The hyperprolactinemia effect on immunohistochemical and morphological characteristics of breast cancer
Автор: Khalimova Zamira Yusupofna, Gumarova Aliya Anvarbekovna
Журнал: Re-health journal @re-health
Рубрика: Нейроэндокринология
Статья в выпуске: 4 (20), 2023 года.
Бесплатный доступ
Objective: Hyperprolactinemia is one of the most common endocrine disorders and can affect both genders at any age. The annual incidence of hyperprolactinemia in women is 8.7 per 100,000 individuals [5], and in the age group of 25-34 years, it increases to 29.3 cases per 100,000 population [3]. The most common causes of hyperprolactinemia include pituitary adenoma, intracranial tumors, medication use, primary hypothyroidism, and chronic kidney failure. Aim: To study the relationship of hyperprolactinemia with the immunohistochemical and morphological characteristics of breast cancer in women. Materials and methods: The study included 100 female patients with breast cancer associated with hyperprolactinemia. To achieve the set objective, all patients were divided into two groups: Group I - 70 women with hyperprolactinemia associated with breast cancer, and Group II - 30 patients with breast cancer and normal prolactin levels. Results: The study included 70 patients with hyperprolactinemia associated with breast cancer (Group I) and 30 women with breast cancer without hyperprolactinemia (Group II, the control group). The age of patients in the first group ranged from 29 to 65 years (mean age in the main group was 49 ± 11.7). In Group II, the age ranged from 19 to 65 years (mean age was 45.2 ± 13.2). Conclusion: Analysis of clinical and laboratory indicators in women with hyperprolactinemia (Group I) associated with breast cancer (BC) revealed a significant predominance of mastalgia (91.4%), nipple edema (53.3%), and breast edema (53.3%) accompanied by the lactoreia-dysmenorrhea syndrome. In contrast, Group II showed a significant prevalence of classical breast cancer symptoms, such as bloody nipple discharge (92%), orange-peel skin (60%), breast redness (40%), and axillary lymph node enlargement (40%). The study of the correlation between histological and molecular subtypes with prolactin levels revealed a predominance of invasive ductal carcinoma in 13 cases (19%), adenocarcinoma in 11 cases (16%), and medullary carcinoma in 10 cases (14.3%) in Group I.
Hyperprolactinemia, breast cancer, prolactin
Короткий адрес: https://sciup.org/14130706
IDR: 14130706
Текст научной статьи The hyperprolactinemia effect on immunohistochemical and morphological characteristics of breast cancer
GIPERPROLAKTINEMIYANING KO‘KRAK BEZI SARATINI IMMUNOHISTOKIMYOVIY VA MORFOLOGIK XUSUSIYATLARIGA TA’SIRI
Dolzarbligi: Mavjud ma'lumotlarga ko'ra, ko'plab tadqiqotlar aylanma prolaktinning yuqori darajalari va ko'krak saratoni rivojlanish xavfi o'rtasida sezilarli bog'liqlikni ko'rsatadi. Maqsad: Giperprolaktinemiya va ayollarda ko'krak bezi saratonining immunogistokimyoviy va morfologik xususiyatlari o'rtasidagi bog'liqlikni o'rganish. Materiallar va usullar: Tadqiqotga TGFRSNMTSOiR, shuningdek, RIEIATM Akademic Turaqulov nomidagi mammologiya bo'limlarida ambulator va statsionar davolanayotgan giperprolaktinemiya bilan bog'liq ko'krak bezi saratoni bilan og'rigan 100 nafar bemor ishtirok etdi. akad. Y. X. To‘raqulova. Ushbu maqsadga erishish uchun barcha bemorlar 2 guruhga bo'lingan: I-asosiy guruh -ko'krak bezi saratoni bilan bog'liq giperprolaktinemiya bilan 70 ayol va II taqqoslash guruhi -normoprolaktinemiya bilan ko'krak bezi saratoni bilan kasallangan 30 bemor. Natijalar: Tadqiqot sub'ektlari ko'krak bezi saratoni va giperprolaktinemiya (asosiy guruh I) bilan bog'liq HPLC bilan kasallangan 70 bemor va giperprolaktinemiyasiz ko'krak bezi saratoni bilan og'rigan 30 ayol (taqqoslash guruhi II) edi. Birinchi guruhdagi yosh 29 yoshdan 65 yoshgacha bo'lgan (asosiy guruhdagi o'rtacha yosh 49 ± 11,7). II guruhda yoshi 19 yoshdan 65 yoshgacha bo'lgan (o'rtacha yoshi 45,2 ± 13,2). Xulosa: Tadqiqot natijalari shuni ko'rsatadiki, BPD erta salbiy oqibatlarning rivojlanishi uchun qo'zg'atuvchi omil hisoblanadi. Bundan tashqari, giperprolaktinemiya bilan ko'krak bezi saratonining noqulay gistostrukturasi va molekulyar pastki turi ko'proq rivojlanadi, bu o'smaning ushbu shakllarini davolashda erta va agressiv yondashuvlarni talab qiladi. Bizning tadqiqotlarimiz shuni ko'rsatadiki, GPRL sharoitida ko'krak bezi saratonining klinik ko'rinishlari xiralashgan va tekislanadi, bu kasallikning kech tashxisiga yordam beradi va shu bilan bemorning omon qolishini kamaytiradi.
