The oxidative-addition reactions of tri(meta-tolyl) antimony and tri(ortho-tolyl)antimony with 5-nitrofurfuraldoxime in the presence of peroxides. The molecular structures of 2-oxo-bis[(5- nitrofurfuraldoximato)tri(meta-tolyl)antimony] and 2-oxo-bis[(5-nitrofurfuraldoximato)tri(ortho- tolyl)antimony]
Автор: Artemeva E.V., Makerova M.S., Sharutin V.V., Sharutina O.K.
Журнал: Вестник Южно-Уральского государственного университета. Серия: Химия @vestnik-susu-chemistry
Рубрика: Химия элементоорганических соединений
Статья в выпуске: 2 т.9, 2017 года.
Бесплатный доступ
The interaction of tri(meta-tolyl)antimony and tri(ortho-tolyl)antimony with 5-nitrofurfural oxime in the presence of an oxidizing agent (hydrogen peroxide or tert-butyl hydroperoxide) leads to the formation of bis(5-nitrofurfuraldoximato)tri(meta-tolyl)antimony (1), μ2-oxo-bis[(5-nitrofurfuraldoximato)tri(meta-tolyl)antimony] (2), bis(5-nitrofurfuraldoximato) tri(ortho-tolyl)antimony (3) and μ2-oxo-bis[(5-nitrofurfuraldoximato)tri(ortho-tolyl)antimony] (4). The molecular structures of complexes 2 and 4 have been determined by X-ray diffraction analysis.
Tri(m-tolyl)antimony, tri(o-tolyl)antimony, 5-nitrofurfuraldoxime, tert-butyl hydroperoxide, hydrogen peroxide, oxidation, bis(5-nitrofurfuraldoximato)tri(m-tolyl) antimony, μ2-oxo-bis[(5-nitrofurfuraldoximato)tri(m-tolyl)antimony], μ2-oxo-bis[(5-nitrofurfuraldoximato)tri(o-tolyl)antimony], molecular structures, x-ray diffraction analysis
Короткий адрес: https://sciup.org/147160389
IDR: 147160389 | УДК: 546.863+546.865+547.152+547.53.024+548.312.5 | DOI: 10.14529/chem170207
Текст научной статьи The oxidative-addition reactions of tri(meta-tolyl) antimony and tri(ortho-tolyl)antimony with 5-nitrofurfuraldoxime in the presence of peroxides. The molecular structures of 2-oxo-bis[(5- nitrofurfuraldoximato)tri(meta-tolyl)antimony] and 2-oxo-bis[(5-nitrofurfuraldoximato)tri(ortho- tolyl)antimony]
It is known that some pentavalent antimony derivatives are biologically active compounds. For example, triphenyl- and trimethylantimony dioximates and dicarboxylates were observed to have bactericidal and antitumor activity [1-6]. One of the methods allowing such compounds to be synthesized is the oxidative synthesis. It is interesting to note that two types of triarylantimony dioximates (Ar 3 SbX 2 or (Ar 3 SbX) 2 О (Ar = Ph, p -Tol, о -Tol; Х = ОNCHR, ОNCRR') can be obtained, depending on the oxime nature and the reaction conditions [7–10].
Oximes that contain two donor atoms (O and N) are ampolydentate ligands. However, according to X-ray diffraction data, the ligands of such antimony derivatives as Ar 3 Sb(ОNCRR’) 2 and (Ar 3 SbОNCRR’) 2 О are usually bound to the antimony atom only via the oxygen atom. In this case, short distances between the antimony atom and the nitrogen atoms of iminoxy-groups, which do not lead to the significant distortion of the trigonal-bipyramidal central atom coordination, are observed [11].
Sometimes the oxime ligand coordination method can be dependent on the nature of the aryl radicals at the antimony atom. For example, furfuraldoxime or 2-hydroxybenzaldoxime performs various structural functions in the derivatives of triphenyl- [8] and tri ( o -tolyl) antimony [10] or triphenyl- and tris (5-bromo-2-methoxyphenyl)antimony [9], respectively.
