The impact of multiple overlapping new generations drug-eluting stents on six-month clinical outcomes in patients with anterior STEMI treated with primary PCI
Автор: Elakabawi Karim, Feng Jiahao, Ullah Hameed, Tawfik Wael, Mahrous Mohamed, Salem Mohamed, Guo Ning, Yuan Zuyi
Журнал: Cardiometry @cardiometry
Рубрика: Original research
Статья в выпуске: 17, 2020 года.
Бесплатный доступ
Background Implantation of multiple (≥ 2) overlapping stents has been associated with adverse outcomes after percutaneous coronary intervention (PCI). However, data regarding the impact of multiple new generations drug-eluting stents (DES) during primary PCI (PPCI) are limited. Objective To evaluate the impact of multiple overlapping stents implantation on the 6-months clinical outcomes of anterior STEMI patients undergoing PPCI with new generations DES. Methods We evaluated a total of 1078 consecutive anterior STEMI patients who underwent PPCI with deployment of new generations DES. The patients were divided according to the number of implanted stents into 2 groups; multiple-stents group having ≥2 overlapping stents (n =388) and single-stent group (n =690). We compared the rates of major adverse cardiovascular events (MACEs; composite of cardiac death, reinfarction, ischemia-driven target vessel revascularization (TVR), definite stent thrombosis (ST), and stroke) between the 2 study groups. Results There was a non-significant trend toward increased in-hospital mortality in the multiple-stents group [3.4% vs 1.7%; P=0.096]related to a significant higher rate of cardiogenic shock [9.5% vs 6.1%; P=0.037] and post-procedural suboptimal TIMI flow [26.3% vs 19.4%; P=0.009] as compared to the single-stent group. Yet, there were no significant differences between the 2 groups in the incidence of 6-month MACEs: [9% vs 7.1%; P=0.259], cardiac death: [4.6% vs 3%; P=0.173], reinfarction: [3.6% vs 3.2%; P=713], TVR: [4.4% vs 4.1%; P=0.799], and definite ST: [3.6% vs 2.8%; P=0.43], respectively. Conclusion The use of multiple new generations DES, if needed, is relatively safe and acceptable during PPCI for STEMI patients.
St elevation myocardial infarction, percutaneous coronary intervention, drug-eluting stents, stent overlap
Короткий адрес: https://sciup.org/148311479
IDR: 148311479 | DOI: 10.12710/cardiometry.2020.17.7684
Текст научной статьи The impact of multiple overlapping new generations drug-eluting stents on six-month clinical outcomes in patients with anterior STEMI treated with primary PCI
Karim Elakabawi, Jiahao Feng, Hameed Ullah, Wael Tawfik, Mohamed Mahrous, Mohamed Salem, Ning Guo, Zuyi Yuan. The impact of multiple overlapping new generations drug-eluting stents on six-month clinical outcomes in patients with anterior STEMI treated with primary PCI. Cardiometry; Issue 17; November 2020; p.76-84; DOI: 10.12710/cardiometry.2020.17.7684; Available from: http://www.
Primary percutaneous coronary intervention (PPCI) with full lesion coverage using a single stent or multiple overlapping stents is the preferred therapeutic option for patients presenting with an acute ST segment elevation myocardial infarction (STEMI).1
Multiple overlapping stents have been used in up to 30% of cases undergoing PCI due to the presence of either long target lesion, accidental dissections, or incomplete target lesion coverage with a single stent. 2 The need of multiple stents and increased total stent(s) length is usually a correlate of extensive coronary artery disease (CAD) and have been associated with worse clinical outcomes after PCI when compared with the single or shorter stents specially in the era of bare-metal stents (BMS) 3,4. However, the effect of multiple drug-eluting stents (DES) showed controversy. In studies assessing the impact of overlapping ≥ 2 paclitaxel-eluting stents (PES) in comparison with BMS, there was improved efficacy, but at the cost of increased rates of peri-procedural infarction (8.3% versus 3.3%, respectively), that was suggested to occur as a result of a reduction in side branch flow. 5,6 In contrast, multiple overlapping sirolimus-eluting stents (SES) demonstrated similar rates of adverse events compared with a single SES.7,8 Newer generations DES carried more favorable long term clinical outcomes than first-generation stents, 9 yet few studies have investigated the implications of multiple stents on the short and long term clinical outcomes. 10,11 Moreover, those prior studies have included mostly elective PCI patients and the prognostic impact of overlapping new generations DES in the context of STEMI patients treated with primary PCI are not well established. In this study we sought to compare the six-month clinical outcomes of PPCI with multiple DESs versus a single DES.
