The influence of nano-additives in the synthesis of eco-friendly polyester plasticizers
Автор: Mazitova A.K., Vikhareva I.N., Aminova G.K., Savicheva Ju.N., Gareeva N.B., Shaikhullin I.R.
Журнал: Nanotechnologies in Construction: A Scientific Internet-Journal @nanobuild-en
Рубрика: Development of new polymer materials
Статья в выпуске: 1 Vol.12, 2020 года.
Бесплатный доступ
Plasticized polymer materials are widely used in all spheres of human life. The most common plasticizers are aromatic compounds-esters of o-phthalic acid. However, their use was limited in accordance with the EU Directive REACH (2009) due to possible toxicity, which contributed to the development of new non-toxic alternatives, which include polyester plasticizers. Polyester plasticizers are classified as special purpose plasticizers. Due to the wide variety of starting materials and the ability to vary the size of the molecule, a wide range of plasticizers can be synthesized. These are mainly polyesters of polyatomic alcohols esterified with dibasic acids and modified with monocarboxylic acid or aliphatic alcohol. Polyesters-based plasticizers contribute to the production of PVC compositions with improved properties such as low volatility, resistance to extraction, excellent flexibility, wear resistance, UV resistance and heat resistance. Also, such plasticizers exhibit an excellent non-sweating property of plastics. This paper describes a method for preparing a polyester compound propylene glycol adipate modified with cyclohexanecarboxylic acid, proposed as a plasticizer of polyvinyl chloride. Conditions of its production with maximum output are given. Physical and chemical properties of the resulting compound were studied. The formulation of PVC-composition on the basis of the received polyester plasticizer is offered. The results of tests of PVC plastic according to state standard 5960-72 are presented. It is shown that the use of propylene adipate modified with cyclohexanecarboxylic acid provides a plasticizing efficiency as high as DOP, while having a reduced migration. This fact allows us to use the developed polyester plasticizer as a non-toxic alternative to industrial PVC plasticizers. It has been found that the use of calcium adipate nano quantities in the production of propylene glycol adipate increases the yield of the desired ester and improves the physical and mechanical properties of PVC plastic.
Adipic acid, cyclohexanecarboxylic acid, cyclohexanoate, esterification, modifying groups, polyester plasticizer, polyvinyl chloride, 1, 2-propanediol, propylene adipate, stabilizer
Короткий адрес: https://sciup.org/142227433
IDR: 142227433 | DOI: 10.15828/2075-8545-2020-12-1-21-26
Текст научной статьи The influence of nano-additives in the synthesis of eco-friendly polyester plasticizers
P olyvinyl chloride is the only large-capacity polymer that is less dependent on the oil market: PVC production consumes no more than 4 % of the world’s oil [1]. In addition, about 30% of chlorine produced as a by-product is involved in the production of PVC, which serves to improve the environmental situation [2–5].
PVC is a universal polymer, the products of which are characterized by durability, resistance to climatic conditions, low flammability [6–8]. A wide range of areas of its application is also due to the fact that polyvinyl chloride is a material that, after molding the product, retains the ability to be recycled [9–15].
The unique combination of properties and low price contributes to the further growth of the use of polyvinyl chloride [3]. The most important additives necessary for processing polyvinyl chloride are plasticizers [16–20]. Traditional phthalate plasticizers are the most widely used all over the world. But their use is gradually limited due to toxicity. The main direction for the replacement of phthalates is the development of non-toxic plasticizers, which are obtained on the basis of adipic and citric acids, polyesters and epoxy compounds [21–28]. Polyester plasticizers based on adipic acid are characterized by high performance, low extractability from the polymer to water and cleaning solutions, organic solvents; oil and gasoline resistance, and minimal migration from the polymer to
DEVELOPMENT OF NEW POLYMER MATERIALS other materials that are in contact with it, as well as low water absorption, increased thermal stability [29]. This type of plasticizers is used in products that have high requirements for toxic safety, such as food packaging, beverage tubes, medical and health products, interior items, children’s toys, wires and cables [30–31].
