The role of EZH2 and ARID1A in the diagnosis of flat urothelial lesions with atypia
Автор: Sameh Reham, Mostafa Naglaa Mostafa, Embaby Ahmed, Raouf Samar Abdel, Abdelwahab Khaled
Журнал: Сибирский онкологический журнал @siboncoj
Рубрика: Лабораторные и экспериментальные исследования
Статья в выпуске: 5 т.20, 2021 года.
Бесплатный доступ
Background. Diagnosis of urothelial carcinoma in situ is of great importance because it has prognostic and therapeutic value. We aim to determine the utility of EZH2 and ARiD1A as a new tool in the diagnosis of carcinoma in situ. Material and Methods. This retrospective cross-sectional study included Twenty-four specimens of flat urothelial lesions, twenty specimens of CiS, and 10 of normal adjacent urothelium that was taken by cystoscopic resection biopsy procedure. immunohistochemical expression of EZH2 and ARiD1A. were evaluated in all studied cases. Results. All normal urothelium specimens showed high nuclear staining for ARiD1A and negative nuclear staining for EZH2. High EZH2 expression was observed in 80 % of CiS specimens compared to 20 % of flat urothelial lesions with atypia (p=0.001 ), while high ARiD1A expression was observed in 70.8 % of flat urothelial lesions with atypia compared to 25 % of CiS specimens (p=0.001). EZH2 was more accurate and specific in the diagnosis of carcinoma in situ. Conclusion. EZH2 and ARiD1A are promising diagnostic markers for urothelial CiS. EZH2 is more accurate and specific than ARiD1A in the diagnosis of carcinoma in situ versus other flat urothelial lesions.
Dysplasia, carcinoma in situ, reactive atypia, immunohistochemistry
Короткий адрес: https://sciup.org/140261336
IDR: 140261336 | УДК: 616.6-006.6-076:577.112 | DOI: 10.21294/18144861-2021-20-5-49-57
Текст научной статьи The role of EZH2 and ARID1A in the diagnosis of flat urothelial lesions with atypia
Urothelial carcinoma is a major health problem. The incidence of urothelial carcinoma worldwide is approximately 4.5 % in males and 1.5 in females [1]. In Egypt, It represents the second mostly diagnosed cancers in men [2]. Early diagnosis of UC may decrease the mortality and morbidity rates [3]. According to the latest WHO 2016 committee, flat urothelial lesions with atypia were classified as reactive urothelial atypia, urothelial proliferation of uncertain malignant potential (UPUMP), urothelial dysplasia and urothelial carcinoma in situ [4]. UPUMP contains no true papillary projections but undulations are common with a thickened urothelium but minimal or no cytological atypia. These entities may be seen de novo, and in this setting, the clinical relevance is unknown. More frequently they are seen in patients who have a history of prior carcinoma or seen adjacent to papillary lesions. It is likely that most represent lateral extension (‘‘shoulder lesion’’) of a papillary neoplasm [5]. Reactive urothelial atypia may in some instances lead to confusion with dysplasia or cause concern for patients. Nucleomegaly is the most prominent finding in reactive urothelial changes, but the cells often have a single prominent nucleolus and evenly distributed vesicular chromatin. The nuclear borders are smooth. The nuclei are frequently round, and nuclear pleomorphism is lacking [6]. Architecturally, the cells maintain their polarity to the basement membrane although a minimal loss of polarity may be evident. The mitotic rate may be increased with mitoses present predominantly in the basal and intermediate urothelium, but atypical forms are not seen. Intraurothelial acute or chronic inflammation is commonly identified [7]. It is important to recognize that even intraepithelial lymphocytes, by themselves, can result in reactive changes. The cytoplasm may become more basophilic or eosinophilic with loss of cytoplasmic clearing. Clinical history of stones, infection, or frequent instrumentation may be present. Reactive urothelium may be denuded with only a single residual layer of basal cells remaining; the residual cells are not hyperchromatic, not enlarged, and do not possess nuclear membrane irregularity
-
[ 8]. Urothelial dysplasia (UD) is defined as the loss of polarity with nuclear rounding and crowding and cytologic atypia that is not severe enough to diagnose CIS. CIS and UD are precursor lesions of invasive urothelial carcinoma and their detection, especially CIS, is associated with a significant risk of progression and recurrence [9]. CIS is often multifocal and can occur in the upper urinary tracts and in the prostatic ducts and urethra. CIS exists in two settings; isolated (primary) CIS and secondary CIS associated with papillary urothelial carcinoma. Isolated CIS was rare, accounting for about 10 % of all CIS and 1 % to 3 % of bladder neoplasm [10]. Although nuclear and architectural features are the primary criteria for differentiation between CIS and other flat epithelial lesions with atypia, it may be difficult in some patients. Expression of markers as EZH2 and ARID1A may be helpful [11, 12].
