Trial risks, scientific competition and politicization: the thorny way of live vaccine against poliomyelitis in the USSR in 1950-1960s
Автор: Smirnova Vera A.
Журнал: Вестник ВолГУ. Серия: История. Регионоведение. Международные отношения @hfrir-jvolsu
Рубрика: Из истории науки и образования
Статья в выпуске: 2 т.28, 2023 года.
Бесплатный доступ
Introduction. The article is devoted to the problems of testing the live poliomyelitis vaccine that took place in the 1950s in the USSR. The problems of the trials have again become topical because of the pandemic of the novel coronavirus. Methods and materials. Using narrative and historical-comparative methods to analyze materials from the archives of the USSR Ministry of Health Care and the digitized archive of the American virologistSabin, the author of the article looked into some problems related to the trials of the live polio vaccine. Analysis . As a result of the analysis of historical sources, the following problems of trials were found out: high risks of mass use of the live vaccine; lack of agreement between scientists and medical officials on the possibility and necessity of testing; difficulties in relations between an organizer of the tests (M. Chumakov) and some officials from the Ministry of Health Care of the RSFSR; the problems in Soviet-American relations which affected the scientific cooperation; difficult relations between the Soviet organizers of the tests (M. Chumakov and A. Smorodintsev); troubles that arose in relations between A. Sabin and A. Smorodintsev in connection with the politicization of the vaccination issue; ethical challenges of human trials. Results . The USSR took huge risks during the mass trials of the live polio vaccine, which was Soviet science and population significant contribution to the world fight against poliomyelitis. Mass trials took place in the USSR during the Cold War, which, however, did not become an obstacle to scientific cooperation between the USSR and the USA in this sphere. The success of the polio vaccine trials was used by the Soviet state to increase its prestige. At some points, representatives of the Soviet state and media even “forgot” that the vaccine was developed in American virology laboratories, calling Soviet organizers of vaccine trials its developers. Ethical norms of experiments on humans in the 1950-1960s were just being formed, so some of them could be called violations by modern standards. However, the success of the tests closed the question of those violations.
Poliomyelitis, vaccine, smorodintsev, chumakov, sabin
Короткий адрес: https://sciup.org/149142907
IDR: 149142907 | УДК: 433.94 | DOI: 10.15688/jvolsu4.2023.2.16
Текст научной статьи Trial risks, scientific competition and politicization: the thorny way of live vaccine against poliomyelitis in the USSR in 1950-1960s
DOI:
Цитирование. Смирнова В. А. Риски испытаний, научное соперничество и политизация: тернистый путь живой вакцины против полиомиелита в СССР в 1950–1960-х годах // Вестник Волгоградского государственного университета. Серия 4, История. Регионоведение. Международные отношения. – 2023. – Т. 28, № 2. – С. 191–204. – (На англ. яз.). – DOI:
Introduction. The COVID-19 pandemic has become a challenge for humanity facing a difficult task of defeating the virus that has changed life on the planet. The problems of this kind are not entirely new. History of medicine knows victories over viruses that cause serious illnesses. Poliomyelitis is an infectious illness that damages the nervous system. The disease is quite serious: in one out of 200 cases poliovirus causes irreversible paralysis. 5–10% of the paralyzed die due to paralysis of the respiratory muscles. The history of victory over poliovirus is a serious lesson for the modern humankind.
Methods and materials. The vast amount of scientific literature is devoted to the history of poliomyelitis and the fight against it (monographs, theses, articles). We review only part of this enormous body of literature in this work.
Poliomyelitis is perhaps the same age as the humankind. Archaeological sources suggest that the disease probably existed in the prehistoric era. M. Chumakov and co-authors mention that outbreaks of this serious disease were described by Hippocrates. In the modern age, the disease was described in the 18th century. In the early
20th century, epidemics were registered in Sweden, Norway, Germany, and the USA [4, p. 7].