Materials and Methods
Study population
This is a retrospective observational study that enrolled 1078 consecutive patients admitted with anterior STEMI at the First Affiliated Hospital of Xi'an Jiaotong University (China) between January 2016 and December 2018. All patients had PPCI within 12 hours from symptom onset. The study was approved by the institutional ethics committee. Anterior STEMI was diagnosed on the basis of typical ischemic chest pain enduring for ≥20 minutes with electrocardiographic (ECG) changes (ST-segment elevations of ≥1mm in ≥2 contiguous precordial leads other than V2,3 or new onset left bundle branch block) with rise in cardiac biomarkers.12 Key exclusions criteria were patients with non-anterior STEMI, acute anterior STEMI with onset >12 hours or unknown, history of coronary artery graft surgery (CABG) or PCI to the LAD, patients treated conservatively or with Percutaneous transluminal coronary angioplasty (PTCA), patients who received thrombolytic therapy prior to angiography and patients lost for follow-up.
Baseline evaluation
Baseline data were collected from the hospital medical records including: full medical history (demographic criteria, coronary risk factors, time from symptom onset, and associated co-morbidities), physical examination, emergency and post-procedural ECG, trans-thoracic-echocardiography, laboratory data, and in-hospital clinical status , together with the related catheterization and PPCI data.
Primary PCI procedure
All patient had 300mg chewable aspirin along with the recommended dose of ticagrelor or clopi-dogrel and a weight-adjusted bolus dose of heparin (100units/kg). Dual oral antiplatelet therapy (DAPT) was prescribed for one year after stent implantation in all patients. Other medications were managed according to the prudence of the doctor in charge and within the scope of recommended clinical approaches. The radial artery was routinely used in almost all cases and the PPCI procedure was done according to the standard techniques [13]. Thrombus aspiration and/or use of glycoprotein inhibitors were restricted to cases with heavy thrombus burden. Implantation of stent(s) to fully cover the culprit lesion was routinely implied with routine optimization using pre and post-dilatation unless the infarct-related artery (IRA) was heavily calcified or the reference luminal diameter was <2.5mm. Intravascular ultrasound (IVUS) or optical coherence tomography (OCT) were utilized, if needed, in selected cases. Second generation permanent polymer DES (PP-DES) (zotarolimus-eluting and everolimus-eluting stents) and biodegradable polymer DES (BD-DES) were used in all patients. The most common cause for using ≥2 DESs was inadequate lesion coverage due to long target lesion, whereas iatrogenic dissections were rare. Two experienced interventional cardiologists evaluated each angiogram with emphasis on type and total length of the stents used, thrombus burden grade, the presence of calcification, residual stenosis or dissections, and final TIMI flow after the procedure.
Multiple overlapping stents were defined as the presence of ≥2 stents used to cover a single culprit lesion with an overlapping area of ≥1 mm. Total ischemic time was the time from symptom-onset to first balloon dilatation. Suboptimal TIMI flow was TIMI ≤2 flow and/or CTFC >27 after finishing the procedure, in the absence of dissection, residual stenosis, or spasm [14].
Study follow up
Information on the Six-month clinical an in-hospital outcomes were obtained from outpatient visits, emergency records, or telephone interviews. We compared the impact of multiple stents versus a single stent on the 6-month clinical outcomes, including cardiac mortality, major adverse cardiovascular events (MACE, defined as being the composite of
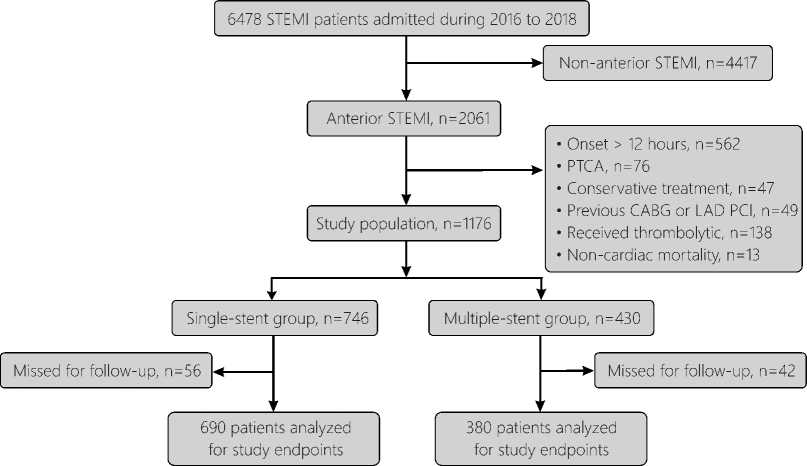
Figure 1. Flowchart of the present study population.
STEMI: ST-elevation myocardial infarction; PTCA: percutaneous transluminal coronary angioplasty; CABG, coronary artery bypass graft; LAD, Left anterior descending; PCI, percutaneous coronary intervention.