Kalit so’zlar: prolactin, giperprolaktinemiya, kukrak bezlari saratoni.
Relevance. Hyperprolactinemia is one of the most common endocrine disorders and can affect both genders at any age. The annual incidence of hyperprolactinemia in women is 8.7 per 100,000 individuals [5], and in the age group of 25-34 years, it increases to 29.3 cases per 100,000 population [3]. The most common causes of hyperprolactinemia include pituitary adenoma, intracranial tumors, medication use, primary hypothyroidism, and chronic kidney failure. Hyperprolactinemia is responsible for menstrual dysfunction in 10% of cases and infertility in 25-40% of cases [4]. According to the World Health Organization (WHO), malignant neoplasms represent the largest burden for women worldwide, with 19.6 million DALYs (Disability-Adjusted Life Years) attributed to breast cancer. Breast cancer is the most frequently diagnosed malignant neoplasm in women globally, with 2.26 million new cases registered in 2020 [95% CI, 2.24–2.79 million] [2]. The prevalence of breast cancer in the context of hyperprolactinemia has not been fully studied. However, the impact of prolactin on mammary glands changes is still not fully understood, as research by various authors has yielded conflicting results. A search on the National Medical Library website using keywords such as prolactin, breast cancer, breast disease, galactorrhea, breast revealed 7433 references [1]. Nevertheless, the most extensively studied effects of prolactin remain its influence on the reproductive organs, especially the mammary gland. Studies related to prolactin have far exceeded those investigating any other hormone in the blood plasma of women with breast cancer or at high risk of developing the disease. Early studies aimed to identify connections between elevated prolactin levels and breast conditions, particularly the association between breast cancer and prolactin levels. There is substantial indirect and direct evidence of prolactin's involvement in carcinogenesis. Most researchers note its association with the severity and duration of the disease [2, 5]. Overall, epidemiological studies indicate a significant correlation between high circulating prolactin levels and the risk of developing breast cancer.
Purpose of the Study: To study the relationship of hyperprolactinemia with the immunohistochemical and morphological characteristics of breast cancer in women, and determine the tactics of their management.
Materials and Methods of the Study: The study included 100 female patients with breast cancer associated with hyperprolactinemia. To achieve the set objective, all patients were divided into two groups: Group I - 70 women with hyperprolactinemia associated with breast cancer, and Group II - 30 patients with breast cancer and normal prolactin levels. All patients underwent clinical examinations, history-taking (with a focus on the age at menarche, cycle regularity, use of oral contraceptives, parity, history of childbirth and pregnancies, breastfeeding, age and duration of menopause), physical examination, and clinical manifestations. The palpation of the breasts was performed to assess lactation and the presence of masses, as well as changes in nipple and areolar condition, thickening or density, and the presence or absence of nipple discharge and its characteristics. Hormonal status was evaluated by measuring prolactin, estradiol (E2), luteinizing hormone (LH), follicle-stimulating hormone (FSH), progesterone, testosterone, thyrotropin, and free thyroxine levels using immunochemiluminescent assays (IHCL). Histological status was determined for all patients through core biopsies, aspiration and incisional biopsies of the breast, and immunohistochemical (IHC) examination, with the expression of ER, PR, HER-2, and Ki-67 receptors being determined.
maging methods included ultrasound examination of the breasts, mammography, and whole-body magnetic resonance imaging (MRI) to detect pituitary adenomas, neoplasms, and the presence of metastases. The inclusion criteria for the study were as follows: women with elevated prolactin levels above 23.3 ng/mL in at least two measurements, confirmed breast cancer, age of the patient between 19 and 65 years. The exclusion criteria included the presence of concomitant endocrine disorders such as hypothyroidism, thyrotoxicosis, type 1 and 2 diabetes, cessation of breastfeeding at least 2 years before the study, stage III breast cancer, liver cirrhosis, and medication use (dopamine agonists, psychotropic drugs, etc.) within a month before the study.