Russia and subsequently the USSR experienced outbreaks of poliomyelitis as well. After the Second World War, the Institute of Neurology of the Academy of Medical Sciences organized expeditions to study the outbreaks in the Baltic republics (1946–47), Eastern Germany (1947–48), and Western Siberia (1949) [4, pp. 89]. In the 1950s, the incidence of poliomyelitis in the USSR began to grow: between 1955 and 1958 it ranged from 17,000 to 22,000 cases. One can see from the graph below that the number of cases increased significantly compared to the first half of the decade.
S. Drozdov and O. Ivanova wrote that in the USA the situation was even graver: in 1950– 1955 annually from 28,500 to 57,200 people a year fell ill with polio. There were up to 200,000 people with post-poliomyelitis disability in the country in 1956 [6, p. 82]. It is not surprising that it was the United States, with their highly developed virology, managed to develop effective vaccines against polio. V. Lashkevich mentions that large-scale use of J. Salk’s inactivated polio vaccine (IPV) began in the USA in 1954. IPV consists of strains of polioviruses of different types which were killed (inactivated) by formalin. In response to vaccination, the human body produces antibodies that in case of infection prevent the spread of the virus to the central nervous system and protect against the development of paralysis. In 1956, the USSR Academy of Medical Sciences institutes (Institute of Experimental Medicine and established in 1955 Institute for the Study of Poliomyelitis) organized the production of IPV [10, p. 5; 36, pp. 3, 4]. The vaccine was quite effective, it significantly reduced the risk of paralysis and death, however, it did not guarantee, that vaccinated people would not get sick. Furthermore, the vaccine was expensive, it required the slaughter of many monkeys (to grow viruses for vaccine, monkey kidney tissue cultures were used); and the protection of the vaccine had been weakening over time.
By 1954, an American virologist A. Sabin had developed a more effective live oral polio vaccine (OPV), which practically guaranteed protection against polio and was much cheaper. OPV consists of live but attenuated strains of poliovirus that build local immunity in the intestinal tract and protect the body more effectively. A. Sabin tested it on a small number of people in the USA, but he failed to obtain permission for mass trials of OPV in the United States. Reasons for rejection of Sabin’s application for trials included satisfactory results of IPV use, the problem of assessing OPV protection in population vaccinated with IPV in the United States, as well as fears of live virus reversion (changes from attenuated strains to dangerous, disease-causing forms). Sabin found foreign partners who organized the trials abroad. The Soviet virologists were among those partners.
The successful project of Soviet-American cooperation was implemented in the following political circumstances. After Stalin’s death in 1953, the USSR and the West made an attempt to reset the relations damaged by the Cold War. In particular, in 1955, the leaders of Western states and the Soviet leader N. Khrushchev met in Geneva. In particular, opportunities for cooperation in the field of vaccinology emerged. It was especially relevant because there was a sharp increase in the number of cases of poliomyelitis in 1955 in the USSR, as one can see in Figure. The Soviet state sent Soviet scientists abroad, where the fight against poliomyelitis was to certain degree successful. In February and March in 1956, Soviet virologists A. Smorodintsev, M. Chumakov, M. Voroshilova visited the leading US research centers involved in the study of poliomyelitis. During the trip, they met
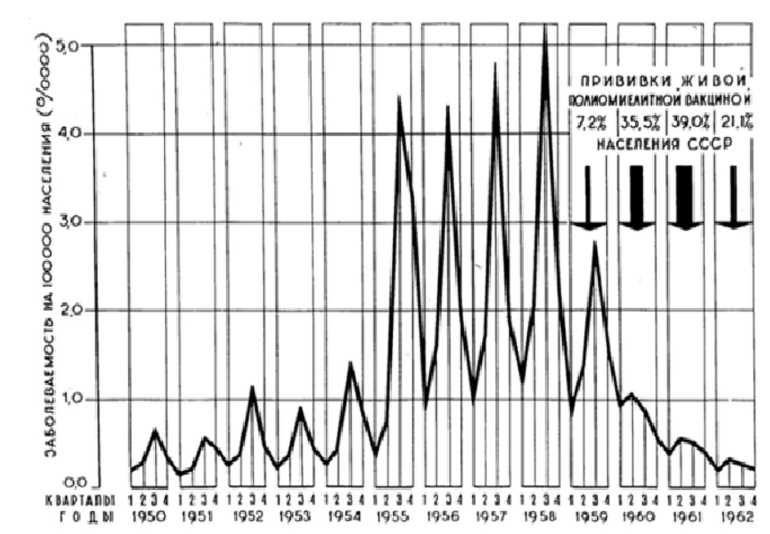
The number of cases per 100,000 people in the USSR in 1950–1962
Source : [6, p. 82].