The Enhancer of Zeste Homolog 2 (EZH2) is a core subunit of the polycomb repressor complex 2 (PRC2), which is overexpressed in numerous cancers and mutated in several others. Notably, EZH2 acts as a critical epigenetic repressor through its role in histone methylation, it is also an activator of gene expression, acting through multiple signaling pathways in distinct cancer types. Increasing evidence suggests that EZH2 is an oncogene and is central to initiation, growth and progression of urological cancers [13]. AT-rich interacting rich domain 1(ARID1A) belongs to a family of proteins containing a highly conserved, approximately 100-amino acid DNA binding domain called ARID (AT-rich interacting domain). Although the ARID domains in general preferentially bind AT-rich DNA sequences, the ARID1A domain of mammalian ARID1A exhibits general DNA binding character without sequence specificity [14]. The ARID1A gene maps to chromosome 1p36.11, a region frequently deleted in cancer. Initial clues that ARID1A was a tumor suppressor came from expression analyses that showed decreased ARID1A expression in 30 % of renal cancer and 10 % of breast cancer [15]. A synthetic lethality relationship between other SWI/SNF components including ARID1A and EZH2 has been revealed revealed in several tumor entities [16]. We evaluate the validity of EZH2 and ARID1A expression in distinction between carcinoma in situ and other flat urothelial lesions
Material and Methods
This retrospective cross-sectional study was performed after approval by the local ethical Committee Zagazig University, Institutional Review Board (IRB) for human studies (reference number is ZU-IRB #:3986-18-9-2017). This study included 54 patients were selected from pathology department archival blocks in collaboration with urology department, Faculty of Medicine, Zagazig University, Egypt, in the period from June 2016 to June 2020. The control group included 10 specimens from adjacent normal mucosa. Twenty-four patients with flat urothelial lesions with atypia (nine specimens of UPUMP, eight specimens of reactive atypia and seven specimens of dysplasia) and 20 specimens of CIS. All specimens were obtained by urethrocystoscopy and biopsies. They were classified according to 2016 WHO/ ISUP [4].
Immunohistochemical staining
Staining was performed with Dako Autostainer link 48 (Dako) according to manufacturer’s guide.
Iry antibodies
-
1 – ARID1A [Rabbit polyclonal antibody (Cat. GTX129433) isotype: IgG, 1:100-1000 dilution, Gene Tex international corporation].
-
2 – EZH2 [Rabbit polyclonal antibody, Clone 144CT2.1.1.5, isotype: IgG, 1:50-100 dilution, US Biological life sciences USA] Normal human testicular tissue was used as positive control for EZH2 while normal human breast and kidney tissue served as positive control for ARID1A. Negative controls were obtained by replacing the primary antibodies with non-immune serum.
The evaluation of ARID1A immunostaining
Nuclear ARID1A expression was considered positive. Immunoreactivity was assessed by considering the extent and the intensity of nuclear staining in the tumor cells. Grades of 0–3 were assigned according to the percentage of positive tumor cells (0=0 %; 1<25 %; 2=25–50 %; 3>51 %) and the intensity of staining in tumor (0=absence of staining; 1=weak; 2=intermedi-ate; 3=strong intensity). The combined score was calculated by multiplication of the percentage and the intensity grade [17]. Receiver operating characteristic (Roc) curve analysis was used for calculating the cutoff point.
The evaluation of EZH2 immunostaining
The EZH2 expression was considered positive in nuclear expression only. The extent of nuclear EZH2 protein staining was graded as follows: 1 (<25 % staining of tumor cells), 2 (25–50 % staining of tumor cells), 3 (50–75 % staining of tumor cells), 4 (>75 % staining of tumor cells). Moreover, the staining intensity was quantified as 0 (indicated no expression), 1 (indicated weak expression), 2 (indicated intermediate expres- sion), 3 (indicated strong expression).
The intensity was multiplied by the extension values to obtain the final immunostaining score (range 0–12) [18]. Receiver operating characteristic (Roc) curve analysis was used for calculating the cutoff point.
Statistical Analysis
Statistical analysis was carried out by using SPSS version «20.0» (SPSS Inc.). Fisher's exact and χ2 tests were applied for comparisons between the nominal variables. Independent student t-test (t) was used to compare two groups of normally distributed data Mann-Whitney U (MW) was used to compare two groups of non-normally distributed data. Sensitivity, specificity, accuracy, and positive predictive values were calculated. The designations true and false were based on the study hypothesis that high EZH2 is expressed in malignancy and true positivity for ARID1A is expected in benign and other flat epithelial lesions.