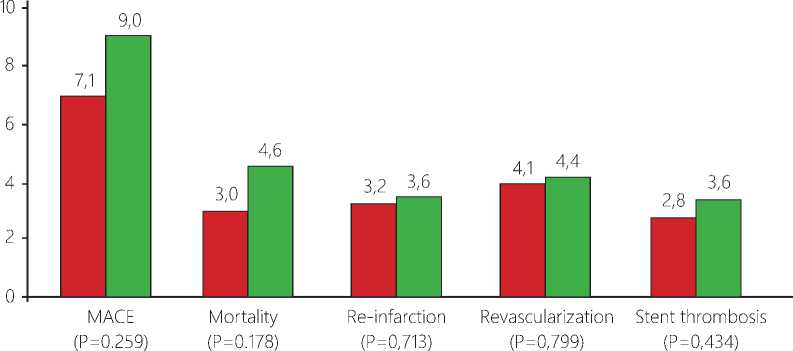
Single stent group □ Multiple stent group
Figure 2. Six-month clinical outcomes.
death, re-infarction, ischemia-driven target vessel revascularization, definite stent thrombosis, and stroke) and post-catheterization reevaluation of general and cardiac status, examining for development of pump failure, cardiogenic shock, mechanical complications or malignant arrhythmias between the two study groups.
Six-month cardiac mortality was defined as death due to cardiac causes during hospitalization or within 6 months post-discharge. Re-infarction was repeated clinical symptoms or development of new ECG changes associated with a new rise of cardiac troponin (cTn) values >99th percentile upper reference limit (URL) in patients with normal baseline values or an increase of cTn values >20% of the base- line value when it is above the 99th percentile URL [12]. Ischemia-driven target vessel revascularization (TVR) is repeated revascularization with PCI or CABG of the IRA driven by symptoms or objective evidence of ischemia [13]. Definite stent thrombosis (ST) was defined based on the Academic Research Consortium (symptoms suggestive of an acute coronary syndrome and angiographic or pathologic confirmation of stent thrombosis) and was subclassified in this study into early ST (0 to 30 days), late ST (31 to 180 days) [15].
Statistical Analysis
Data management and statistical analysis were done using SPSS vs.21. (IBM, Armonk, New York,
Table 1. Clinical Characteristics
|
Variables |
Single stent (n = 690) |
Multiple stents (n = 388) |
P value |
|
|
Age (years) Age ≥65 |
Mean ±SD Yes n (%) |
58.1±12.5 218 (31.6) |
59±12.5 122 (31.4) |
0.243 0.959 |
|
Sex |
Females n (%) Males n (%) |
116 (16.8) 574 (83.2) |
63 (16.2) 325 (83.8) |
0.808 |
|
Weight (kg) |
Mean ±SD |
71.5 ±11.8 |
72.3 ±11.7 |
0.284 |
|
Hypertension |
Yes n (%) |
299 (43.3) |
196 (50.5) |
0.023 |
|
Diabetes |
Yes n (%) |
130 (18.8) |
82 (21.1) |
0.363 |
|
Smoking |
Yes n (%) |
381(55.2) |
220 (56.7) |
0.638 |
|
Previous IHD |
Yes n (%) |
57 (8.3) |
31 (8) |
0.876 |
|
Previous CVS |
Yes n (%) |
39 (5.7) |
18 (4.6) |
0.476 |
|
Total ischemic time, hr |
Mean ±SD |
5.5 ±3 |
5.4 ±3.2 |
0.537 |
|
Admission Heart rate, bpm |
Mean ±SD |
82.9 ±13.9 |
82.6 ±12.7 |
0.765 |
|
Systolic blood pressure, mm Hg |
Mean ±SD |
133.1±20.5 |
133.9±23.9 |
0.604 |
|
Diastolic blood pressure, mm Hg |
Mean ±SD |
86.6 ±13.3 |
86.5 ±14.5 |
0.952 |
|
Killip classification |
One n (%) >1 n (%) |
535 (77.5) 155 (22.5) |
306 (78.9) 82 (21.1) |
0.613 |
|
Cardiac arrest on presentation |
Yes n (%) |
33(4.8) |
15(3.9) |
0.484 |
|
Serum Creatinine, umol/L |
Mean ±SD |
66.1 ±17.3 |
67.3 ±20 |
0.333 |
|
Triglyceride, mmol/L |
Mean ±SD |
1.7 ±1.1 |
1.6 ±1.1 |
0.073 |
|
HDL cholesterol, mmol/L |
Mean ±SD |
1 ±0.27 |
1 ±0.29 |
0.614 |
|
LDL cholesterol, mmol/L |
Mean ±SD |
2.6±1 |
2.6 ±0.9 |
0.655 |
|
LPa, mg/dL |
Mean ±SD |
215 ±188.1 |
209.5 ±168.9 |
0.638 |
|
Hemoglobin, g/L |
Mean ±SD |
147 ±17.1 |
145.4 ±17.9 |
0.143 |
|
Platelets count, (x109/L) |
Mean ±SD |
219 ±66.8 |
209.3 ±62.3 |
0.020 |
|
White blood cells count, (x109/L) |
Median (range) |
10.7(3.2 – 26.1) |
10.4 (4.3 – 25.8) |
0.012 |
|
TNT-hs, ng/mL |
Median (range) |
0.4 (0.01 – 10.1) |
0.4 (0.01 – 10.2) |
0.795 |
|
CK_MB |
Median (range) |
52 (8- 700) |
47.5 (9 - 671) |
0.512 |
|
NT-ProBNP, pg/mL |
Median (range) |
248.5 (5 - 13256) |
284(5 - 15428) |
0.128 |
|
hsCRP, mg/L |
Median (range) |
2.8 (0.02 – 10.4) |
2.5 (0.09 - 10) |
0.845 |
IHD : Ischemic heart disease; CVS: Cerebrovascular stroke; HDL: high-density lipoprotein; LDL: low-density lipoprotein; LPa: lipoprotein (a); TNT-hs: Troponin T-high sensitive; CK-MB: Creatine kinase- myocardial band; NT-ProBNP: N-terminal proB-type Natriuretic Peptide; hsCRP= High-sensitivity C-reactive Protein.