For the statistical analysis of the study results, data from all 100 patients were entered into a "database" created using electronic Excel spreadsheets. The obtained results were processed using standard IBM SPSS Statistics packages.
T he Results of the Study: The study included 70 patients with hyperprolactinemia associated with breast cancer (Group I) and 30 women with breast cancer without hyperprolactinemia (Group II, the control group). The age of patients in the first group ranged from 29 to 65 years (mean age in the main group was 49 ± 11.7). In Group II, the age ranged from 19 to 65 years (mean age was 45.2 ± 13.2). Table 1 presents the age distribution of breast cancer patients based on prolactin levels.
Table 1
Age distribution of women with breast cancer depending on the level of prolactin
|
Age |
Hyperprolactinemia (I group) |
Normoprolactinemia (II group) |
Total |
|||
|
n |
% |
n |
% |
n |
% |
|
|
<20 |
0 |
0 |
1 |
3,33 |
1 |
1 |
|
20-29 |
1 |
1,43 |
3 |
10 |
4 |
4 |
|
30-39 |
5 |
7,14 |
5 |
16,67 |
10 |
10 |
|
40-49 |
20 |
28,57 |
10 |
33,3 |
30 |
30 |
|
50-59 |
32 |
47,14 |
6 |
20 |
28 |
22 |
|
60-65 |
12 |
15,72 |
5 |
16,63 |
27 |
25 |
|
Total |
70 |
100 |
30 |
100 |
100 |
100 |
As seen in Table 1, the majority of cases in Group I were in the age range of 50-59 years (47.14%), while in Group II, women in this age range accounted for 20% of cases.
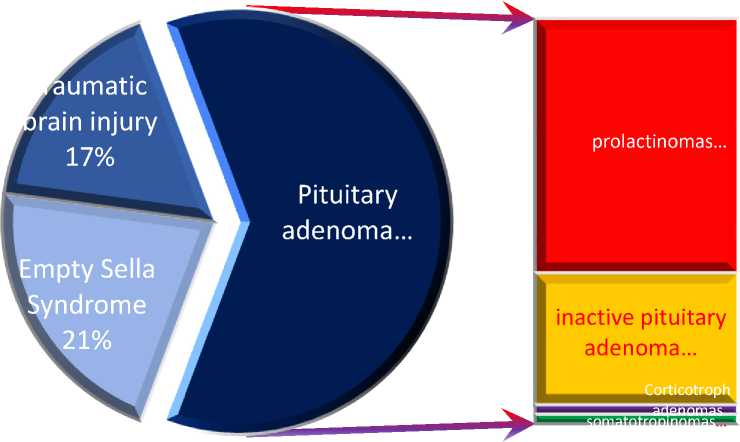
The analysis of the main disease indicators in the study groups revealed the following: among the 70 patients in Group I, the level of prolactin ranged from 96.1 ng/mL to 532.2 ng/mL (mean ± 113.2), while in Group II, it ranged from 9.4 to 17.8 ng/mL (mean ± 3.66) (p<0.001). Magnetic resonance imaging of the sellar region identified the following findings: microadenomas were detected in 43 patients (62%), with prolactinomas present in 27 of them (62.7%), non-functioning pituitary adenomas in 14 (32.5%), and somatotropinomas and Corticotroph adenomas in 1 case each (2.32%) respectively. Empty sella syndrome was observed in 15 patients (21.4%), and no pituitary pathology was found in 12 patients (17.4%), which was attributed to intracranial hypertension of post-traumatic origin (Figure 1).
inactive pituitary adenoma… adenomas somatotropinomas… prolactinomas…
Pituitary
* adenoma…
J Empty Sella
Syndrome
21% umatic rain injury
17%
Fig. 1. Brain changes in MRI examination of Group I.