A. Sabin [36, p. 43], and this acquaintance predetermined the subsequent vaccine trials in the USSR. Successful trials of OPV in the USSR and several other countries predetermined its further success around the world, as a result of which entire continents were declared free from poliomyelitis. Trials organized by A. Smorodintsev and M. Chumakov in the USSR became a huge contribution to the fight against poliomyelitis on the planet. Textbooks and documentaries tell us this story often painting a perfect picture of the trials. This quite understandable glorification and simplification though played its role during coronavirus vaccine trial campaign. The opponents of coronavirus vaccine trial (with its cut corners) argued that in the USSR, the trials were organized perfectly, were carried out without haste and didn’t involve any risks. It seemed interesting to find out whether everything really went so smoothly.
Smorodintsev and others wrote about the general course of polio vaccine trials in the USSR, whereas other authors analyzed the situation with polio in individual republics: for example, V. Boyko described the situation with polio incidence in Uzbekistan before and after OPV vaccination [3], V. Evdoshenko analyzed the effectiveness of OPV using the example of the Kyrgyz SSR [8]. S. Drozdov notes that OPV was successfully used in developing countries, in particular, noting the importance of Soviet assistance in supplying OPV to Ghana, Niger and Cambodia in 1962 [7].
D. Vargha studied the history of the OPV trials in Eastern Europe in the context of international politics, noting the impact of the confrontation between the blocs on the willingness of the respective states to invest resources in the development of science. Vargha pointed out the photographs of patients’ bodies damaged by polio contradicted the idea of a communist paradise, and therefore, despite the relatively small numbers of morbidity and mortality, it was polio exactly that attracted so much attention. Prevention was one of the cornerstones of medicine in the socialist countries, and vaccination against infectious diseases was an ideal area for demonstrating the power of the socialist state. Vargha noted that the successful eradication of polio in socialist countries raised uncomfortable questions about the positive side of communist regimes – their ability to effectively control epidemics [33].
M. Hortsmann described the West’s distrust towards the results of Soviet trials of OPV. She wrote how the World Health Organization sent its representative to assess the quality of trials, and that only after this mission the United States recognized the effectiveness of OPV [9].
The purpose of this article is to add new data to information known to us from the academic literature about OPV trials via the use of new sources. The article is focused upon the following tasks: to identify the problems that vaccine trial organizers had to face; to establish mechanisms they used to overcome the obstacles; the risks taken by the trial organizers and administrators of the Soviet health care system; to find out who exactly was the pioneer of OPV trials in the USSR; to assess the impact of competition between scholars on the course of research as well as its role in subsequent historical representations of scientific achievements; to reveal the role of the experience of previous interaction between state bodies and scholars on subsequent trust and relations; to identify the ethical challenges of OPV trials; to establish how the politicization of the issue of vaccine trials affected the context of international cooperation in the field of vaccinology.
To achieve the goal and to perform the tasks we studied the sources: materials of fund 8009 (USSR Ministry of Health) of the State Archive of the Russian Federation, the digitized archive of the American virologist A. Sabin on the website of the University of Cincinnati [31], as well as personal records – the memories by employees and relatives about Mikhail Chumakov [35].
Analysis. Cooperation between US and USSR virologists. Cooperation between Russian and American virologists on the polio vaccine began during the Khrushchev thaw. All the contacts were coordinated at the high political level. In January 1956, A. Sabin received a letter from the US Department of Health, Education and Social Security about the upcoming visit of Soviet virologists in February 1956. The purpose of the visit was to study the treatment of poliomyelitis in the United States and the technology IPV production [17]. M. Chumakov, his wife M. Voroshilova, A. Smorodintsev visited both A. Sabin’s laboratory and his house. During that visit Chumakov and Smorodintsev established close ties with Sabin.