the United States). Statistical data were calculated as means and standard deviations for normally distributed numerical data or medians and ranges for abnormally distributed data. Categorical data were calculated as numbers and percentages. Comparisons between groups (multiple-stents vs. single-stent) were done using independent t-test or Mann Whitney U-test for normally and non-normally distributed numeric variables, respectively. Categorical data were compared using the Chi-square test or Fisher exact tests, as appropriate. All P-values were two-sided. P-values ≤0.05 were considered statistically significant.
ResultsClinical characteristics
As shown in 1, a total of 1078 anterior STEMI patients were included in the present study, of whom 690 patients (64%) were treated with a single stent (single-stent group) and 388 (36%) patients were treated with ≥2 overlapping stents (multiple-stents group). The clinical features, risk factors, and laboratory data of the 2 study groups are shown in Table 1. Compared to patients treated with a single stent, patients required multiple stents tended to be more hypertensive with lower baseline platelets and white blood cells counts. The other baseline clinical and laboratory variables showed no significant differences between the 2 groups.
Angiographic data andPrimary PCI Procedure
Patients of the multiple-stents group had significant higher left main or multi-vessels disease involvements, a higher prevalence of lesion calcification, and a smaller mean implanted stent diameter. There was also a significant increase in the final suboptimal TIMI flow and the need for hemodynamic support using in-tra-aortic balloon pump (IABP) in the multiple-stents group, while the thrombus aspiration catheters were used more often in patients with a single stent. Between groups analysis did not show significant differences in other parameters. Table 2.
Table 2. Angiographic Data and Primary PCI Procedure
|
Variables |
Single stent (n = 690) |
Multiple stents (n = 388) |
P value |
|
|
Left main involvement |
Yes n (%) |
52 (7.5) |
47 (12.1) |
0.013 |
|
Number of diseased vessels |
One n (%) Two n (%) Three n (%) |
248 (35.9) 242 (35.1) 200 (29.0) |
80 (20.6) 153 (39.4) 155 (39.9) |
<0.001 |
|
Heavy thrombus burden (grade 4) |
Yes n (%) |
106 (15.4) |
59 (15.2) |
0.946 |
|
Moderate/severe calcification |
Yes n (%) |
58 (8.4) |
51 (13.1) |
0.013 |
|
Initial TIMI flow grade ≤1 |
Yes n (%) |
488 (70.7) |
276 (71.1) |
0.887 |
|
Stent type:
|
Yes n (%) |
384(65.5) 306(62.2) |
202(34.5) 186(37.8) |
0.256 |
|
Total Stent length, mm |
Mean ±SD |
27.7 ±7.3 |
53.1 ±10.9 |
<0.001 |
|
Mean stent diameter, mm |
Mean ±SD |
3.07 ±0.37 |
2.96 ±0.32 |
<0.001 |
|
Final TIMI flow grade ≤2 |
Yes n (%) |
134 (19.4) |
102 (26.3) |
0.009 |
|
Approach |
Radial n (%) Femoral n (%) |
665 (96.4) 25 (3.6) |
367 (94.6) 21 (5.4) |
0.163 |
|
Thrombus Aspiration |
Yes n (%) |
183(26.5) |
82(21.1) |
0.049 |
|
Glycoprotein IIb/IIIa inhibitor |
Yes n (%) |
96 (13.9) |
64 (16.5) |
0.252 |
|
IC Nitroprusside |
Yes n (%) |
54 (7.8) |
37 (9.5) |
0.332 |
|
IC Adrenaline |
Yes n (%) |
32 (4.6) |
19 (4.9) |
0.847 |
|