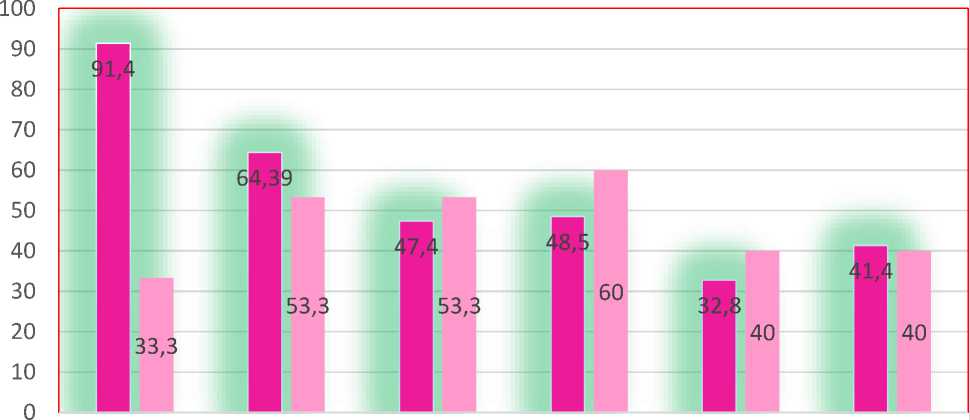
The clinical presentation of the studied patients consisted of the following symptoms: nipple discharge was observed in 100% of patients in Group I, characterized as follows: milk in 28 cases (40%), colostrum in 22 cases (31.4%), bloody discharge in 10 cases (14.2%), and mixed discharge in 10 cases (14.2%) respectively. Breast tenderness was present in 64 patients (91.4%) in Group I, compared to 10 patients (33.3%) in the control group. Breast edema was observed in 45 cases (64.28%) and 16 cases (53.3%) respectively, while skin dimpling was found in 33 cases (47.4%) and 16 patients (53.3%). Nipple retraction was present in 42 cases (60%) compared to 19 cases (63.3%) in the control group. Skin dimpling (orange peel appearance) was seen in 34 cases (48.5%) and 18 cases (60%) in the respective groups. Redness of the breast was noted in 23 cases (32.8%) and 12 cases (40%), and finally, enlarged axillary lymph nodes were observed in 29 cases (41.4%) and 12 cases (40%) in Groups I and II, respectively (Fig. 2).
mastalgia *** swelling of the swelling of the orange peel on get red zof mammary internal mammary mammary gland *** nipple* breasts** glands * lymphadenopathy*
■ I group ■ II group
Note: *P <0.05; **P <0.01; ***P <0.001
Fig. 2. Frequency of occurrence of main symptoms in patients of Group I and II in comparative aspect (n=100)
As seen in the figure, mastalgia was significantly more common in patients of Group I, while classical symptoms of breast cancer (breast and nipple edema, skin dimpling, and redness) predominated in Group II, indicating a more pronounced and evident course of the disease. In cases of hyperprolactinemic association with breast cancer, these manifestations were attenuated.
Furthermore, we conducted an analysis of histological characteristics, describing the odds ratios with 95% confidence intervals and the corresponding p-values (Table 2).
Table
№2
Occurrence of various histological subtypes of breast cancer depending on the level of prolactin (n=100)
|
№ |
Parameters |
Hyperprolactinemia (n=70) |
Normoprolactinemia (n=30) |
Total |
-OR; -95% CI -p-value |
||
|
n |
% |
n |
% |
n (%) |
|||
|
1. |
Invasive ductal carcinoma |
13 |
18,6 |
1 |
3,3 |
14 (14%) |
22,1 2,6-18,7 <0,001 |
|
2 |
Apocrine carcinoma |
8 |
11,4 |
1 |
3,3 |
9 (9%) |
20,5 1,27-9,06 0,01 |
|
3. |
Adenoid cystic carcinoma |
11 |
15,7 |
1 |
3,3 |
12 (12%) |
16,7 2,0-14,8 0,01 |
|
4. |
Intraductal carcinoma in situ |
4 |
5,7 |
7 |
23,3 |
11 (11%) |
0,5 0,131-1,9 0,03 |
|
5. |
Lobular carcinoma in situ |
4 |
5,7 |
8 |
26,7 |
12(12 %) |
0,5 0,131-1,9 0,03 |
|
6. |
Lobular carcinoma |
6 |
8,6 |
1 |
3,3 |
7 (7%) |
7,25 1,8-6,4 0,044 |
|
7. |
Tubular carcinoma |
6 |
8,6 |
1 |
3,3 |
7 (7%) |
7,25 1,8-6,4 0,044 |
|
8. |
Colloid carcinoma |
7 |
10,0 |
1 |
3,3 |
8 (8%) |
8,82 1,01-7,6 0,02 |
|
9. |
Medullary carcinoma |
10 |
14,3 |
2 |
6,7 |
12 (12%) |
7,0 1,3-3,5 0,01 |
|
10. |
Papillary carcinoma |
1 |
1,4 |
7 |
23,3 |
8 (8%) |
0,13 0,01-1,9 0,02 |
|
Total |
70 |
100 |
30 |
100* |
100(10 0%) |
||