It is interesting that these were not only Soviet virologists who had to report in detail to the state authorities about this Soviet scholars’ visit to the USA. Although McCarthyism had been done within the USA by the mid-1950s, the state control of cooperation with the USSR in the scientific field was quite tight: one can find in Sabin’s correspondence references to detailed reports on conversations with the Soviet scholars for the Department of the Army [12]; reports on Soviet virology laboratories for the US State Department after a trip to the USSR in summer of 1956 [18]; as well to the need to get authorization from the US Department of Defense for the transfer of attenuated polio strains to M. Chumakov [11].
These were hard times for Soviet-American relations, but not for personal relations between Soviet and American virologists: the letters by which M. Chumakov and A. Sabin exchanged were incredibly warm; these were the conversations of people among whom not only scientific, but also emotional ties developed. Probably, it was exactly the fact that scientists were representatives of conflicting superpowers very different in cultural, economic and political respect that gave their relationship a very special character: each of them wanted to show the best in himself both as a scientist and as a person.
Interestingly, there was no special focus on live polio vaccine in the correspondence between A. Sabin and M. Chumakov in the first half of 1956. A. Sabin himself tested it on several dozens of prisoners sometime later – in the early 1957. But in the USSR, this direction of A. Sabin’s research was closely observed from the very beginning. In January 1957, N.I. Grashchenkov, a member of the board of the USSR Ministry of Health, wrote to Sabin that Chumakov was interested in the results of trials planned for the early 1957. On March 15, 1957, A. Sabin informed M. Chumakov about the success of polio vaccine trials on 100 adult volunteers, as well as the plans to give the vaccine to his daughters, wife, neighbors and their children [19].
The pioneer of OPV in the USSR: Chumakov or Smorodintsev? Authors of works on the history of polio vaccine trials in the USSR usually evade what looks like the complicated relationship between Mikhail Chumakov, director of Moscow Institute for the Study of Poliomyelitis, and his teacher [34], Anatoly Smorodintsev, head of virology department, Leningrad Institute of Experimental Medicine. Meanwhile, the consequences of this complex relationship are still felt. While the website of St. Petersburg Smorodintsev Research Institute of Influenza [1] mentions Chumakov in the context of organizing OPV trials, the website of the Moscow Chumakov Institute of Poliomyelitis doesn’t mention Smorodintsev’s name in the section “history” [24] at all. One has to remember that it was precisely the meeting between M. Chumakov, A. Smorodintsev and A. Sabin in the USA that became the starting point for Soviet-American cooperation in OPV trials, no matter how complicated the relation between A. Sabin and A. Smorodintsev became later.
A. Smorodintsev, in his work published in 1960, mentions, in fact, his priority in promoting the idea of the OPV trials in the USSR, arguing that until January 1959, the Department of Virology of the Institute of Experimental Medicine of the USSR Academy of Medical Sciences was the only scientific team in the USSR involved in the OPV trials. According to Smorodintsev, it was only in late 1958 that Chumakov began to actively pursue the idea of the OPV trials by his institute [36, pp. 44-46].
Archival materials confirm Smorodintsev’s priority in terms of obtaining permission to test OPV. In March 1957, the Minister of Health of the USSR M. Kovrigina received a letter from the Academy of Medical Sciences with a request to allow A. Smorodintsev to test the OPV obtained from A. Sabin [25, l. 1]. In April 1957, A. Smorodintsev obtained the permission to conduct trials in closed institutions in Leningrad [25, l. 4]. Thus, it was exactly A. Smorodintsev who actually pioneered OPV trials in the USSR.
M. Chumakov also demonstrated interest in OPV. According to American archival material, he was thinking about the trials at least since April 1957 (when Smorodintsev already had the permission for them), as Sabin mentioned in a letter to one of his respondents [21]. By the autumn of 1957, the interest had transformed into intention: in November 1957, M. Chumakov wrote to A. Sabin that his institute was preparing to organize OPV trials [13]. However, M. Chumakov’s institute was the enterprise that produced IPV which took a lot of effort and resources, therefore, for some time it couldn’t go further than interest and preparation for trials. So, Chumakov’s Institute started OPV trials later than Smorodintsev’s group. Moreover, the relations between Chumakov and Ministry of Health of the RSFR deteriorated due to problems with IPV production in 1958 (we’ll cover this story later in this article) which to some degree narrowed down the options for Chumakov.