Obviously, the oxidative-addition reactions of triarylantimony with oximes and the structure of the obtained products require further study.
The present work concerns the investigation of the interaction between tri( m -tolyl)- or tri( o -tolyl)antimony and 5-nitrofurfuradoxime in the presence of an oxidizing agent (hydrogen peroxide or t -butyl hydroperoxide) and the establishment of the product molecular structures.
ExperimentalSynthesis of bis(5-nitrofurfuraldoximato)tri(m-tolyl)antimony (1).
100 mg of tri( m -tolyl)antimony (0.25 mmol) and 79 mg (0.5 mmol) of 5-nitrofurfuraldoxime were dissolved in the mixture of diethyl ether (15 mL) and heptane (15 mL), then 28 mg of 30 % aqueous solution of hydrogen peroxide (0.25 mmol) were added. The mixture was kept for 24 hours at 20 °C. After the solvent evaporation, the light-yellow crystals of 1 were obtained (m = 0.132 g (78 %), MP = 78 °C).
IR spectrum, ν, cm–1: 3155, 3138, 3049, 2957, 2922, 2860, 1558, 1520, 1475, 1456, 1377, 1348, 1300, 1244, 1097, 1016, 970, 937, 810, 773, 739, 706, 689, 545, 505, 474, 426.
Synthesis of µ 2 -oxo- bis [(5-nitrofurfuraldoximato)tri( m -tolyl)antimony] (2).
-
а) 100 mg of tri( m -tolyl)antimony (0.25 mmol) and 39 mg (0.25 mmol) of 5-nitrofurfuraldoxime were dissolved in the mixture of diethyl ether (25 mL) and heptane (5 mL), then 28 mg of 30 % aqueous solution of hydrogen peroxide (0.25 mmol) were added. The mixture was kept for 24 hours at 20 °C. After the solvent evaporation, the yellow crystals of 2 were obtained (m = 0.134 g (96 %), MP = 143 °C).
IR spectrum (ν, cm–1): 3176, 3161, 3049, 2866, 2826, 1570, 1522, 1479, 1381, 1350, 1298, 1246, 1163, 1099, 1018, 966, 914, 860, 810, 775, 739, 689, 552, 484, 426.
-
b) 100 mg of tri( m -tolyl)antimony (0.25 mmol) and 39 mg (0.25 mmol) of 5-nitrofurfuraldoxime were dissolved in the mixture of diethyl ether (15 mL) and heptane (15 mL), then 22 mg of 70 % aqueous solution of tert -butyl peroxide (0.25 mmol) were added. The mixture was kept for 24 hours at 20 °C. After the solvent evaporation, the yellow crystals of 2 were obtained (m = 0.127 g (91 %), MP = 144 °C).
Synthesis of bis (5-nitrofurfuraldoximato)tri( o -tolyl)antimony (3).
-
a) 100 mg of tri( o -tolyl)antimony (0.25 mmol) and 79 mg (0.5 mmol) of 5-nitrofurfuraldoxime were dissolved in diethyl ether (40 mL), then 28 mg of 30 % aqueous solution of hydrogen peroxide (0.25 mmol) were added. The mixture was kept for 24 hours at 20 °C. When the solvent was evaporated, fine-crystalline precipitate was crystallized from benzene with the addition of heptane (5:1 vol.) to give light-yellow crystals of 3 (m = 0.139 g (83 %), MP = 154 °C, with destr.).
IR spectrum ( ν , cm–1) : 1576, 1560, 1526, 1477, 1456, 1375, 1348, 1298, 1242, 1205, 1180, 1159, 1123, 1018, 964, 924, 864, 837, 808, 746, 737, 704, 683, 631, 582, 548, 507, 480, 436, 412.