Atropine |
Yes n (%) |
56(8.1) |
41 (10.6) |
0.177 |
|
Noradrenaline |
Yes n (%) |
148 (21.4) |
81 (20.9) |
0.825 |
|
IABP |
Yes n (%) |
52 (7.5) |
46 (11.9) |
0.018 |
DES: drug-eluting stents; TIMI: thrombolysis in myocardial infarction; IC: intracoronary; IABP: Intra-aortic balloon pump
Table 3. In-hospital clinical outcomes
|
Single stent (n = 690) |
Multiple stents (n = 388) |
P value |
||
|
In-hospital Mortality |
Yes n (%) |
12 (1.7) |
13 (3.4) |
0.096 |
|
Cardiogenic shock |
Yes n (%) |
42 (6.1) |
37 (9.5) |
0.037 |
|
Heart Failure |
Yes n (%) |
147 (21.3) |
97 (25.0) |
0.164 |
|
Malignant arrhythmias |
Yes n (%) |
87 (12.6) |
41 (10.6) |
0.320 |
|
Bradyarrhythmias |
Yes n (%) |
54 (7.8) |
36 (9.3) |
0.408 |
|
Bleeding |
Yes n (%) |
24 (3.5) |
23 (5.9) |
0.059 |
|
Creatine kinase peak, U/L |
Median (range) |
4628 (685 - 16721) |
4572 (680 - 15078) |
0.222 |
|
ST segment resolution |
<70% n (%) >70% n (%) |
268 (38.8) 422 (61.2) |
151 (38.9) 237 (61.1) |
0.980 |
|
Ejection faction (%) |
Mean ±SD |
45.5 ±6.9 |
45.9 ±6.6 |
0.339 |
|
Hospital stay (days) |
Mean ±SD |
4.6 ±2.4 |
4.9 ±2.5 |
0.047 |
DES: drug-eluting stents; TIMI: thrombolysis in myocardial infarction; IC: intracoronary; IABP: Intra-aortic balloon pump
Table 4. Six-month clinical outcomes
|
Single stent (n = 690) |
Multiple stents (n = 388) |
P value |
|||
|
MACE |
Yes |
n (%) |
49 (7.1) |
35 (9.0) |
0.259 |
|
Mortality (cardiac) - Total |
Yes |
n (%) |
21 (3) |
18 (4.6) |
0.178 |
|
- In-hospital |
12 (1.7) |
13 (3.4) |
0.096 |
||
|
Re-infarction |
Yes |
n (%) |
22 (3.2) |
14 (3.6) |
0.713 |
|
Revascularization |
Yes |
n (%) |
28 (4.1) |
17 (4.4) |
0.799 |
|
Stent thrombosis (ST) Timing of ST: |
Yes |
n (%) |
19 (2.8) |
14 (3.6) |
0.434 |
|
- Early |
Yes |
n (%) |
5 (0.7) |
7(1.8) |
0.105 |
|
- Late |
14(2.0) |
7(1.8) |
0.798 |
||
|
Ischaemic Stroke |
Yes |
n (%) |
0 (0.0) |
2 (0.2) |
0.059 |
MACE: Major adverse cardiovascular events (cardiac death, reinfarction, ischemia-driven target vessel revascularization, stent thrombosis, or ischemic stroke).
In-hospital clinical outcomes
Patients with multiple stents had a significant higher rate of cardiogenic shock [9.5% versus 6.1%, respectively; P=0.037] and a more prolonged hospital stay [4.9 ±2.5 versus 4.6 ±2.4 days, respectively; P=0.047] than the single-stent group. There was also a trend, albeit not statistically significant, toward increased rates of in-hospital death [3.4% versus 1.7%; P=0.096], heart failure [25% versus 21.3%; P=0.164], and bleeding complications [5.9% versus 3.5%; P=0.056] in the multiple-stents group. Table 3.
Six-month clinical outcomes
The six-month clinical outcomes are listed in Table 4. During the follow-up period (196.4±36.2 days), there were no significant differences between the multiple-stents group and the single-stent group in the incidence of 6-month cardiac death: [18 (4.6%) and 21 (3%), respectively; P=0.173], MACEs: [35 (9%) and 49 (7.1%), respectively; P=0.259], re-infarction: [14 (3.6%) and 22 (3.2%), respectively; P=713], TVR: [17 (4.4%) and 28 (4.1%), respectively; P=0.799], and definite ST: [14 (3.6%) and 19 (2.8%), respectively; P=0.434].