Thus, there is still a need for polyester plasticizers, which show the optimal combination of plasticization and the necessary properties of PVC plastics, since too large a molecular weight of the polyester plasticizer can degrade the properties of PVC plastic, for example, increase the viscosity and processing time of the composition, and also leads to a decrease in plasticity. Therefore, the adjustment of the structure of the polyester oligomer is an important condition for the development of this type of plasticizers.
In this regard, we obtained and tested a new polyester plasticizer with low toxicity and migration, and also investigated the effect of small amounts of calcium adipate on the yield of the target compound during synthesis and the physical and mechanical parameters of PVC plastic.
MAIN PART
Previously, we have obtained and described alkoxy-lated alcohol adipates [32–33]. This paper presents a synthesis method and some physicochemical properties of a new polyester compound – poly(1,2-propylene glycol) adipate with terminal cyclohexanoate groups. It is shown that the use of nano quantities of calcium adipate in the preparation of this compound increases the yield of the target ester and improves the properties of the obtained PVC plastics. The results of tests of the developed polyester plasticizer in PVC composition for construction purposes are presented.
The target polyester plasticizer was prepared in two stages. The general scheme of obtaining poly(1,2-propyl-ene glycol) adipate modified with cyclohexanecarboxylic acid has the following form:
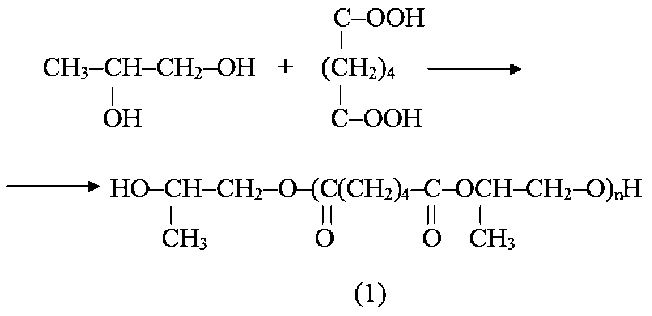
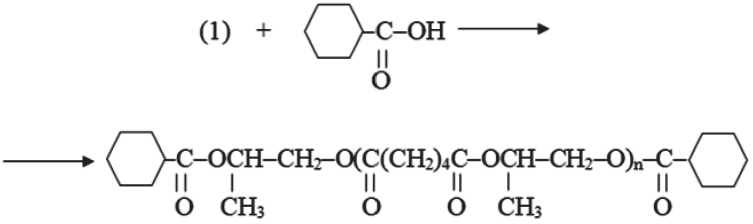
Synthesis of the polyester plasticizer was carried out by a two-stage method of condensation telomerization, since in this case oligoesters are more homogeneous in molecular weight and with a lower content of diesters compared to the one-stage method [34]. First, polycondensation of 1,2-propanediol and adipic acid was performed to obtain an oligoester with terminal hydroxyl groups, and then the resulting oligomer was esterified with cyclohexanecarboxylic acid.
Method of preparation of polyester oligomer poly(1,2-propylene glycol) adipate
In a round bottom flask equipped with a magnetic stirrer, distillation column filled with a six-inch mesh of stainless steel distillation head with receiving flask and an inlet for nitrogen (100 ml/min) load 146 g (1 mol) of adipic acid, 119,2 g (1.6 mol) 1,2-propanediol, 0.44 g (0.3% wt. in relation to the mass of adipic acid) of cal-
DEVELOPMENT OF NEW POLYMER MATERIALS
Table 1
Physical and chemical properties poly (1,2-propylene glycol) adipate/dicyclohexanoate (PPA/DCG)
The resulting ester is a viscous yellow liquid.
Method of preparation of polyester oligomer of poly(1,2-propylene glycol) adipate modified with cyclohexanecarboxylic acid
In a round-bottomed flask equipped with a stirrer, a thermometer, a reverse refrigerator, the calculated amount of cyclohexanecarboxylic acid and the resulting oligoester is loaded at a molar ratio of 1:2,2. The process is carried out at a temperature of 80–90оC and at a residual pressure of 0.14-0.4 atm in the presence of a catalyst, 0.49 g of tetrabutoxytitanium (C 4 H 9 O) 4 Ti and 0.98 g of activated carbon. The amount of catalyst is calculated 0.33% wt in relation to the mass of adipic acid, the amount of activated carbon – 0.66% wt. To facilitate the distillation of the released water, nitrogen is passed through the reaction mass in a small amount. Synthesis in the presence of a catalyst is completed in 4 hours. Upon completion of the synthesis, the volatile components, including the entrainment agent, are distilled with acute superheated steam. Dicyclohexanoate obtained is treated with water to transfer the titanium compounds in the insoluble form. The finished ester is purified from activated carbon by filtration.