-
b) 100 mg of tri( o -tolyl)antimony (0.25 mmol) and 79 mg (0.5 mmol) of 5-nitrofurfuraldoxime were dissolved in diethyl ether (40 mL), then 22 mg of 70 % aqueous solution of tert -butyl peroxide (0.25 mmol) were added. The mixture was kept for 24 hours at 20 °C. When the solvent was evaporated, fine-crystalline precipitate was crystallized from benzene with the addition of heptane (5:1 vol.) to give light-yellow crystals of 3 (m = 0.139 g (83 %), MP = 154 °C, with destr.).
Synthesis of µ 2 -oxo- bis [(5-nitrofurfuraldoximato)tri( m -tolyl)antimony] (4).
-
a) 100 mg of tri( o -tolyl)antimony (0.25 mmol) and 39 mg (0.25 mmol) of 5-nitrofurfuraldoxime were dissolved in heptane (40 mL), then 28 mg of 30 % aqueous solution of hydrogen peroxide (0.25 mmol) were added. The mixture was kept for 24 hours at 20 °C. After the solvent evaporation, the colorless crystals of 4 were obtained (m = 0.138 g (99 %), MP = 164 °C, with destr.).
IR spectrum (ν, см - 1): 1587, 1570, 1550, 1516, 1473, 1377, 1352, 1304, 1242, 1206, 1178, 1155, 1121, 1031, 1013, 982, 968, 943, 860, 822, 808, 798, 748, 723, 698, 674, 665, 542, 503, 471, 438, 409.
-
b) 100 mg of tri( o -tolyl)antimony (0.25 mmol) and 39 mg (0.25 mmol) of 5-nitrofurfuraldoxime were dissolved in heptane (40 mL), then 22 mg of 70 % aqueous solution of tert -butyl peroxide (0.25 mmol) were added. The mixture was kept for 24 hours at 20 °C. After the solvent evaporation, the yellow crystals of 4 were obtained (m = 0.138 g (99 %), MP = 164 °C, with destr.).
IR spectra of compounds 1 - 4 were recorded on a Shimadzu IRAffinity-1S FTIR spectrometer (pellets with KBr; 4000 - 400 cm–1).
X-ray diffraction analysis of crystalline substances 2 , 4 was performed on Bruker D8 QUEST automatic four-circle diffractometer (Mo K α - emission, λ = 0.71073 Å, graphite monochromator).
Data collection and editing, the refinement of unit cell parameters, and correction for absorption were carried out in SMART and SAINT-Plus software [10]. All calculations aimed at solving and refining the structures of 2 , 4 were performed in SHELXL/PC software [11]. The structures of 2 , 4 were
Химия элементоорганических соединений
determined using direct methods and refined with LS method in the anisotropic approximation for nonhydrogen atoms. The selected crystallographic data and the structure refinement results are listed in Table 1. Selected bond lengths and bond angles are summarized in Table 2.
The full tables of atomic coordinates, bond lengths, and bond angles were deposited with the Cambridge Crystallographic Data Centre (CCDC 1048763, 1048167, 1048131 for compounds 2 and 4, respectively; ; .
