyed the effect of various exercise training on the HDL, and an increased rate and composition of HDL have been confirmed [3]. However, few studies have been conducted on other stages of reverse cholesterol transport. For example, only one study has been reported an increased removal of cholesterol from cells in trained individuals [1]. Another study has reported an increase in apolipoprotein A-I as a result of exercise. The training protocol was not performed, and participants were classified into training and without training groups via a questionnaire [13]. Few studies have been conducted on ABCA1. One study showed that 12-week aerobic training resulted in the upregulation of ABCA1 in rat intestine [11]. The other study concluded that Treadmill exercise training enhanced ABCA1 gene expression in rat liver [5]. Also, it has been demonstrated that a 6-week endurance training overexpressed liver ABCA1 in rats [10] and showed that a single session of circuit-resistance exercise affected human peripheral blood lymphocyte ABCA1 expression in female students [6]. The other study surveyed the effect of a high-intensity treadmill running program on rat heart tissue ABCA1 and PPAR-α genes expression [4].
It is clear from the review studies that despite the importance of the process of reverse cholesterol transport and the role of ABCA1 in the prevention of cardiovascular disease, little training study has been conducted in this area, especially with the short-term and single-session protocol. So, the present study investigates the effect of a single bout of exercise on liver ABCA1 in the rat.
MATERIAL AND METHODS
Animals. Twenty four male Wistar rats (weight 338 ± 31 g) were acquired from Pasteur's Institute (Tehran, Iran). They were kept in standard conditions (temperature: 22 ± 2 °C, lightdark cycle: 12:12 h, relative humidity: 50% and access to water and food). After a week of familiarity to a Lab space and manipulations by the researcher, the rats were randomly divided into the experiment (n = 12) and control (n = 12) groups with the same weight. The ethics committee of Urmia University approved the study (UMSU.REC.IR.1394.451).
Exercise Protocol. The training involved running exercise on a rodent treadmill. After a few training sessions to make rats familiar with the lab and observe the overload principle, we conducted an experiment where the rats ran on a treadmill at 18 m/min for 120 minutes. The control group also walked on a treadmill at 8 m/min for 10 min, three times during two weeks of familiarity.
Blood and Tissue Sampling. Food was removed from cages 4 h before training to take the blood samples in a relatively fasting mode. Immediately, 2 and 24 hours after the last training session, 8 rats (4 experimental and 4 control) were anesthetized with intraperitoneal administration of pentobarbital sodium (6 mg/100g body weight) and sampling of blood, the adipose tissue and liver were collected. Blood sampling was done by perforating heart and syringe. The adipose tissue and liver were excised, transported to RNAase free and DNAase microtubes and frozen in liquid nitrogen. Blood samples were centrifuged in EDTA contained falcon tubes and stored at -80 °C together with tissue samples.
Liver ABCA1 Gene Expression. Semi-quantitative RT-PCR was done for ABCA1 gene expression in the Liver tissue. For this purpose, 20 mg of tissues were separated and served for RNA extraction reaction. RNA refining was done using a kit of MN firm (Germany). Firstly, tissues were autolyzed by adding 350 µ l RA1 and 3/5 µ l mercaptan solution. Then, this solution with bufferlyse was added to a column (containing red ring) and centrifuged at 11000 cycles for a minute. The solution passed from the column was collected in a microtube. Then the condition for pasting RNA became suitable by adding 350 microlitres 70% alcohol, and, in this phase, the composition was mixed with a pipet. Above homogenized solution was added to a refined RNA column (blue ring) and centrifuged at 11000 cycles for 30 seconds. Then, 350 µ l of MDB buffer were added and centrifuged at 11000 cycles for a minute. Using 95 µ l of DNase solution and 15-minute incubation, DNAs accompanied by RNA, which linked to membrane, was removed. After, 200 µ l of RA2 solution and following 600 µ l of RA3 were added to the column and centrifuged at 11000 cycles for 30 seconds. In the next phase, 250 µ l of RA3 were added and centrifuged at 11000 cycles for 2 minutes. 60 µ l of RNase free water were added to the column and centrifuged at 11000 cycles for a minute and stored at –70 °C. The quantitative rate of RNA extraction with its Optical density (OD) at 260 nm was determined in biophotometer. To make cDNA in a tube without RNase, 100–400 ng total RNA and 0/5 µ g oligo primer dT were added to it. The last volume reaches 11 µ l by aqua pure without RNase. The collection was incubated at 70 °C for 5 minutes and then was cooled immediately on ice. (5X)RT buffer 4 µ l, dNTP mix (10mM) 2 µ l, and (RNasin) Ribonuclease inhibitor 20 unit were added and reached to the last volume of 19 µ l by aqua pure without RNase and incubated for 5 minutes at 37 °C. Then 200 units of reverse transcriptase enzyme (M-Mul-V) were added and incubated for an hour at 42 °C. In the next phase, it was exposed to 70 °C for 10 minutes which inactivated the enzyme. Then immediately transported to the ice pot. A cDNA product was stored at –70 °C. Using the aboveprepared cDNA sample, PCR was conducted according to the following protocol. In this process, beta-actin was used as an intrinsic control, and a PCR reaction was conducted with beta-actin primers for every sample.