Results
Mean age of the cases was 39.8 ± 1.3, most cases were males (79.2 %). According to Roc curve analysis the cutoff point of EZH2 immunoreactivity in all studied cases was 4 (Fig. 1). They were reclassified into two group; low expression (score<4,) and high expression (score≥4 was). However, the cutoff point
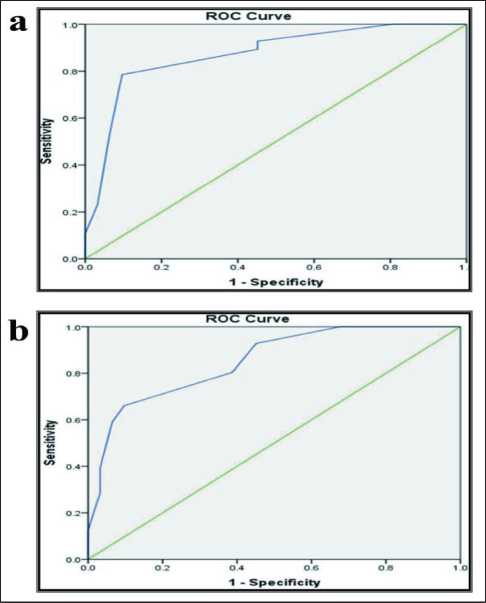
Рис. 1. а) ROC-кривая для оценки EZH2; b) ROC-кривая для оценки EZH2
Fig. 1. a) ROC curve for EZH2 cut-off point estimation; b) ROC curve for EZH2 cut-off point estimation
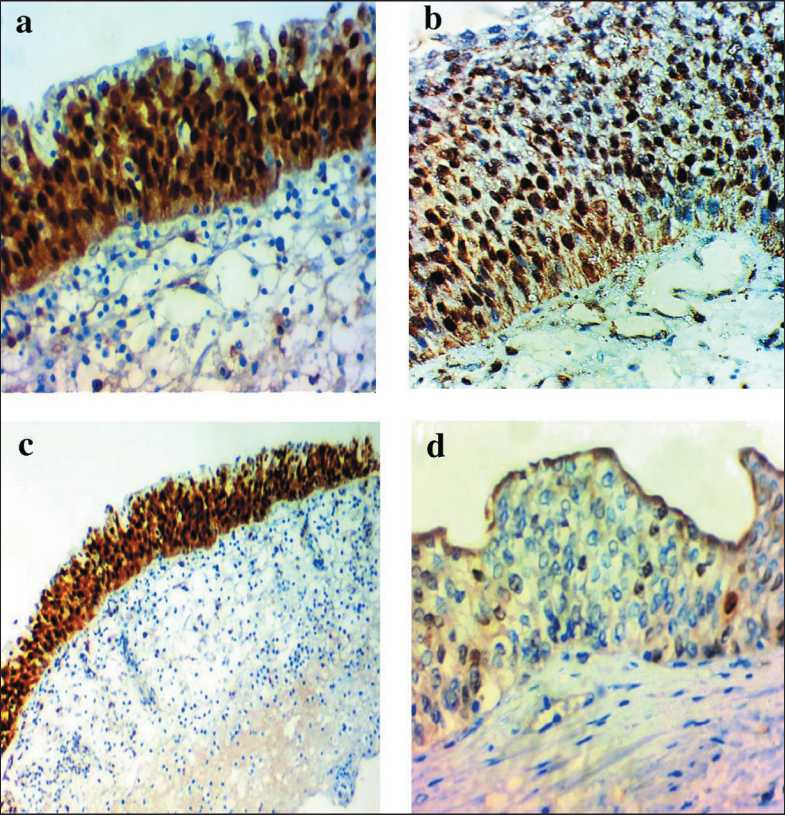
Рис. 2. Микрофото: a) высокая ядерная экспрессия ARID1A в нормальном эпителии; b) высокая ядерная экспрессия
ARID1A в случае UPUMP;
c) высокая ядерная экспрессия ARID1A в случае реактивной атипии;
d) низкая экспрессия ARID1A в случае CIS
Fig. 2. Microphoto: a) high ARID1A nuclear expression in normal epithelium; b) high ARID1A nuclear expression in a case UPUMP; c) high ARID1A nuclear expression in a case reactive atypia; d) low ARID1A expression in a case of CIS
of ARID1A immunoreactivity in all studied cases was (Fig. 1).