Discussion
The principal findings of the current study can be summarized as follows:
-
(1) The 6-month rates of cardiac mortality, MACE, re-infarction, TVR, or definite ST
showed no significant differences between anterior STEMI patients treated with multiple stents and patients treated with a single stent. (2) There was a non-significant increase in in-hospital mortality in the multiple-stents group attributable mainly to increased rates of cardiogenic shock and a significant higher post-procedural incidence of suboptimal TIMI flow as compared to the single-stent group.
Generally, Primary PCI in the setting of a ruptured plaque with thrombus and atherosclerotic debris leads to distal embolization of microthrombi and plaque contents.16 The use of multiple longer stents and the associated pre and post-dilation may increase the likelihood of mechanical fragmentation with subsequent dislodgement of thrombus and plaque contents.17 These mechanisms explain the higher prevalence of suboptimal flow in the multiple stents group (26.5% vs. 19.4% in the single-stent group; P=0.009). Subop-timal TIMI flow is associated with larger infarct size, lower LVEF, and increased rates of heart failure, cardiac rupture, cardiogenic shock, and mortality. 18,19 Using the shortest possible stent(s), avoidance of unnecessary balloon dilatations and direct stenting if feasible with the liberal use of glycoprotein IIb/IIIa inhibitors, aspiration catheters, and coronary vasodilators in selected cases can significantly improve distal final TIMI flow and decrease the rates of adverse in-hospital outcomes in those high risk patients requiring ≥2 stents.20,21
Previous studies stated that the use of multiple overlapping stents with increased total stents length was associated with worse outcomes and increased rates of MACEs in BMS 3 and even in some reports from the era of first and second-generations DES. 6-10
This study is unique in that it demonstrated comparable 6-month clinical outcomes between patients treated with multiple overlapping DES and those treated with a single stent, suggesting that the strategy of treating diffuse or complicated LAD lesions during PPCI with multiple overlapping new generations DESs is feasible, when required, with acceptable rates of MACEs in the mid-term follow-up. This study was restricted to STEMI patients, which can partly explain the difference between the results of the present study and previous reports that mainly included patients with stable CAD and we particularly investigated patients presenting with an acute anterior STEMI to minimize the impact of different anatomy, vessel size, and amount of subtended myocardium on the clinical outcomes.
The occurrence of acute STEMI in a coronary artery with diffuse atherosclerotic disease and multiple complex coronary plaques can promote plaques destabilization through the enhanced systemic inflammation and the accelerated progression of atherosclerosis. 22 Thereby, a full lesion coverage in case of a long diffuse or complex lesion with multiple stents as required could help preventing future adverse cardiovascular events known to be more associated with STEMI patients than in patients with stable CAD. 23
The use of multiple longer stents with overlap has been recognized in many reports to be significantly associated with stent thrombosis. 24,25 However, in the present study, patients with multiple stents showed no significant increase in the rates of stent thrombosis. This may be explained in part due to the use of either second-generation DESs or the newer DESs with biodegradable polymer for all patients. Sec ond-generation DESs are designed with thinner stent struts, more biocompatible polymer coatings and the new anti-proliferative drugs (everolimus / zotaroli-mus). These factors combined together significantly reduced thrombogenicity compared to the first generation DES and BMS.26,27 DES with biodegradable polymer also showed favorable clinical outcome that was non-inferior to the second-generation DES in a large meta-analysis.28 Moreover, we routinely prescribed dual antiplatelets therapy for all cases with STEMI which has provided a solid superior efficacy in preventing ST. 29
In our study, there were also no significant differences in the need for TVR at 6-month follow up between patients treated with multiple stents and those treated with a single stent. These results are in accordance with a recent study conducted by Fukutomi et al, 30 where they investigated the effects of the stent length on the short and long-term outcomes in patients with STEMI, and they reported a TVR rate of 5.5% in their long DES subgroup that contained longer and multiple stents, and it didn’t differ from the rates of other groups with deployed single and shorter stents. However, Tsagalou et al 4 observed a significant higher rate of binary restenosis (19.6%) when they studied the effect of multiple ultra-long DES implantation in diffuse LAD lesion at 6-month follow-up. This increased rates of stent failure in their study can be explained as a result of the inclusion of a large proportion of patients with chronic total occlusion lesions with complex anatomy (20%) and increased total implanted stents length (mean length 80mm versus 53.1mm in our overlapping-stents group), and also they used earlier generations DESs (SES and PES) in their study.
Although we observed similar rates of mortality, MACEs, ST, and TVR among patients with single and multiple stents, further studies with more patients and longer follow-up would be required in the future to statistically establish the safety and efficacy of multiple DESs in STEMI patients, particularly after the end of the first year when the DAPT is terminated.
Study limitations
This was a retrospective observational study and the selection of patients was not randomized to receive either multiple stents or single stents. The study also might be confounded by selection bias of the stent types. We evaluated only clinical outcomes and control angiographic follow-up was not performed. Final- 82 | Cardiometry | Issue 17. November 2020
ly, follow up of outcomes beyond the first 6 months was not established.