The analysis of the frequency of occurrence of histological subtypes of breast cancer revealed a predominant frequency of invasive ductal carcinoma at 14%, followed by adenocarcinoma, lobular carcinoma in situ, and medullary carcinoma at 12% each. However, a separate analysis considering the prolactin level showed that in women with hyperprolactinemia, the most common subtypes of cancer were invasive and aggressive forms, such as invasive ductal carcinoma at 18.6%, adenocarcinoma at 15.7%, and medullary carcinoma at 14.3%. In contrast, in cases of normal prolactin levels, these forms of breast cancer were significantly lower at 3.3%, 3.3%, and 6.7% respectively. Instead, the majority of cases in the normal prolactin group were relatively benign histotypes, including lobular carcinoma in situ, ductal carcinoma in situ, and papillary carcinoma, accounting for 23.3%, 26.7%, and 23.3% respectively.
Moreover, a comparative study of the frequency of benign and malignant histotypes based on prolactin levels revealed that unfavorable subtypes were present in 87% of patients in Group I compared to 26.6% in Group II. Conversely, favorable subtypes constituted the majority of patients in Group II at 73.3%, while only 12.5% of patients in Group I had favorable subtypes (Figure № 3).
I group (n=70)
II group (n=30)
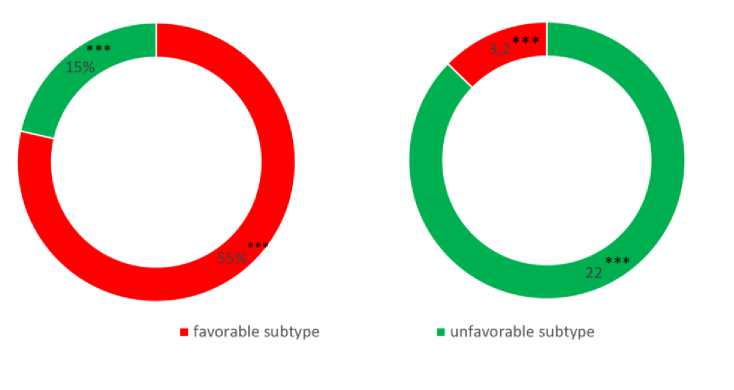
Note: ***Significant differences when comparing the control group, p<0.001 Fig. 3. Distribution of breast cancer histological subtypes depending on the level of prolactin
Further, we analyzed the distribution of molecular subtypes in relation to the prolactin level. A significant difference was observed in the distribution of triple-negative breast cancer in Group I compared to Group II, with proportions of 32.9% and 3.3%, respectively. We also observed a notable difference in the Luminal B HER2+ subtype, with 20% in Group I compared to 3.3% in Group II. As for the Non-luminal type, our results showed 14.3% in Group I and 3.3% in Group II. The most significant difference was found in the Luminal A subtype, with 32% in breast cancer cases associated with normal prolactin levels compared to 14.3% in cases with hyperprolactinemia. Below is the contingency table of breast cancer molecular subtypes and prolactin levels (Table № 3).
Table №3
Molecular Subtypes of Breast Cancer in Normal and Hyperprolactinemia Conditions
|
№ |
Parametrs |
Hyperprolactinemia (n=70) |
Normorprolactinemia (n=30) |
Total |
Pearson's r; OR; 95% CI; p-value |
||
|
n |
% |
n |
% |
n(%) |
|||
|
1. |
Triple-negative type |
23 |
32,9 |
1 |
3,3 |
24 (24%) |
0,74 23,0 3,3-15,9 <0,001 |
|
2 |
Luminal B HER2/neu-negative |
13 |
18,6 |
5 |
16,7 |
17 (17%) |
0,33 4,9 1,38-17,8 0,01 |
|
3. |
Luminal B HER2/neu-positive |
14 |
20 |
1 |
3,3 |
15 (15%) |
0,5 25,3 3,0-21,1 <0,001 |
|
4. |
Non-luminal type |
10 |
14,3 |
1 |
3,3 |
11 (11%) |
0,4 14,0 1,7-12,2 0,003 |
|
5. |
Luminal A |
10 |
14,3 |
22 |
73,3 |
32 (32%) |
-0,401 0,1 0,06-0,55 0,002 |
|
Total |
70 |
100 |
30 |
100* |
100(100%) |
||
In addition, we conducted an analysis that correlated the molecular subtype with the size of the tumor, histological type, patient's age, and the stage of breast cancer.