Furthermore, they were reclassified into two groups: low expression (score<3) and high expression (score≥3). High ARID1A expression was significantly low in CIS (25 %) compared to its high expression in
100 % of normal epithelium and 70.8 % of all flat lesions with atypia with a highly statistically significance difference between them (p-value=0.0002) (Table 1) (Fig. 2).
ARID1A expression level was significantly lower in CIS compared to normal, UPUMP and reactive aty- таблица 1/table 1
сравнение eZh2 и aRid1a между cis и другими плоскими уротелиальными опухолями с атипией comparison of eZh2 and aRid1a between cis and other flat urothelial lesions with atypia
|
No |
EZH2 Immunoreactivity |
p-value |
ARID1A Immunoreactivity |
p-value |
|||
|
Low (cut off <4) |
High (cut off ≥4) |
Low (cut off <3) |
High (cut off ≥3) |
||||
|
Normal/Нормальные |
10 |
10 (100.0 %) |
0 (0.0 %) |
0 (0 %) |
10 (100 %) |
||
|
Плоские эпителиальные опухоли с атипией/ Flat epithelial lesions with atypia |
24 |
20(80 %) |
4(20 %) |
7 (29.2 %) |
17 (70.8 %) |
||
|
• UPUMP1 |
9 |
9 (100 %) |
0 (0 %) |
<0.001** |
1 (11.1 %) |
8 (88.9 %) |
<0.001** |
|
• Реактивная атипия/ • Reactive atypia |
8 |
7 (87.5 %) |
1(12.5 %) |
2 (25.0 %) |
6 (75.0 %) |
||
|
• Дисплазия/Dysplasia |
7 |
4 (57.1 %) |
3 (42.9 %) |
4 (57.1 %) |
3 (42.9 %) |
||
|
CIS2 |
20 |
4 (20 %) |
15 (80 %) |
16 (75 %) |
5 (25 %) |
||
Примечание: 1 –UPUMP: уротелиальная пролиферация с неопределенным злокачественным потенциалом; 2 – CIS: карцинома in situ ;
** – различия статистически значимые (p≤0,001).
Note: 1– UPUMP: urothelial proliferation of uncertain malignant potential, 2 – CIS: Carcinoma in situ , ** – p≤0.001 is highly significant.
таблица 2/table 2
Экспрессия eZh2 в aRid1a в cis по сравнению с другими плоскими опухолями expression of eZh2 in aRid1a in cis versus other flat lesions
|
Варианты/ Variable |
Versus |
EZH2 level |
ARID1A level |
||||
|
Mean ± SD |
Медиана (Диапазон)/ Median (Range) |
p-value |
Mean ± SD |
Медиана (Диапазон)/ Median (Range) |
p-value |
||
|
CIS1 vs нормы/ CIS1 vs normal |
CIS Плоский нормальный/ Flat normal |
6.5 ± 3.1 1.7 ± 1.1 |
6 (2–12) 1 (1–4) |
0.001** |
3.3 ± 1.7 7.1 ± 1.8 |
3 (0.0–6) 6 (4–9) |
0.001** |
|
CIS vs UPUMP2 |
CIS UPUMP |
6.5 ± 3.1 1.9 ± 1.2 |
6 (2–12) 1.5 (1–4) |
0.001** |
3.3 ± 1.7 5.7 ± 2.4 |
3 (0.0–6) 5 (4–9) |
0.004* |
|
CIS vs реактивной атипии/ CIS vs reactive atypia |
CIS Реактивная атипия/ Reactive atypia |
6.5 ± 3.1 2.0 ± 1.4 |
6 (2–12) 1.5 (1–4) |
0.01* |
3.3 ± 1.7 5.3 ± 1.6 |
3 (0.0–6) 5 (4–8) |
0.02* |
|
CIS vs дисплазии/ CIS vs Dysplasia |
CIS Дисплазия/ Dysplasia |
6.5 ± 3.1 2.5 ± 1.4 |
6 (2–12) 2.5 (1–4) |
0.006* |
3.3 ± 1.7 3.7 ± 2.1 |
3 (0.0–6) 3.5 (1–7) |
0.6 |
Примечание: ^ = Тест Манна-Уитни; 1 – CIS: карцинома in situ ; 2 – UPUMP: уротелиальная пролиферация с неопределенным злокачественным потенциалом; * – различия статистически значимые (p<0,05); ** – различия статистически значимые (p≤0,001).