Conclusion
The need of ≥2 overlapping stents occurs in a considerable proportion of patients undergoing primary PCI for STEMI. In this observational study, there was a non-significant trend toward increased in-hospital mortality in the multiple-stents group. However, similar rates of mortality, MACEs, ST, and TVR among patients with and without overlapping stents were reported at 6-month follow-up. We hypothesized that the use of multiple overlapping new generations DES, when required, is relatively safe and acceptable during PPCI of STEMI patients.
Acknowledgements
The authors acknowledge the support of China Scholarship Council (CSC) and all the staff members of cardiology department in Xi’an Jiaotong University Hospitals.
Funding
Statement on ethical issues
Research involving people and/or animals is in full compliance with current national and international ethical standards.
Conflict of interest
None declared.
Author contributions
The authors read the ICMJE criteria for authorship and approved the final manuscript.
Список литературы The impact of multiple overlapping new generations drug-eluting stents on six-month clinical outcomes in patients with anterior STEMI treated with primary PCI
- Steg PG, Goldberg RJ, Gore JM, Fox KAA, Eagle KA, Flather MD, et al. Baseline characteristics, manage-ment practices, and in-hospital outcomes of patients hospitalized with acute coronary syndromes in the Global Registry of Acute Coronary Events (GRACE). Am J Cardiol 2002;90:358–63.
- Schampaert E, Moses JW, Schofer J, Schlüter M, Gershlick AH, Cohen EA, et al. Sirolimus-Eluting Stents at Two Years: A Pooled Analysis of SIRIUS, E-SIRIUS, and C-SIRIUS With Emphasis on Late Re-vascularizations and Stent Thromboses. Am J Cardiol 2006;98:36–41.
- Applegate RJ, Sacrinty MT, Kutcher MA, Santos RM, Gandhi SK, Little WC. Effect of length and diam-eter of drug-eluting stents versus baremetal stents on late outcomes. Circ Cardiovasc Interv 2009;2:35–42.
- Tsagalou E, Chieffo A, Iakovou I, Ge L, Sangiorgi GM, Corvaja N, et al. Multiple overlapping drug-elut¬ing stents to treat diffuse disease of the left anteri¬or descending coronary artery. J Am Coll Cardiol. 2005;45(10):1570–3.
- Dawkins KD, Grube E, Guagliumi G, Banning AP, Zmudka K, Colombo A, et al. Clinical efficacy of polymer-based paclitaxel-eluting stents in the treat¬ment of complex, long coronary artery lesions from a multicenter, randomized trial: support for the use of drug-eluting stents in contemporary clinical practice. Circulation 2005;112:3306–13.
- Stone GW, Ellis SG, Cannon L, Mann JT, Greenberg JD, Spriggs D, et al. Comparison of a Polymer-Based Paclitaxel-Eluting Stent With a Bare Metal Stent in Pa¬tients With Complex Coronary Artery Disease. JAMA 2005;294:1215.
- Kereiakes DJ, Wang H, Popma JJ, Kuntz RE, Dono¬hoe DJ, Schofer J, et al. Periprocedural and Late Con-sequences of Overlapping Cypher Sirolimus-Eluting Stents: Pooled Analysis of Five Clinical Trials. J Am Coll Cardiol 2006;48:21–31.
- Shirai S, Kimura T, Nobuyoshi M, Morimoto T, Ando K, Soga Y, et al. Impact of Multiple and Long Si-rolimus-Eluting Stent Implantation on 3-Year Clinical Outcomes in the j-Cypher Registry. JACC Cardiovasc Interv 2010;3:180–8.
- Konishi H, Miyauchi K, Dohi T, Tsuboi S, Ogita M, Naito R, et al. Impact of stent length on clinical outcomes of first-generation and new-generation drug-eluting stents. Cardiovasc Interv Ther 2016;31:114–21.
- Honda Y, Muramatsu T, Ito Y, Sakai T, Hirano K, Yamawaki M, et al. Impact of ultra-long second-gen-eration drug-eluting stent implantation. Catheter Car¬diovasc Interv 2016;87:E44–53.
- Choi IJ, Koh Y-S, Lim S, Kim JJ, Chang M, Kang M, et al. Impact of the Stent Length on Long-Term Clinical Outcomes Following Newer-Generation Drug-Eluting Stent Implantation. Am J Cardiol 2014;113:457–64.
- Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, et al. Fourth universal defini¬tion of myocardial infarction (2018). Russ J Cardiol 2019;24:107–38.
- Levine G, Bates E, Blankenship J, Bailey S, Bittl J, Cercek B, et al. 2015 ACC / AHA / SCAI Focused Up¬date on Primary PCI for Patients with STEMI : Circu¬lation 2016;133:1135–47.