Table №4
Frequency of occurrence of molecular subtypes depending on the stage of breast cancer and prolactin level.
|
tumor characteristics |
Group of Patients I group (n=70) II group (n=30) |
Luminal A |
Luminal B HER2/neu-negative |
Luminal B HER2/neu-positive |
Nonluminal type |
Triplenegative type |
N (%) |
p-value |
|
disease stage |
||||||||
|
I stage T1N0M0 |
I группа (n=70) |
1 (1,4%) |
1 (1,4%) |
- |
1 (1,4%) |
1 (1,4%) |
4 (5,7%) |
<0,001 |
|
II группа |
5 (16,7%) |
- |
1 (3,3%) |
- |
1 (3,3%) |
7 (23,3%) |
||
|
II stage T1N1M0 |
I группа |
2 (2,9%) |
- |
1 (1,4%) |
1 (1,4%) |
1 (1,4%) |
5 (7,1%) |
0,072 |
|
T2N0M0 T2N1Mo |
II группа |
15 (49,9) |
3 (10%) |
3 (10%) |
1 (3,3%) |
- |
22 (73,3) |
|
|
III stage T3N1M0 |
I группа |
3 (4,29% |
5 (7,14) |
5 (7,14) |
2 (2,9%) |
12 (17,14) |
27 (38,6) |
0,005 |
|
II группа |
- |
- |
- |
- |
- |
(0%) |
||
|
IV stage T1N1M1 N2N1M1 T3N1M1 T4N1M1 |
I группа |
5 (7,14) |
7 (10%) |
8 (11,43%) |
5 (7,14) |
9 (12,9%) |
34 (48,6%) |
<0,001 |
|
II группа |
- |
1 (3,3%) |
- |
- |
- |
1 (3,3%) |
There was a significant difference in the distribution of patients across all four stages in both patient groups. In the first stage, the distribution was 5.7% versus 23.3% (p<0.001), in the second stage, it was 7.1% versus 73.3% (p<0.072), in the third stage, it was 38.6% versus 0% (p=0.005), and in the fourth stage, it was 48.6% versus 3.3% (p<0.001). This means that with hyperprolactinemia, the disease progresses rapidly, leading to rare diagnoses of breast cancer at stages I and II, while its frequency sharply increases at stages III and IV, which accounted for 38.6% and 48% respectively (Table №4).
On the other hand, when considering the relationship between tumor size and molecular subtype, no significant association was found (p=0.717), indicating that the tumor size does not influence the molecular subtype of breast cancer. The data are presented in Table №5.
Table №5
Study of molecular subtypes and tumor size in breast cancer depending on prolactin level.
|
tumor size |
Group of Patients I group (n=70) II group (n=30) |
Luminal A |
Luminal B HER2/neu-negative |
Luminal B HER2/neu-positive |
Nonluminal type |
Triple-negati ve type |
N (%) |
p-value |
|
0,717 |
||||||||
|
<2 cm |
I группа |
5 |
6 |
4 |
3 |
3 |
21 (30%) |
|
|
II группа |
9 |
3 |
2 |
1 |
1 |
16 (53,3%) |
||
|
2-5 cm |
I группа |
6 |
6 |
7 |
8 |
14 |
41 (58,6%) |
|
|
II группа |
10 |
3 |
4 |
- |
- |
14 (46,7%) |
||
|
>5 cm |
I группа |
3 |
1 |
1 |
1 |
2 |
8 (11,4%) |
|
|
II группа |
- |
- |
- |
- |
- |
0 (0%) |
Table № 6
Frequency of breast cancer occurrence considering molecular and histological subtypes depending on prolactin level (n=100).