FORWARD PRIMER:
TGACACCAAAACCCTCATCA
REVERSE PRIMER:
AGCCCGGGAATGAAGTCCA
Beta actin (in leptin RT-PCR)
BA-for:
(5’-AGTAGGCTTTGTGGTTGATG-3’)
BA-rev:
(5’-CTGTCAGGAAAGGAGAAATC-3’)
PCR product length: 429 bp
For evaluating PCR results, we used the electrophoresis of a PCR product on Agarose gel and UVtech software. ANOVA test with repeated measurements was used to evaluate the effect of training on ABCA 1 gene expression (p < 0.05), and all statistical analyses were conducted in SPSS (version 21).
RESULTS
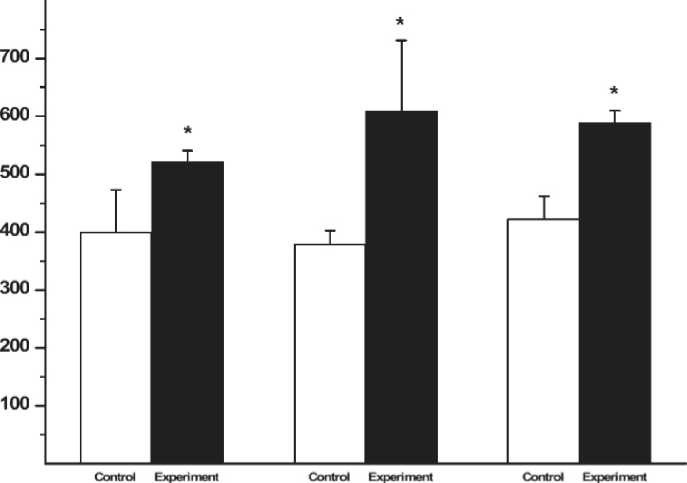
The results showed that exercise training increases liver ABCA1 gene expression immediately, 2 and 24 h after exercise.
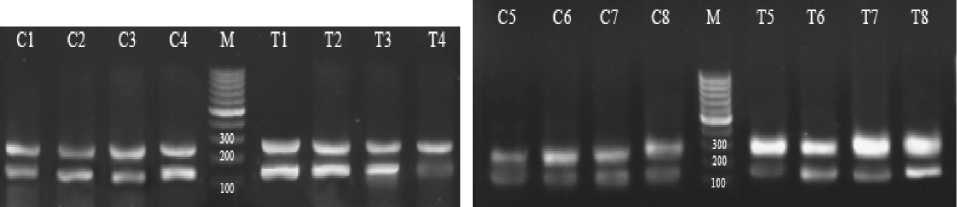
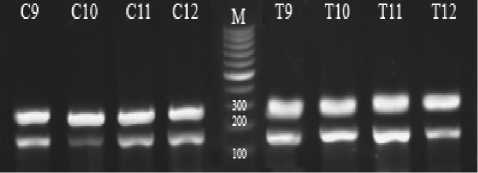
The results of the liver ABCA1 gene expression has been shown in figure 1. The expression of this gene was measured in comparison with the expression of the internal control gene (betaactin). Letter C and T on the bands indicate the control and exercise group, respectively. In Time 0, the bands from left to right are C1 to C4 for control and T1 to T4 for the trained rats (fig. 1a). In Time 2, C5 to C8 and T5 to T8 include the control and experimental groups, respectively (fig. 1b). In Time 24, C9 to C 12 is the control and T9 to T 12 is the experimental group (fig. 1 c). M is also the marker of molecular weight. The weight of the important bands has been shown in the fig. Accordingly, the results of this research on the gene expression of ABCA1 in the liver showed a significant increase in the experimental group compared with the control group immediately, 2 and 24 hours after exercise (p < 0.05) (fig. 2).
DISCUSSION
The main finding of the present study was higher liver ABCA1 gene expression immediately, 2 and 24 h after exercise. To our knowledge, this is the first study that investigates the genetic variations in tissue using such an exercise protocol. In the present study, the increase of ABCA1 expression was in line with the results of previously reported findings on different subjects with different training protocols [5, 8, 10].
Ghanbari-Niaki investigated the effect of a 12-week endurance exercise (intensity: 25 m/min, duration: 60 min/session, and five days a week), that resulted in ABCA1gene expression increase in the rat's heart. Plasma apo-A and pre-β-HDL concentrations were significantly increased [7]. Also, it has been shown that eight weeks of low-intensity exercise (walking) significantly up-regulated ATP-binding cassette transporters A1 (ABCA1) [15].