Physical and chemical properties of synthesized polyester are given in table 1.
The resulting product has a high flash point and a low pour point, the acid number meets the requirements for ester plasticizers.
DISCUSSION OF RESULTS
The sample with the developed plasticizer was tested in a PVC composition for construction purposes (table 2).
To determine the effectiveness of the developed plasticizer, the optimal ratio of plasticizer: PVC was determined, at which the best characteristics of PVC-plasticate are achieved. Shore a hardness of plasticizer concentration (plasticizer : PVC – 1:100) – 84.
Table 2
The formulation of PVC composition
|
Structure of composition |
Mass parts |
|
PVC |
100 |
|
Plasticizer |
70 |
|
Epoxidized soybean oil |
5 |
|
Stabilizer |
2 |
|
Сalcium adipate |
0.4 |
Based on the calculation of the quantitative substitution factor (FS), which determines the required amount of poly(1,2-propylene glycol) adipate /dicyclohexanoate in comparison with dioctyl phthalate (DOP) and provides the necessary hardness of flexible PVC at room conditions, it was found that the resulting compound is not inferior to DOP in plasticizing efficiency.
The resulting sample of plastic was tested in PVC composition for construction purposes according to state standard 5960-72. DOP was selected as a control sample. The test results are given in table 3.
Hygienic parameters were determined according to state standard R 50962-96: smell, taste, color change and transparency of water extract (table 3).
DEVELOPMENT OF NEW POLYMER MATERIALS
Table 3
The test results plasticizer in the formulation of cable plastic grade O-40
|
Indicators |
Plasticizer |
||
|
Norm according state standart 5960-72 (1 class) |
Control sample |
PPA/DCG |
|
|
Specific volume electric. resistance at 20оС, Ohm•cm |
not less 1•1010 |
9.0•1012 |
4.5•1013 |
|
Tensile strength, kgf/cm2 |
not less 110 |
147 |
248 |
|
Thermal stability at 180оС, min |
state standart 14041-91 |
2 h 15 min |
2 h 25 min (2 h 15 min)* |
|
Temperature of fragility, оC |
Not above –40 |
Stand the test |
Stand the test |
|
Water absorption, % |
Not above 0.45 |
0.08 |
0.05 |
|
Weight loss at 160оC for 6 hours, % |
Not above 3.0 |
2.2 |
0.7 |
|
Gas resistance (weight loss after holding at 25оС for 48 h), % |
state standart 12020-2018 |
Not above 10 |
6.0 |
|
Oil resistance (weight loss after maintenance at 25оС for 48 h),% |
state standart 12020-2018 |
Not above 10 |
9.0 |
|
Hygienic parameters |
state standart R 50962-96 |
– |
Stand the test |
* – PVC plastic that does not contain calcium adipate.
From the given data it is visible that the offered plasticizer does not concede to the control sample on the basic physical and mechanical indicators and corresponds to state standard; the introduction of nano quantities of calcium adipate improves some of the physical and mechanical properties of PVC plastic, namely the thermal stability index.
Список литературы The influence of nano-additives in the synthesis of eco-friendly polyester plasticizers
- Ul’yanov V.M., Rybkin E.P., Gudkovich A.D., Pishin G.A. Polivinilkhlorid [Polyvinylchloride]. Moscow, Khimiya, 2000. 288 р. (In Russian).
- Fadina Yu.I. Analiz rossiiskogo rynka polimerov i dal’neishie puti ego razvitiya [Analysis of the Russian polymer market and its further development] // Biznes-obrazovanie v ekonomike znanii = Business Education in the Knowledge Economy. 2017, no. 1, рр. 99–101.