Note: ^= Mann-Witenny test; 1 – CIS: carcinoma in situ ; 2 – UPUMP: urothelial proliferation of uncertain malignant potential; * – p˂0.05 is significant, ** – p≤0.001 is highly significant.
pia (0.001, 0.004, 0.02) respectively. However, there was no statistically significant difference in ARID1A expression levels in differentiating CIS from dysplasia (p=0.6) (Table 2) (Fig. 3).
In contrast, Overexpression of EZH2 was higher in CIS (80 %) compared to its overexpression in 20 % of all flat epithelial lesions with atypia and 0 % of normal epithelium, with a highly statistically significant difference between them (p=0.001) (Table 1) (Fig. 4).
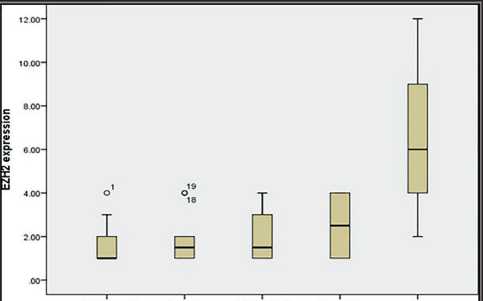
Accent noimal UPUMP Reactive atypia Dysplasia CIS urothelium
Flat epithelial lesions with atypia
Рис. 3. Прямоугольная диаграмма для уровней иммунореактивности EZH2 в нормальных образцах, плоских эпителиальных опухолях и CIS
Fig. 3. Box plot chart for EZH2 immunoreactivity levels in normal, flat epithelial lesions and CIS
Moreover, EZH2 expression levels were statistically increased in CIS compared to normal, UPUMP, reactive atypia and dysplasia (0.001, 0.001, 0.01, 0.006) respectively (Table 2) (Fig. 5).
Sensitivity, Specificity, and accuracy of high EZH2 expression in diagnosis of carcinoma in situ versus other flat urothelial lesions were 80 %, 88.24 %, 85.19 % respectively while sensitivity, specificity, and accuracy of low ARID1A expression were 75 %. 79.41 %, 77.78 % respectively (Table 3).
Discussion
Early detection of CIS would help in reduction of bladder cancer incidence, morbidity and mortality. The differentiation of CIS from other flat lesions with atypia is critical because it has both therapeutic and prognostic importance [19, 20] Even after publication of the 4th edition of WHO/ISUP classifications, the distinction between reactive and dysplastic changes has not been resolved, There are still no definite morphological criteria to diagnose CIS, and there is great
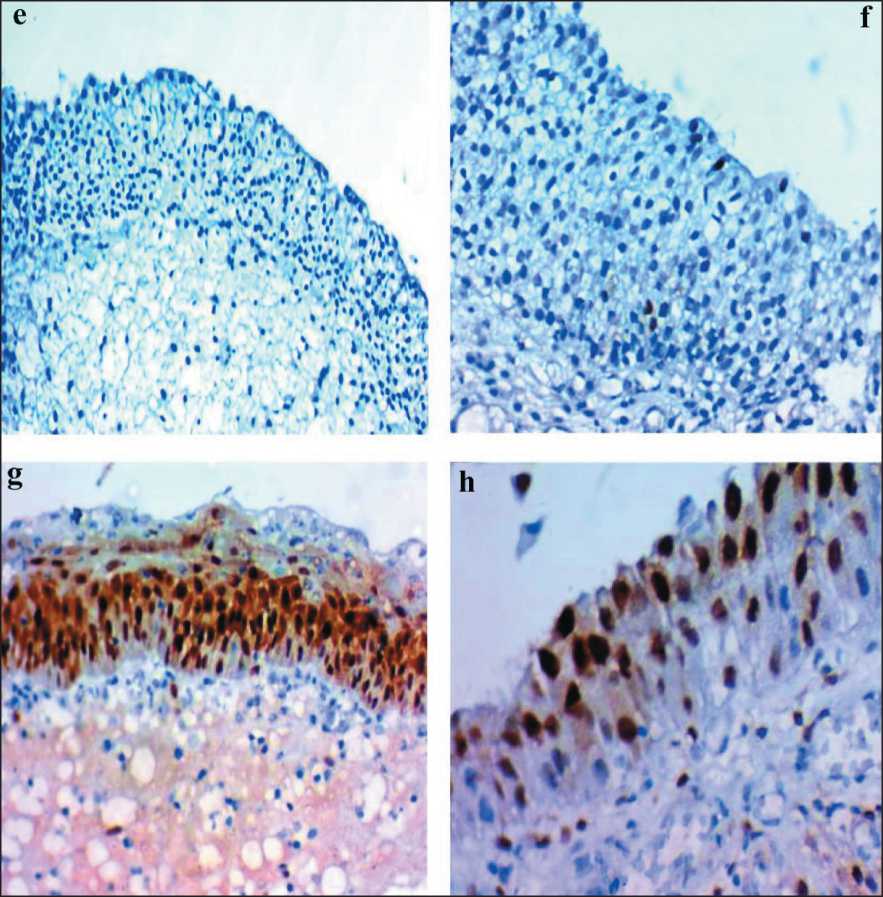
Рис. 4. Микрофото: e) низкая экспрессия EZH2 в нормальном эпителии;
f) низкая экспрессия EZH2 в случае UPUMP; g) высокая экспрессия EZH2 в случае умеренной дисплазии; h) высокая экспрессия EZH2 в случае CIS Fig. 4. Microphoto: e) low EZH2 expression in normal epithelium; f) low EZH2 expression in a case of UPUMP;
g) high EZH2 expression in acase of moderate dysplasia; h) high EZH2 expression in a case of CIS