a)
b)
c)
Fig. 1. Semi-quantitative RT-PCT of liver’ ABCA1 and β-actin mRNA expression in control and trained male rats and ABCA1/ B-actin mRNA expression ratio: a – time 0/ immediately after exercise, b – time 2/2 hours after exercise, c – time 24/24 hours after exercise
Time 0 time 2h time 24h
Fig 2. The changes in the gene expression of ABCA1 in the liver immediately (Time 0), 2 hours (Time 2), and 24 hours (Time 24) after exercise
Hoang et al. reported that participants with high physical activity had higher levels of ABCA1 expression in leukocytes [8]. Ghanbari-Niaki et al. reported that a single session of circuit-resistance exercise (CRE) increased peripheral blood lymphocyte ABCA1 expression and plasma HDL-C level in human [6]. The mechanism of how circuit-resistance exercise increases lymphocyte ABCA1 expression is unclear. A higher ABCA1 expression might be attributed to an exercise-induced lymphocytosis, particularly after a resistance exercise which has been reported by previous studies [17]. According to Simonson and Jackson, a resistance exercise (8 exercises, 3 sets, and 8–10 repetitions at 75% of 1RM) increased all leukocyte subpopulations which was accompanied by a significant increase in lymphocytes counts. They suggested that this leukocytosis was due to an increase in circulating lymphocytes [17]. Rashidlamir et al. reported that two months of cardiac rehabilitation training elevated ABCA1 expression in the lymphocytes of patients undergoing coronary artery bypass graft operation [14].
ABCA1 is a cell membrane transporter that facilitates the delivery of phospholipids from cell membranes to lipid-poor apoA-1 with the formation of discoidal apoA-I- containing HDL, and it plays a pivotal role in forming plasma HDL. The mechanism(s) by which the exercise can influence liver ABCA1 gene expression is (are)
poorly understood. However, several possible mechanisms could be considered. It has been suggested that the modulating effect of fatty acids (FA) is mediated by peroxisome proliferator-activated receptors (PPARs), and it is also well known that PPAR is a nuclear receptor like liver X receptor (LXR) and retinoid X receptor (RXR) that regulates the expression of genes controlling lipid and glucose metabolism [13].
Contrary to this research, Ghanbari-Niaki et al. showed that a high-intensity running program (34 m/min (0% grade), 60 min/day, 5 days/week) on a motor-driven treadmill for eight weeks did not significantly change heart ABCA1 and PPAR α genes expression [4]. One of the reasons for this difference may be related to the duration and intensity of the exercise training in these studies.
Increased ABCA1 expression after 24 h shows that the positive effects of training sustain even after 24 h. Increased ABCA1, which is the main responsible for reverse cholesterol transport and phospholipids to apo A-I, indicates that more apoA-I is lipidated [10]. This can explain the part of the positive effect of training through affecting blood lipids. The cause of increased ABCA1 as a result of training can indicate increased LCAT and pre-b-HDL, which have occurred in response to training [10].
Finally, it can be concluded that the increase of ABCA1 gene expression in the liver subse- quent to one session of long term aerobic exercise supports the regulating and coordinating role of this factor after exercise training and show the positive effects of acute exercise on fat metabolism. Of course, in this study, the lipid profile of plasma was not studied due to some limitations; higher training intensity with more sample number can inspire a more precise insight in this respect.
Acknowledgment. We would like to appreciate the department of biotechnology in the school of medicine of Tarbiat Moddares University in Tehran for its sincere cooperation; particularly the authors wish to thank Dr. Fatemeh Rahbari Zadeh for her help in conducting biochemical experiments and RT-PCR. Dr. Mahdi Jabbari is appreciated for statistical counseling.
Список литературы The acute effect of running exercise on liver ABCA1 gene expression in male Wistar rats
- Brites F., Verona J., De Geitere C., Fruchart J.-C., Castro G., Wikinski R. Enhanced Cholesterol Efflux Promotion in Well-Trained Soccer Players // Metabolism, 2004, vol. 53 (10), pp. 1262-1267. DOI: 10.1016/j.metabol.2004.05.002
- Brunham L.R., Kruit J.K., Iqbal J., Fievet C., Timmins J.M., Pape T.D. et al. Intestinal ABCA1 Directly Contributes to HDL Biogenesis in Vivo // J Clin Invest, 2006, vol. 116 (4), pp. 1052-1062. DOI: 10.1172/JCI27352
- Durstine J.L., Grandjean P.W., Davis P.G., Ferguson M.A., Alderson N.L., DuBose K.D. Blood Lipid and Lipoprotein Adaptations to Exercise: a Quantitative Analysis // Sports Medicine (Auckland, NZ), 2001, vol. 31 (15), pp. 1033-1062. DOI: 10.2165/00007256-200131150-00002
- Ghanbari-Niaki A., Ghanbari-Abarghooi S., Rahbarizadeh F., Zare-Kookandeh N., Gholizadeh M., Roudbari F. et al. Heart ABCA1 and PPAR- α Genes Expression Responses in Male Rats: Effects of High Intensity Treadmill Running Training and Aqueous Extraction of Black Crataegus-Pentaegyna // Research in Cardiovascular Medicine, 2013, vol. 2 (4), pp. 153-159. DOI: 10.5812/cardiovascmed.13892
- Ghanbari-Niaki A., Khabazian B.M., Hossaini-Kakhak S.A., Rahbarizadeh F., Hedayati M. Treadmill Exercise Enhances ABCA1 Expression in Rat Liver // Biochemical and Biophysical Research Communications, 2007, vol. 361 (4), pp. 841-846. DOI: 10.1016/j.bbrc.2007.07.100