|
Characteristi cs of Tumors |
Group of Patien ts I group (n=70) II group (n=30) |
Lumin al A |
Luminal B HER2/ne u-negative |
Luminal B HER2/ne u-positive |
Nonlumin al type |
Triple-negativ e type |
N (%) |
p-value |
|
Histological type |
<0,00 1 |
|||||||
|
Invasive ductal carcinoma |
I групп а |
4 |
3 |
2 |
0 |
4 |
13 (18,6 %) |
<0,00 1 |
|
II групп а |
1 |
0 |
0 |
0 |
0 |
1 (3,3%) |
||
|
Apocrine carcinoma |
I групп а |
1 |
1 |
2 |
2 |
2 |
8 (11,4 %) |
<0,00 1 |
|
II групп а |
0 |
0 |
0 |
1 |
0 |
1 (3,3%) |
||
|
Adenoid cystic carcinoma |
I групп а |
0 |
2 |
4 |
2 |
3 |
11 (15,7 %) |
<0,00 1 |
|
II групп а |
1 |
0 |
0 |
0 |
0 |
1 (3,3%) |
||
|
Intraductal carcinoma in situ |
I групп а |
0 |
0 |
1 |
0 |
3 |
4 (5,7%) |
<0,00 1 |
|
II групп а |
5 |
2 |
0 |
0 |
0 |
7 (23,3 %) |
||
|
Lobular carcinoma in situ |
I групп а |
0 |
1 |
1 |
0 |
2 |
4 (5,7%) |
<0,00 1 |
|
II групп а |
5 |
2 |
1 |
0 |
0 |
8 (26,7 %) |
||
|
Lobular carcinoma |
I групп а |
0 |
1 |
0 |
2 |
3 |
6 (8,6%) |
<0,00 1 |
|
II групп а |
1 |
0 |
0 |
0 |
0 |
1 (3,3) |
||
|
Tubular carcinoma |
I групп а |
3 |
1 |
0 |
1 |
1 |
6 (8,6%) |
<0,00 1 |
|
II групп а |
0 |
1 |
0 |
0 |
0 |
1 (3,3%) |
||
|
Colloid carcinoma |
I групп а |
1 |
1 |
1 |
2 |
2 |
7 (10%) |
<0,00 1 |
|
II групп а |
1 |
0 |
0 |
0 |
0 |
1 (3,3%) |
||
|
Medullary carcinoma |
I групп а |
1 |
3 |
3 |
0 |
3 |
10 (14,3 %) |
<0,00 1 |
|
II групп а |
2 |
0 |
0 |
0 |
0 |
2 (6,7%) |
||
|
Papillary carcinoma |
I групп а |
0 |
0 |
0 |
1 |
0 |
1 (1,4%) |
<0,00 1 |
|
II групп а |
6 |
0 |
0 |
0 |
1 |
7 (23,3 %) |
The study investigated the histological type of tumors in 100 patients (Table № 6), with the predominant type being invasive ductal carcinoma at 14% of all cases (Group I - 92.8%, Group II - 7.14%). The second most common histological types were three types: medullary carcinoma at 12% (Group I - 83.3% vs. 16.6% in Group II), adenoid-cystic carcinoma at 12% overall (Group I - 91.6%, Group II - 8.3%), and ductal carcinoma in situ at 12% (33.3% in Group I and 66.6% in Group II). It should be noted that papillary carcinoma of the breast was present in 8% of all histological types, with 12.5% in Group I and 87.5% in Group II, respectively. From this, it can be inferred that breast cancer subtypes with more favorable prognosis are more prevalent in Group II, while Group I primarily had histological types associated with an unfavorable prognosis (p <0.001).
In patients with hyperprolactinemia, the most prevalent histological type was ductal carcinoma in situ, accounting for 41.89% of cases. The frequency of the more unfavorable triple-negative breast cancer subtype was higher in patients with hyperprolactinemia. In the group of patients with normoprolactinemia, no predominance of a specific histological type or its biological subtype was observed. Therefore, the main predisposing factor for breast cancer associated with hyperprolactinemia is the severity of hyperprolactinemia symptoms related to pituitary adenoma.
High expression of prolactin is characteristic of Luminal subtypes of tumors and is associated with smaller tumor size in Luminal B HER2-negative breast cancer. Additionally, the level of prolactin expression depends on the age of the patients, but this relationship is opposite for Luminal A and Luminal B HER2-negative and triple-negative subtypes of breast cancer.
All of the above indicates a significant role of prolactin in the development and progression of these breast cancer subtypes. In Luminal B HER2-positive breast cancer, prolactin likely acts as an oncogene, while in Luminal B HER2-negative and Luminal A subtypes, it may play a role as a tumor suppressor.