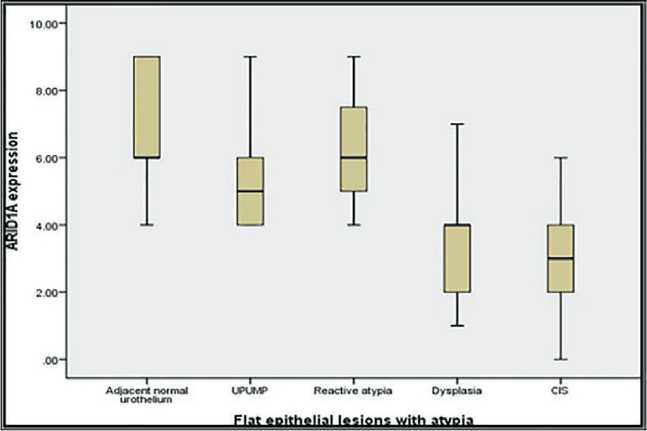
Рис. 5. Прямоугольная диаграмма для уровней иммунореактивности ARID1A в поражениях плоского эпителия и CIS Fig. 5. Box plot chart for ARID1A immunoreactivity levels in flat epithelial lesions and CIS
inter- and interobserver disagreement [4]. Some forms of CIS may be difficult in diagnosis such as clinging and pagetoid type. So morphology alone is frequently insufficient to differentiate CIS from other flat lesions with atypia [21]. There is always a thorough work to discover a diagnostic marker of CIS to differentiate it from other flat epithelial lesions. Moreover, UP-UMP has 40 % risk to turn into low grade urothelial carcinoma after 5 years [4]. Earlier studies suggested the use of CK20, CD44, Ki67, p53, p16 and CK5/6 immunostains as a diagnostic panel for problematic flat lesions with atypia [22], this is a large panel with a high cost and the discriminatory performance may be unsatisfactory in some cases.
EZH2-immunohistochemistry was used in distinction between malignant and reactive mesothelial cells, with 95.5 % sensitivity providing a promising diagnostic marker for malignancy [23]MOC-31, CEA, or B72.3. Also, ARID1A was used as a diagnostic marker for atypical endometrial hyperplasia to таблица 3/table 3
валидность eZh2 и aRidia в диагностике cis по сравнению с нормальными и плоскими поражениями с атипией
Validity of eZh2 and aRidia in diagnosis of cis versus normal and flat lesions with atypia
There are no previous data about the role of EZH2 and ARID1A in distinction between carcinoma in situ and other flat urothelial lesions. In current study, we investigated the use of EZH2 and ARID1A as a diagnostic marker of CIS. We reported that expression of high EZH2 was observed in (80 %) of cases of CIS compared to 20 % of flat epithelial lesions with atypia and 0 % of adjacent normal epithelium with a highly statistically significant difference between them (p-value=0.001), suggesting that EZH2 is a diagnostic marker for CIS. This observation goes in agreement with Warrick et al. (2016) study which found that EZH2 expression was common in bladder cancer with greatest expression seen in CIS in comparison to normal urothelium and recommended EZH2 as a specific marker of CIS and invasive bladder cancer compared to benign urothelium [11]. J.D. Raman et al., (2005) also demonstrated that EZH2 protein levels were higher in UC compared with benign urothelium [25].