Table 1
Crystallographic data and the experimental and structure refinement parameters for compounds 2, 4
|
Parameter |
Value |
|
|
2 |
4 |
|
|
Empirical formula |
C 52 H 48 N 4 O 9 Sb 2 |
C 26 H 24 N 2 O 4.5 Sb |
|
Formula weight |
1116.44 |
558.22 |
|
Т , К |
273.15 |
273.15 |
|
Crystal system |
monoclinic |
monoclinic |
|
Space group |
P2 1 /c |
C2/c |
|
a , Å |
22.472(2) |
20.5292(11) |
|
b , Å |
13.1397(11) |
10.1119(6) |
|
c, Å |
21.1657(18) |
27.9617(15) |
|
α , deg |
90.00 |
90.00 |
|
β, deg |
110.646(3) |
91.075(2) |
|
γ , deg |
90.00 |
90.00 |
|
V , Å3 |
5848.3(9) |
5803.5(6) |
|
Z |
4 |
8 |
|
ρ (calcd.) , g/сm3 |
1.268 |
1.278 |
|
µ , mm–1 |
0.974 |
0.981 |
|
F (000) |
2248.0 |
2248.0 |
|
θ Range of data collection, deg |
6.5–38.12° |
7–49.5° |
|
Range of refraction indices |
–20 ≤ h ≤ 20, –12 ≤ k ≤ 12, –19 ≤ l ≤ 19 |
–24 ≤ h ≤ 19, –11 ≤ k ≤ 11, –32 ≤ l ≤ 31 |
|
Measured reflections |
32451 |
9023 |
|
Independent reflections |
4701 |
4648 |
|
R int |
0.0476 |
0.0428 |
|
Refinement variables |
610 |
307 |
|
GOOF |
1.117 |
1.078 |
|
R factors for F2 > 2 σ (F2) |
R 1 = 0.0738, wR 2 = 0.2106 |
R 1 = 0.0792, wR 2 = 0.2043 |
|
R factors for all reflections |
R 1 = 0.0906, wR 2 = 0.2430 |
R 1 = 0.1291, wR 2 = 0.2323 |
|
Residual electron density (min/max), e /Å3 |
1.54/–0.41 |
1.05/–0.47 |
Table 2
Selected bond lengths and bond angles in the structures of compounds 2, 4
|
Bond \ |
d , Å \ |
Angle \ |
ω , deg |
|
2 |
|||
|
Sb(1)–С(1) |
2.156(17) |
O(1)Sb(1)O(2) |
176.5(4) |
|
Sb(1)–С(11) |
2.107(19) |
C(11)Sb(1)C(1) |
121.5(7) |
|
Sb(1)–С(21) |
2.051(16) |
C(21)Sb(1)C(11) |
118.3(7) |
|
Sb(1)–О(1) |
1.971(9) |
C(1)Sb(1)C(21) |
119.1(7) |
|
Sb(1)–O(2) |
2.130(10) |
N(1)O(2)Sb(1) |
107.1(9) |
|
О(2)–N(1) |
1.409(17) |
O(2)N(1)C(35) |
108.2(13) |
|
O(6)–N(3) |
1.332(17) |
Sb(1)O(1)Sb(2) |
146.0(5) |
|
N(1)–C(35) |
1.267(19) |
N(3)O(6)Sb(2) |
116.3(8) |
|
N(3)–C(75) |
1.206(17) |
O(6)N(3)C(75) |
113.9(12) |
Table 2 (end)
|
Bond |
d , Å |
Angle |
ω , deg |
|
Sb(2)–С(41) |
2.119(15) |
C(61)Sb(2)C(51) |
120.9(6) |
|
Sb(2)–С(51) |
2.088(13) |
C(51)Sb(2)C(41) |
121.0(5) |
|
Sb(2)–С(61) |
2.092(14) |
C(41)Sb(2)C(61) |
116.9(6) |
|
Sb(2)–О(1) |
1.975(8) |
O(1)Sb(1)C(11) |
92.8(7) |
|
Sb(2)–O(6) |
2.127(9) |
O(1)Sb(1)C(21) |
94.8(5) |
|
Sb(1)–N(1) |
2.88(2) |
O(1)Sb(2)C(61) |
96.6(4) |
|
Sb(2)–N(3) |
2.97(1) |
O(1)Sb(2)C(41) |
91.0(5) |
|
4 |
|||
|
Sb(1) – С(1) |
2.097(10) |
O(1)Sb(1)O(2) |
173.6(2) |
|
Sb(1) – С(11) |
2.116(16) |
C(11)Sb(1)C(1) |
123.3(5) |
|
Sb(1) – С(21) |
2.107(17) |
C(21)Sb(1)C(1) |
122.1(6) |
|
Sb(1) – О(1) |
1.960(8) |
C(11)Sb(1)C(21) |
114.0(6) |
|
Sb(1) – O(2) |
2.193(8) |
N(1)O(2)Sb(1) |
111.3(6) |
|
О(2) – N(1) |
1.335(11) |
O(4)N(2)C(34) |
116.9(14) |
|
O(5)–N(2) |
1.187(14) |
Sb(1)O(1)Sb(2) |
180.00(3) |
|
N(1)–C(35) |