- Obzory Rynka INVENTRA: Polivinilkhlorid (PVKh-S). Itogi goda 2018 [INVENTRA Market Reviews: Polyvinyl Chloride (PVC-C). Results of the year 2018]. URL: https://plastinfo.ru/information/articles/671/.
- Pomerantsev E.G. Ekologicheskie problemy proizvodstva, pererabotki, potrebleniya i utilizatsii PVKh i izdelii iz nego (obzor) [Environmental problems of production, processing, consumption and disposal of PVC and its products (overview)]. Plasticheskie massy = Plastics. 1995, no. 2.
- Kovriga V.V. Polivinilkhlorid – yasnaya ekologicheskaya perspektiva [Polyvinyl Chloride – Clear Environmental Outlook]. Plasticheskie massy = Plastics. 2007, no. 7, рр. 52–54.
- Uilki Ch., Sammers D., Daniels Ch. Polivinilkhlorid [Polyvinylchloride]. St. Petersburg, Professiya, 2007. 728 р.
- Mazitova A.K., Aminova G.K., Nafikova R.F., Deberdeev R.Ya. Osnovnye polivinilkhloridnye kompozitsii stroitel’nogo naznacheniya [Basic polyvinyl chloride building compositions]. Ufa, 2013. 130 р.
- Wilkes C.E., Summers J.W., Daniels C.A., Berard M.T. PVC Handbook. Hanser Publications, 2005. 723 р.
- Moroz P.A., Askadskii A.A., Matseevich T.A., Solov’eva E.V., Askadskii A.A. Primenenie vtorichnykh polimerov dlya proizvodstva drevesno-polimernykh kompozitov [Use of secondary polymers for the production of wood-polymer composites]. Plasticheskie massy = Plastics. 2017, no. 9–10, рр. 56–62.
- Vagner A. Otkhody PVKh: neobkhodim retsikling [PVC waste: recycling required]. Tverdye bytovye otkhody = Municipal solid waste. 2015, no. 11, рр. 11.
- The European PVC industry commitment to Sustainability. Vinyl 2010. 2011, 32 p.
- Erenkov O.Yu., Bogachev, A.P., Polyakova, A.A. Povyshenie effektivnosti vtorichnoi pererabotki otkhodov plastmass [Improving Recycling of Plastic Waste]. Uchenye zametki TOGU = Scientific notes POSU. 2014, Vol. 5, no. 1, рр. 48–54.
- Incentives to collect and recycle. Recovinyl.com. Retrieved on 28 January 2016. URL: https://www.recovinyl.com/.
- URL: https://vinylplus.eu/progress/annual-progress/.
- Potapova E.V. Problema utilizatsii plastikovykh otkhodov [Problem of plastic waste disposal]. Izvestiya Baikal’skogo gosudarstvennogo universiteta = News of Baikal State University. 2018, Vol. 28, no. 4, рр. 535–544.
- Erythropel H.C., Maric M., Nicell J.A., Leask R.L., Yargeau V. Leaching of the plasticizer di(2-ethylhexyl)phthalate (DEHP) from plastic containers and the question of human exposure. Appl. Microbiol. Biotechnol. 2014, Vol. 98, no. 24, рр. 9967–9981.
- Rahman M., Brazel C.S. The plasticizer market: An assessment of traditional plasticizers and research trends to meet new challenges. Prog. Polym. Sci. 2004, Vol. 29, no. 12, рр. 1223–1248.
- Mazitova A.K., Nafikova R.F., Aminova G.K. Plastifikatory polivinilkhlorida [Polyvinylchloride plasticizers]. Nauka i epokha: monografiya; pod obshchei red. prof. O.I. Kirikova. Voronezh. 2011, рр. 277–297.
- Mazitova A.K., Aminova G.K., Maskova A.R., Yagafarova G. G., Mazitov R.M. Novye plastifikatory dlya PVKh-kompozitsii stroitel’nogo naznacheniya [New plasticizers for PVC construction compositions]. Nanotekhnologii v stroitel’stve = Nanotechnologies in Construction. 2017, Vol. 9, no. 4, pp. 48–63, DOI: 10.15828/2075-8545-2017-9-4-48-63.