Concerning ARID1A our results revealed that ARID1A expression was significantly low in CIS cases (either isolated or concomitant), compared to its high expression in 100 % of normal epithelium and 70.8 % of flat epithelial lesions with atypia with a highly statistically significance difference between them (p-value=0.0002) suggesting the use of ARID1A as a diagnostic marker for CIS. Similarly, T. Aso et al., (2015) reported that loss of ARID1A tumor suppressor gene may play an important role in carcinogenesis and progression of gastric dysplastic lesions to overt carcinoma [26]. This observation goes in agreement with Q. Cao et al. (2019) who reported that ARID1A expression was significantly downregulated in carci- noma tissues compared with normal tissues (p=0.002) suggesting that ARID1A may act as a tumor suppressor in the development of UC and its downregulation may play a role in progression of flat urothelial lesions with atypia to carcinoma [21].
Список литературы The role of EZH2 and ARID1A in the diagnosis of flat urothelial lesions with atypia
- Ferlay J., Colombet M., Soerjomataram I., Mathers C., Parkin D.M., Piñeros M., Znaor A., Bray F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019 Apr 15; 144(8): 1941–1953. doi: 10.1002/ijc.31937.
- Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018 Nov; 68(6): 394–424. doi: 10.3322/caac.21492.
- Cheng L., Lopez-Beltran A., MacLennan G.T., Montironi R., Bostwick D.G. Neoplasms of the urinary bladder. Urologic Surgical Pathology. Elsevier, 2008. p. 258–351.
- Humphrey P.A., Moch H., Cubilla A.L., Ulbright T.M., Reuter V.E. The 2016 WHO Classification of Tumours of the Urinary System and Male Genital Organs-Part B: Prostate and Bladder Tumours. Eur Urol. 2016 Jul; 70(1): 106–119. doi: 10.1016/j.eururo.2016.02.028.
- Moch H., Cubilla A.L., Humphrey P.A., Reuter V.E., Ulbright T.M.
- The 2016 WHO Classification of Tumours of the Urinary System and Male Genital Organs-Part A: Renal, Penile, and Testicular Tumours. Eur Urol. 2016 Jul; 70(1): 93–105. doi: 10.1016/j.eururo.2016.02.029.
- Nguyen J.K., Przybycin C.G., McKenney J.K., Magi-Galluzzi C. Immunohistochemical staining patterns of Ki-67 and p53 in florid reactive urothelial atypia and urothelial carcinoma in situ demonstrate significant overlap. Hum Pathol. 2020; 98: 81–88. doi: 10.1016/j.humpath. 2020.02.008.
- Poropatich K., Yang J.C., Goyal R., Parini V., Yang X.J. Nuclear size measurement for distinguishing urothelial carcinomas from reactive urothelium on tissue sections. Diagn Pathol. 2016 Jun; 11(1): 57. doi: 10.1186/s13000-016-0501-7.
- Lowenthal B.M., Sahoo D., Amin M.B., Hansel D.E. Urothelial Proliferation of Unknown Malignant Potential Involving the Bladder: Histopathologic Features and Risk of Progression in De Novo Cases and Cases With Prior Neoplasia. Arch Pathol Lab Med. 2020; 144(7): 853–62. doi: 10.5858/arpa.2019-0005-OA.
- Cheng L., Zhang S., MacLennan G.T., Williamson S.R., Lopez-Beltran A., Montironi R. Bladder cancer: translating molecular genetic insights into clinical practice. Hum Pathol. 2011 Apr; 42(4): 455–81. doi: 10.1016/j.humpath.2010.07.007.
- Takenaka A., Yamada Y., Miyake H., Hara I., Fujisawa M. Clinical outcomes of bacillus Calmette-Guérin instillation therapy for carcinoma in situ of urinary bladder. Int J Urol. 2008 Apr; 15(4): 309–13. doi: 10.1111/j.1442-2042.2008.02012.x.
- Warrick J.I., Raman J.D., Kaag M., Bruggeman T., Cates J., Clark P., DeGraff D.J. Enhancer of zeste homolog 2 (EZH2) expression in bladder cancer. Urol Oncol. 2016 Jun; 34(6): 258.e1-6. doi: 10.1016/j.urolonc.2016.02.011.
- Pavlidou E.N., Balis V. Diagnostic significance and prognostic role of the ARID1A gene in cancer outcomes. World Acad Sci J. 2020; 2: 49–64. doi: 10.3892/wasj.2020.37.
- Liu X., Wu Q., Li L. Functional and therapeutic significance of EZH2 in urological cancers. Oncotarget. 2017 Jun 6; 8(23): 38044–38055. doi: 10.18632/oncotarget.16765.
- Kelso T.W.R., Porter D.K., Amaral M.L., Shokhirev M.N., Benner C., Hargreaves D.C. Chromatin accessibility underlies synthetic lethality of SWI/SNF subunits in ARID1A-mutant cancers. Elife. 2017 Oct 2; 6: e30506. doi: 10.7554/eLife.30506.