1.302(13) |
O(5)N(2)C(34) |
119.7(13) |
|
N(2)–C(34) |
1.395(16) |
O(1)Sb(1)C(11) |
95.1(3) |
|
Sb(1)–N(1) |
2.95(1) |
O(1)Sb(1)C(21) |
91.4(4) |
Results and Discussion
We have studied the reactions of tri( m -tolyl) - and tri( o -tolyl)antimony with 5-nitrofurfuraloxime at various molar ratios in the presence of an oxidizing agent. It has been found that at molar ratio 1:2 of triarylantimony and 5-nitrofurfuraldoxime the product of the reactions, regardless of the nature of the oxidant, is bis (5-nitrofurfuraloximato)triarylantimony: m -Tol 3 Sb[ON=CHC 4 H 2 O(NO 2 -5)] 2 ( 1 ) or о -Tol 3 Sb[ON=CHC 4 H 2 O(NO 2 -5)] 2 ( 3 ):
Ar 3 Sb + 2 HON=CHC 4 H 2 O(NO 2 -5) + ROOH → Ar 3 Sb[ON=CHC 4 H 2 O(NO 2 -5)] 2 + 2 ROH Ar = m -Tol, о -Tol; R = H, Bu- t
At the equimolar ratio of the reagents, in the presence of both hydrogen peroxide and tert -butyl hydroperoxide, μ 2 -oxo-bis[(5-nitrofurfuraldoxymato) tri( m -tolyl)antimony[ m -
Tol 3 SbON=CHC 4 H 2 O(NO 2 -5)] 2 O (2) or μ 2 -oxo- bis [(5-nitrofurfuraldoxymato)tri( o -tolyl) antimony] [ o -Tol 3 SbON=CHC 4 H 2 O(NO 2 -5)] 2 O (4) is formed:
2 Ar 3 Sb + 2 HON=CHC 4 H 2 O(NO 2 -5) + 2 ROOH → [Ar 3 SbON=CHC 4 H 2 O(NO 2 -5)] 2 O + 2 ROH + H 2 O Ar = m -Tol, о -Tol; R = H, Bu- t
Compounds 1 – 4 are crystalline substances, which are resistant to the effect of moisture and air oxygen and freely soluble in aromatic and aliphatic hydrocarbons.
The synthesized triarylantimony dioximates have been identified by infrared spectroscopy and X-ray diffraction analysis.
In the IR-spectrum of compounds 1–4 there are intensive absorption bands, which characterize nitro group vibrations. Thus, the absorption band due to NO 2 -group asymmetric vibrations is at 1520, 1522, 1526 и 1516 cm-1, while the band, corresponding to NO 2 -group symmetric vibrations appears at 1348, 1350, 1348 и 1352 cm–1 in the spectra of 1 – 4 , respectively. The C–NO 2 vibrations are characterized by the bands at 810, 810, 808 и 808 cm–1. In addition, there are bands at 426, 426, 436 и 438 cm–1, which have been attributed to the Sb–C(Ar) vibration of the С 3 -symmetric [12] SbC 3 fragment. The characteristic bands at 1558–1587 cm–1 (C=N bonds), 964–970 cm–1 (N–O bonds) have also been found.
According to the X-ray diffraction analysis data, the antimony atoms in the molecules of compounds 2 and 4 have distorted trigonal-bipyramidal coordination with oxygen atoms in axial positions (Fig. 1 - 2). Molecule 4 is centrosymmetric. In binuclear molecules 2, 4 the Sb (1) and Sb (2) atoms are connected by the bridging oxygen atom, Sb(1)O(1)Sb(2) bond angles are equal to 146.0(3)° и 180.0(0)°, respectively. The oximate ligands in molecules 2, 4 are monodentate as in the case of μ 2 -oxo- bis [(furfuraloxymat)tri( o -tolyl) antimony] [10].