- Mazitova A.K., Aminova G.K., Maskova A.R., Sabitov I.N., Nedoseko I.V. Novye plastifikatory polivinilkhlorida [New plasticizers of polyvinylchloride]. Nanotekhnologii v stroitel’stve = Nanotechnologies in Construction. 2017, Vol. 9, no. 6, pp. 168–180, DOI: 10.15828/20758545-2017-9-6-168-180.
- Lithner D., Larsson А., Dave G. Environmental and health hazard ranking and assessment of plastic polymers based on chemical composition. Sci. Total Environ. 2011, Vol. 409, no. 18, рр. 3309–3324.
- Hines C.J., Hopf N.B., Deddens J.A., Silva M.J., Calafat A.M. Occupational exposure to diisononyl phthalate (Di NP) in polyvinyl chloride processing operations. Int. Arch. Occup. Environ. Health. 2012, Vol. 85, no. 3, рр. 317–325.
- Chiellini F., Ferri M., Morelli A., Dipaola L., Latini G. Perspectives on alternatives to phthalate plasticized poly(vinyl chloride) in medical devices applications. Prog. Polym. Science. 2013, Vol. 38, no. 7, рр. 1067–1088.
- Vikhareva I.N., Builova E.A., Gatiyatullina D.R., Arslanov V.R., Gilem’yanov D.A., Mazitova A.K. Sintez i svoistva slozhnykh efirov adipinovoi kisloty [Synthesis and properties of adipic acid esters]. Bashkirskii khimicheskii zhurnal = Bashkir chemical journal. 2019, Vol. 26, no. 2, рр. 33–36.
- Vieira M.G.A., Silva M.A.D., Santos L.O., Beppu M.M. Natural-based plasticizers and biopolymer films: A review. Eur. Polym. J. 2011, Vol. 47, рр. 254–263.
- Vijayendran B.R., Benecke H., Elhard J.D., McGinniss V.D., Ferris K.F. Environmentally Friendly Plasticizers for Polyvinyl Chloride (PVC). Resins Antec, Dallas, Texas. 2001, 604 p.
- Perry N.L. Exopy plasticizers-stabilizers. Ind. Eng. Chem. 1958, Vol. 50, рp. 862.
- Tricresyl phosphate. International Program on Chemical Safety. Environmental Health Criteria 110. URL: http://www. inchem.org/documents/ehc/ehc/ehc110.htm.
- An Y., Ding Y. Tan J., Yang W. Influences of polyester plasticizers on the properties of oil resistance flexible poly(vinyl chloride) and powder nitrile butadiene rubber blends. Adv. Sci. Lett. 2011, Vol. 4, рр. 875–879.
- Noti A.F., Grob K. Migration of plasticizers from PVC gaskets of lids for glass jars into oily foods: Amount of gasket material in food contact, proportion of plasticizer migrating into food and compliance testing by simulation. Trends Food Sci. Technol. 2006, Vol. 17, рр. 105–112.
- Audic J.L., Brosse J.C. Migration of additives from food grade polyvinyl chloride (PVC) films: Effect of plasticization by polymeric modifiers instead of conventional plasticizers. J. Appl. Polym. Sci. 2003, Vol. 89, no. 5, рр. 1291–1299.
- Mazitova A.K., Vikhareva I.N., Aminova G.K., Timofeev A.A., Builova E.A., Distanov R. Sh. Issledovanie vliyaniya kolichestva dobavok na svoistva efirov adipinovoi kisloty [Investigation of the effect of the amount of additives on the properties of adipic acid esters]. Nanotekhnologii v stroitel’stve = Nanotechnologies in construction. 2019, Vol. 11, no. 4, рр. 437–446.
- Vikhareva I.N., Il’yasova A.D., Likhacheva O.G., Zapotylok G.Yu., Mazitova A. K. Di-(2-etilgeksiloksi)etiladipinaty [Di-(2-ethylhexyloxy)adipates]. Bashkirskii khimicheskii zhurnal = Bashkir chemical journal. 2019, Vol. 26, no. 2, рр. 90–91.
- Ovchinnikov Yu.V., Stesikov V.P., Stupen’ P.V. Vysokomolekulyarnye soedineniya [High-molecular compounds]. 1973, Vol. 15, seriya B, 278–282 р.