- Ding S, Pu J, Qiao ZQ, Shan P, Song W, Du Y, et al. TIMI myocardial perfusion frame count: A new meth-od to assess myocardial perfusion and its predictive value for short-term prognosis. Catheter Cardiovasc Interv 2010;75:722–32.
- Laskey WK, Yancy CW, Maisel WH. Thrombosis in coronary drug-eluting stents: report from the meet-ing of the Circulatory System Medical Devices Advi¬sory Panel of the Food and Drug Administration Cen¬ter for Devices and Radiologic Health, December 7-8, 2006. Circulation 2007;115:2352–7.
- Jaffe R, Dick A, Strauss BH. Prevention and treat¬ment of microvascular obstruction-related myocardi¬al injury and coronary no-reflow following percuta¬neous coronary intervention: A systematic approach. JACC Cardiovasc Interv 2010;3:695–704.
- Bae JH, Kwon TG, Hyun DW, Rihal CS, Lerman A. Predictors of slow flow during primary percuta¬neous coronary intervention: An intravascular ultra¬sound-virtual histology study. Heart 2008;94:1559–64.
- Ito H. Etiology and clinical implications of micro¬vascular dysfunction in patients with acute myocardi¬al infarction. Int Heart J 2014;55:185–9.
- Ndrepepa G, Tiroch K, Keta D, Fusaro M, Seyfarth M, Pache J, et al. Predictive factors and impact of no reflow after primary percutaneous coronary interven¬tion in patients with acute myocardial infarction. Circ Cardiovasc Interv 2010;3:27–33.
- Bouleti C, Mewton N, Germain S. The no-reflow phenomenon: State of the art. Arch Cardiovasc Dis 2015;108:661–74.
- Prati F, Capodanno D, Pawlowski T, Ramazzotti V, Albertucci M, La Manna A, et al. Local delivery versus intracoronary infusion of abciximab in patients with acute coronary syndromes. JACC Cardiovasc Interv 2010;3:928–34.
- Wang H, Eitzman DT. Acute myocardial infarction leads to acceleration of atherosclerosis. Atherosclero-sis 2013;229:18–22.
- Keeley EC, Mehran R, Brener SJ, Witzenbi¬chler B, Guagliumi G, Dudek D, et al. Impact of multiple complex plaques on short- and long-term clinical outcomes in patients presenting with ST-segment elevation myocardial infarction (from the Harmonizing Outcomes With Revas¬cularization and Stents in Acute Myocardial In¬farction [HORIZONS-AMI] Trial). Am J Cardiol 2014;113:1621–7.
- Suh J, Park D-W, Lee J-Y, Jung IH, Lee S-W, Kim Y-H, et al. The Relationship and Threshold of Stent Length With Regard to Risk of Stent Thrombosis After Drug-Eluting Stent Implantation. JACC Cardiovasc Interv 2010;3:383–9.
- Tada T, Byrne RA, Simunovic I, King LA, Cassese S, Joner M, et al. Risk of Stent Thrombosis Among Bare-Metal Stents, First-Generation Drug-Eluting Stents, and Second-Generation Drug-Eluting Stents: Results From a Registry of 18,334 Patients. JACC Car¬diovasc Interv 2013;6:1267–74.
- Kolandaivelu K, Swaminathan R, Gibson WJ, Ko¬lachalama VB, Nguyen-Ehrenreich K-L, Giddings VL, et al. Stent thrombogenicity early in high-risk inter¬ventional settings is driven by stent design and deploy-ment and protected by polymer-drug coatings. Circu¬lation 2011;123:1400–9.
- De Luca G, Smits P, Hofma SH, Di Lorenzo E, Vla¬chojannis GJ, van’t Hof AWJ, et al. Everolimus eluting stent vs first generation drug-eluting stent in primary angioplasty: A pooled patient-level meta-analysis of randomized trials. Int J Cardiol 2017;244:121–7.
- Pandya B, Gaddam S, Raza M, Asti D, Nalluri N, Vazzana T, et al. Biodegradable polymer stents vs sec-ond generation drug eluting stents: A meta-analysis and systematic review of randomized controlled trials. World J Cardiol 2016, 8 (2): 240-246.
- Schömig A, Neumann F-J, Kastrati A, Schühlen H, Blasini R, Hadamitzky M, et al. A Randomized Com-parison of Antiplatelet and Anticoagulant Therapy af¬ter the Placement of Coronary-Artery Stents. N Engl J Med 1996;334:1084–9.
- Fukutomi M, Takahashi M, Toriumi S, Ogoyama Y, Oba Y, Funayama H, et al. Evaluation of stent length on the outcome of ST-segment elevation myocardial infarction receiving primary percutaneous coronary intervention. Coron Artery Dis 2019;30:196–203.