Химия элементоорганических соединений
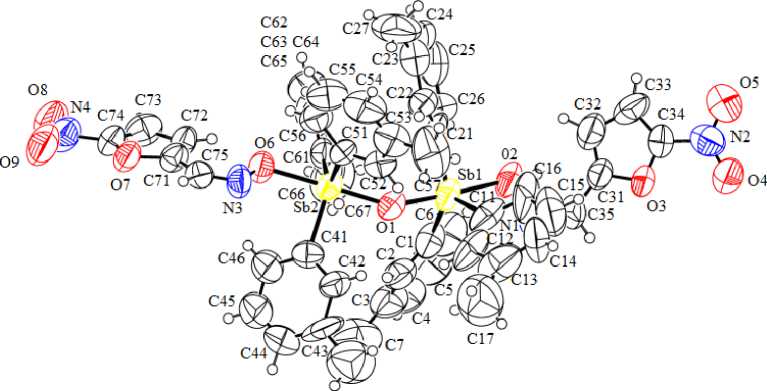
Fig. 1. The structure of compound 2
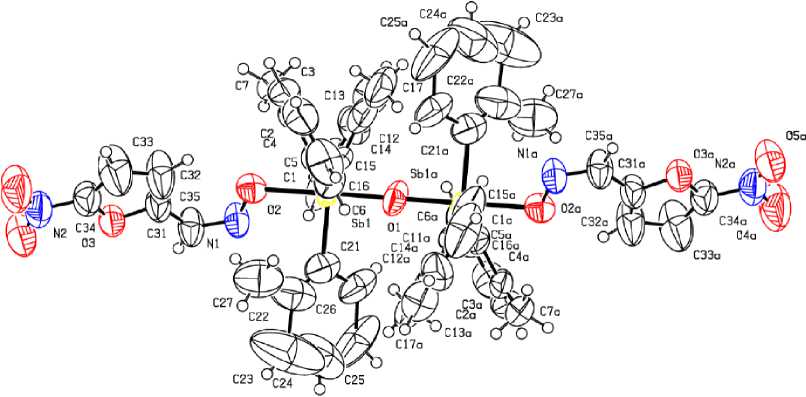
Fig. 2. The structure of compound 4
The sums of СSbC bond angles are equal to 358.9(7)°, 358.8(6)° ( 2 ), 359.4(6)° ( 4 ), at that the values of the individual angles differ from the theoretical angle not more than by 6º. The Sb atoms are shifted from the correlated planes [C 3 ] at 0.002 Å ( 1 ), 0.091, 0.098 Å ( 2 ), 0.052 Å ( 3 ). The axial OSbO angles are equal to 176.5(4)°, 176.1(3) ( 2 ), 173.6(2)° ( 4 ) . The OSbC angles vary within the ranges 92.8(7)° - 94.8(5) ° , 91.0(5)°-96.6(4)° ( 2 ), 91.4(4)° - 95.1(3)° ( 4 ).
The lengths of Sb–C bonds lie within the ranges of 2.051(16)-2.156(17) Å, 2.088(13)–2.119(15) Å ( 2 ), 2.097(10)–2.116(16) Å ( 4 ). The Sb–O bridging bond lengths in 2 (1.971(9), 1.975(8) Å) are longer in comparison with the same characteristic in 4 (1.960(8) Å), which correlates with the previously discovered pattern for the molecules with the similar structure: the closer the angle SbOSb is to linear, the shorter the Sb–O bond is [11]. The lengths of the Sb–O terminal bonds are 2.130 (10), 2.127 (9) Å (2), and 2.195 (8) Å (4). It should be noted that the values of angles for the antimony atom in molecule 4 are approximately equal to the corresponding values in the fragment of the molecule of μ 2 -oxo- bis [(furfuraloxime)tri( o -tolyl)antimony], where the Sb - O - Sb fragment is linear, but the internuclear distances Sb–C and Sb–O in these molecules are different. Thus, the bonds Sb–O (1.9706 (3), 2.112 (6) Å [10]) in the latter compound are shorter than in 4 , which can be explained by the presence of the nitro group in the furan ring of its oximate ligand.