- Suryo Rahmanto Y., Jung J.G., Wu R.C., Kobayashi Y., Heaphy C.M., Meeker A.K., Wang T.L., Shih Ie.M. Inactivating ARID1A Tumor Suppressor Enhances TERT Transcription and Maintains Telomere Length in Cancer Cells. J Biol Chem. 2016 Apr 29; 291(18): 9690–9. doi: 10.1074/jbc.M115.707612.
- Luchini C., Veronese N., Solmi M., Cho H., Kim J.H., Chou A., Gill A.J., Faraj S.F., Chaux A., Netto G.J., Nakayama K., Kyo S., Lee S.Y., Kim D.W., Yousef G.M., Scorilas A., Nelson G.S., Köbel M., Kalloger S.E., Schaeffer D.F., Yan H.B., Liu F., Yokoyama Y., Zhang X., Pang D., Lichner Z., Sergi G., Manzato E., Capelli P., Wood L.D., Scarpa A., Correll C.U. Prognostic role and implications of mutation status of tumor suppressor gene ARID1A in cancer: a systematic review and metaanalysis. Oncotarget. 2015 Nov 17; 6(36): 39088–97. doi: 10.18632/oncotarget.5142.
- Li J., Lu S., Lombardo K., Monahan R., Amin A. ARID1A alteration in aggressive urothelial carcinoma and variants of urothelial carcinoma. Hum Pathol. 2016 Sep; 55: 17–23. doi: 10.1016/j.humpath.2016.04.006.
- Zhou X., Liu N., Zhang J., Ji H., Liu Y., Yang J., Chen Z. Increased expression of EZH2 indicates aggressive potential of urothelial carcinoma of the bladder in a Chinese population. Sci Rep. 2018 Dec; 8(1): 17792. doi: 10.1038/s41598-018-36164-y.
- Kunju L.P., Lee C.T., Montie J., Shah R.B. Utility of cytokeratin 20 and Ki-67 as markers of urothelial dysplasia. Pathol Int. 2005 May; 55(5): 248–54. doi: 10.1111/j.1440-1827.2005.01821.x.
- Lopez-Beltran A., Henriques V., Montironi R., Cimadamore A., Raspollini M.R., Cheng L. Variants and new entities of bladder cancer. Histopathology. 2019 Jan; 74(1): 77–96. doi: 10.1111/his.13752.
- Cao Q., Wang C., Ding Y., Xu D., Qian S., Shen H., Qi J. ARID1A upregulation predicts better survival in patients with urothelial bladder carcinoma. J Int Med Res. 2020 Apr; 48(4): 300060519895687. doi: 10.1177/0300060519895687.
- McKenney J.K., Desai S., Cohen C., Amin M.B. Discriminatory immunohistochemical staining of urothelial carcinoma in situ and nonneoplastic urothelium: an analysis of cytokeratin 20, p53, and CD44 antigens. Am J Surg Pathol. 2001 Aug; 25(8): 1074–8. doi: 10.1097/00000478-200108000-00013.
- Ang P.P., Tan G.C., Karim N., Wong Y.P. Diagnostic Value of the EZH2 Immunomarker in Malignant Effusion Cytology. Acta Cytol. 2020; 64(3): 248–255. doi: 10.1159/000501406.
- Raffone A., Travaglino A., Saccone G., Cieri M., Mascolo M., Mollo A., Insabato L., Zullo F. Diagnostic and prognostic value of ARID1A in endometrial hyperplasia: a novel marker of occult cancer. APMIS. 2019 Sep; 127(9): 597–606. doi: 10.1111/apm.12977.
- Raman J.D., Mongan N.P., Tickoo S.K., Boorjian S.A., Scherr D.S., Gudas L.J. Increased expression of the polycomb group gene, EZH2, in transitional cell carcinoma of the bladder. Clin Cancer Res. 2005 Dec 15; 11(24 Pt 1): 8570–6. doi: 10.1158/1078-0432.CCR-05-1047.
- Aso T., Uozaki H., Morita S., Kumagai A., Watanabe M. Loss of ARID1A, ARID1B, and ARID2 Expression During Progression of Gastric Cancer. Anticancer Res. 2015 Dec; 35(12): 6819–27.
- Alldredge J.K., Eskander R.N. EZH2 inhibition in ARID1A mutated clear cell and endometrioid ovarian and endometrioid endometrial cancers. Gynecol Oncol Res Pract. 2017 Oct 31; 4: 17. doi: 10.1186/s40661-017-0052-y.