Thus, patients with hyperprolactinemia-associated breast cancer require close attention from the moment of diagnosis. The study results show that prolactin is a triggering factor in the development of early unfavorable outcomes. Moreover, in hyperprolactinemia, breast cancer forms with unfavorable histological structure and molecular subtype develop more frequently, requiring early and aggressive treatment approaches for these tumor forms. Our research suggests that in conditions of hyperprolactinemia, clinical manifestations of breast cancer may be masked and downplayed, leading to delayed disease diagnosis and thereby reducing patient survival.
Conclusions:
Analysis of clinical and laboratory indicators in women with hyperprolactinemia (Group I) associated with breast cancer (BC) revealed a significant predominance of mastalgia (91.4%), nipple edema (53.3%), and breast edema (53.3%) accompanied by the lactoreia-dysmenorrhea syndrome. In contrast, Group II showed a significant prevalence of classical breast cancer symptoms, such as bloody nipple discharge (92%), orange-peel skin (60%), breast redness (40%), and axillary lymph node enlargement (40%). The study of the correlation between histological and molecular subtypes with prolactin levels revealed a predominance of invasive ductal carcinoma in 13 cases (19%), adenocarcinoma in 11 cases (16%), and medullary carcinoma in 10 cases (14.3%) in Group I. In contrast, Group II showed an increased frequency of in situ intraductal and papillary carcinoma in 7 cases (23.3%) and 8 cases (26.7%) respectively, compared to 5.7%, 5.7%, and 1.4% in hyperprolactinemia (p<0.001).
Comparative study of the immunohistochemical characteristics of the receptor status in breast cancer tissue in the investigated groups revealed a significant increase in HER2/neu (67.2%) and Ki-67 (52.7%) in Group I compared to (9.9%) and (9.6%) respectively in Group II (p<0.001). Estrogen receptors (ER) and progesterone receptors (PR) predominated significantly in women of Group II, with 81.6% and 59.6%, respectively, compared to 24.8% and 19.3% in Group I (p<0.001). These findings suggest that in cases of normal prolactin levels, breast cancer is highly differentiated with low proliferative activity and minimal aggressive progression. The study of the immunohistochemical characteristics of the receptor status of breast tissue, taking into account the prolactin level and molecular subtype of the tumor, revealed a predominance of triple-negative subtype in 32.9% compared to 3.3%, as well as non-luminal subtype in 20% compared to 3.3% in Group I. Conversely, in Group II, the majority of cases were luminal A in 73.3% compared to 14.3% of molecular subtypes (p<0.001). The correlation analysis between prolactin levels and clinical, hormonal, histological, molecular subtypes, and immunohistochemical indicators showed a positive correlation with mastalgia (r=0.71), Ki-67 (r=0.6), HER2/neu (r=0.7), metastasis (r=0.56), estradiol (0.63), and invasive ductal carcinoma (r=0.6) and adenocarcinoma (r=0.5), while showing a negative correlation with ER (-0.67) and PR (-0.59), as well as papillary carcinoma (r=-0.78), in situ ductal carcinoma (r=-0.69), and in situ intraductal carcinoma (r=-0.65) respectively, with a significance of p=0.001.
Список литературы The hyperprolactinemia effect on immunohistochemical and morphological characteristics of breast cancer
- Porter, P. Westernization of Risks for Women? Breast Cancer in LowIncome Countries. N. Engl. J. Med. 2008, 358, 213–216.
- World Health Organization. Global Health Estimates 2016: Disease Burden by Cause, Age, Sex, by Country and by Region, 2000–2016; World Health Organization: Geneva, Switzerland, 2018.
- Гилев А.А., Лусевич А.И., Щербакова Е.С.: Синдром гиперпролактинемии у женщин. Современные проблемы науки и образования. – 2021. – № 6
- Исах И.А., Юсуф Р., Исах Х.С., Рандава А.Дж., Адесиюн А.Г. Гиперпролактинемия и женское бесплодие: клиническая картина в третичном медицинском учреждении в Северной Нигерии. Сахель Мед Дж. 2018; 21 :1–5.
- Сергеева Н. И. Участие пролактина в маммогенезе и канцерогенезе молочной железы / Сергеева Н.И., Дзеранова Л.К., Меских Е.В., Рожкова Н.И. и др. // Акушерство и гинекология. – 2005. – № 3. – С. 13–17.