In molecules 2, 4 the Sb···N distances between the Sb atom and N atoms of iminoxy groups [2.88(8), 2.97(1) Å (2), 2.95(1) Å (4)] are considerably less than the sum of Van der Waals radiuses of the Sb and N atoms (3.8 Å [15]). Obviously, there is no correlation between the Sb–O bond lengths and strength of Sb···N contacts. Thus, in the molecule of μ2-oxo-bis[(furfuraloximatio)tri(o-tolyl)antimony], the distance Sb···N (2.951(1) Å [10]) coincides with the comparable distance in 4. The decrease of the Sb···N distances does not result in the expected N-O bond lengthening [1.409(17), 1.332(17) Å (2), 1.335(11) Å (4)].
T he s tr u c tu r e or g a niza t ion of c r y stals 2 , 4 is due to weak intermolecular О···Н hydrogen bonds between the oxy g e n a toms of ni tr o groups and the hydrogen atoms of methyl groups or a roma tic ring s, as well as due to С–Н··· π int e ra ction s.
Conclusions
The s truc t ur e of the pr o d uc ts o f the rea ction s of t r i( m -tolyl)- and tri( o -tolyl) antimony with 5-n itr of u r f u r aldox ime de pends on the molar ratio of the reactants. Regardless of th e nature of th e o xida n t, t he r ea c t ion o f t r iar y la nt imony with 5-nitrofurfuraloxime at molar ratio 1:2 leads to the for ma tion of trito ly la nt imony dioxyma te s. At the s t oic h iom etric ratio of the reagents the antimony o r g a nic p r odu c t w ith the bri dg ing oxygen a tom is f or me d.
The s tr uctu r e o f mole c u l e s is determined by the aryl radical nature, thu s, the μ 2 -oxo- bis [(5-nitrofurfuraldoxymato)tri( m - t oly l) a n timony mole c ul e] ha s th e a ng ular structure of the central fragment, and the μ 2 -oxo- bis [(5- nitrof urf u r aldox ima to) t r i ( o -tolyl) antimony] molecule is centrosymmetric and has a l inea r st r ucture . T h e ox i me lig ands are monodentate in both cases; they are bo und to the a n timony atom via the oxygen atom.
Список литературы The oxidative-addition reactions of tri(meta-tolyl) antimony and tri(ortho-tolyl)antimony with 5-nitrofurfuraldoxime in the presence of peroxides. The molecular structures of 2-oxo-bis[(5- nitrofurfuraldoximato)tri(meta-tolyl)antimony] and 2-oxo-bis[(5-nitrofurfuraldoximato)tri(ortho- tolyl)antimony]
- Bajpai, K. Synthesis and reactions of o-triorganoantimony dioximates/K. Bajpai, R.C. Srivastava//Synth. Inorg. Met. -Org. Chem. -1981. -V. 11, № 1. -P. 7-13.
- Li, J.S. Synthesis and biological activity of some triarylantimony dipyrazolecarboxylates/Y.-Q. Ma, L. Yu, J.-S. Li//Heteroatom Chemistry. -2002. -V. 13, № 4. -P. 299-301.
- Synthesis and in vitro antitumor activity of some triarylantimony di(N-phenylglycinates)/L. Yu, Y.-Q. Ma, G.-C. Wang. et al.//Heteroatom Chemistry. -2004. -V. 15, №1. -P. 32-34.
- Synthesis and spectroscopic characterization of biologically active triarylantimony (V) carboxylates containing germanium/M.K. Khosa, M. Mazhar, S. Ali//Turkish Journal of Chemistry. -2006. -V. 30, № 3. -P. 345-354.
- Handong, Y. Synthesis, spectroscopic and structural aspects of triphenylantimony(V) complex with internally functionalized acetylferroceneoxime: Crystal and molecular structures of 2SbPh3 and C5H5FeC5H4C(CH3)=NOH/Y. Handong, Q. Li, L. Linwei//Inorganic Chemistry Communications. -2008. -№ 11. -P. 1121-1124.
- Novel triphenylantimony (V) and triphenylbismuth (V) complexes with benzoic acid derivatives: structural characterization, in vitro antileishmanial and antibacterial activities and cytotoxicity against macrophages/A. Islam, J.G. Da Silva, F.M. Berbet et al.//Molecules. -2014. -V. 19, № 5. -P. 6009-6030.
- Синтез и строение оксиматов трифенилсурьмы/В.А. Додонов, А.В. Гущин, Д.А. Горькаев и др.//Изв. РАН. Сер. хим. -2002. -№ 6. -С. 965-971.
- Синтез и строение µ-оксобис/В.В. Шарутин, О.К. Шарутина, О.В. Молокова и др.//Журн. общ. химии. -2001. -Т. 71, вып. 9. -С. 1507-1510.
- Шарутин, В.В. Особенности взаимодействия трис(5-бром-2-метоксифенил)сурьмы с 2-оксибензальдоксимом. Строение бис(μ3-2-оксибензальдоксимато-О,О',N)-(μ2-оксо)-бис(5-бром-2-метоксифенил)дисурьмы/В.В. Шарутин, О.К. Шарутина//Журн. неорган. химии. -2014. -Т. 59, № 11. -С. 1507-1511 DOI: 10.7868/S0044457X14110221
- Реакции окислительного присоединения три(2-метилфенил)сурьмы/В.В. Шарутин, О.В. Молокова, О.К. Шарутина и др.//Журн. неорган. химии. -2012. -Т. 57, № 9. -С. 1334-1340.
- Молекулярные структуры органических соединений сурьмы (V): монография/О.К. Шарутина, В.В. Шарутин. -Челябинск: Издательский центр ЮУрГУ, 2012. -395 с.
- Bruker. SMART. Bruker molecular analysis research tool, Versions 5.625 Bruker AXS, Madison, Wisconsin, USA, 2000.
- Bruker. SAINT Plus data reduction and correction program versions 6.02a, Bruker AXS, Madison, Wisconsin, USA, 2000.
- Doak, G.O. The infrared spectra of some phenylsubstituted pentavalent antimony compounds/G.O. Doak, G.G. Long, L.D. Freedman//J. Organomet. Chem. -1965. -V. 4, № 1. -P. 82-91.
- Бацанов, С.С. Атомные радиусы элементов/С.С. Бацанов//Журн. неорган. химии. -1991. -Т. 36, вып. 12. -С. 3015-3037.

![The oxidative-addition reactions of tri(meta-tolyl) antimony and tri(ortho-tolyl)antimony with 5-nitrofurfuraldoxime in the presence of peroxides. The molecular structures of 2-oxo-bis[(5- nitrofurfuraldoximato)tri(meta-tolyl)antimony] and 2-oxo-bis[(5-nitrofurfuraldoximato)tri(ortho- tolyl)antimony] The oxidative-addition reactions of tri(meta-tolyl) antimony and tri(ortho-tolyl)antimony with 5-nitrofurfuraldoxime in the presence of peroxides. The molecular structures of 2-oxo-bis[(5- nitrofurfuraldoximato)tri(meta-tolyl)antimony] and 2-oxo-bis[(5-nitrofurfuraldoximato)tri(ortho- tolyl)antimony]](/file/cover/147160389/the-oxidative-addition-reactions-of-tri-meta-tolyl-antimony-and-tri-ortho-tolyl.png)