After small-scale successful initial trials organized by A. Smorodintsev, larger trials started. Applications for trial permissions were submitted by scientific institutions Chumakov and Smorodintsev in parallel. Interestingly, M. Chumakov always requested permission to recruit more trial participants than A. Smorodintsev. A. Smorodintsev was apparently more cautious and less inclined to take risks. For example, in August 1958, Deputy Minister of Health V.M. Zhdanov received applications for permission to test OPV on 20,000 people from A. Smorodintsev and 100,000 people from M. Chumakov [25, l. 22].
The institutes headed by M. Chumakov and A. Smorodintsev worked in different regions of the USSR, in fact, dividing the Soviet republics into spheres of influence of their research centers: for example, in 1958, the Chumakov’s group worked in Estonia and Lithuania, whereas Smorodintsev’s team conducted trials in Latvia [25, l. 117-119]. In February 1959, Chumakov organized a vaccination campaign in the Uzbek and Kazakh SSR; while Smorodintsev was ordered to carry out trials in the Moldavian and Belorussian SSR in March 1959 [25, l. 118, 122]. Receiving state assignments to carry out OPV trials in certain regions meant getting more power and resources, as well as an increase in the influence of the relevant research institutes and their leadership.
Risk assessment of OPV use. Just like nowadays, vaccine trials were not entirely free from risk and uncertainty. These risks were well recognized and discussed at the meeting of the Poliomyelitis Vaccination Committee of the USSR Ministry of Health in August 1958. The virologist Professor N.A. Zeitlenok, who supported the OPV trials, in general, spoke about the need to make sure that the reversion of the virus and its uncontrolled spread is ruled out. Reversion means that the weakened virus becomes capable of causing serious disease [25, l. 24]. To reduce those risks, initial trials were conducted in closed facilities (orphanages) [25, l. 3, 6].
Another category of medical-biological professionals that traditionally draws attention to risks of vaccine trials was physicians. Neurologist, Professor M.B. Zucker pointed to the risks of immunization in cities without sewerage. Neurologist, Professor D.S. Futer called for caution and careful monitoring, pointing out that medical authorities in the United States are hesitant to conduct OPV trials in their country [25, l. 24]. Deputy Minister of Health V.M. Zhdanov replied that IPV was more expensive than OPV, which was allegedly unprofitable for American pharmaceutical companies and supposedly was an obstacle to trials in the USA [25, l. 24]. The same reason was later used by Soviet propagandists who criticized the United States for refusing to conduct mass OPV trials.
Discussion participants present at the meeting of Poliomyelitis Vaccination Committee of the USSR Ministry of Health in 1958 understood that OPV use could also have unpredictable consequences. So, somebody must be responsible for all bad things that could happen as a result of the trials. A. Smorodintsev and M. Chumakov took personal responsibility for the risks of trials [25, l. 25-27]. Even in a country that had just denounced Stalin’s cult of personality and his methods (at the 20th Communist Party Congress in 1956), this was an enormous responsibility, a very brave decision that could cost them not only their scientific careers, but also their freedom.
The burden of this responsibility was so huge that not all trial organizers were strong and persuaded enough to carry it and to take risks. In October 1958, O.V. Baroyan, the head of Epidemiology Department of Research Institute of Virology (Academy of Medical Sciences), assigned to participate in the trials, wrote a letter to the USSR Ministry of Health. In this letter he mentioned the risk of reversion and proposed to reduce the number of trial participants to 20,000, as well as offered to conduct trials only in cities with weakened contacts with the rest of the USSR – Prokopievsk and Anzhero-Sudzhensk [25, l. 90]. Anxiety about the unpredictability of trial consequences and the desire to secure his position under conditions of uncertainty and risk were probably behind this letter by O.V. Baroyan. This was definitely the evidence of fear that things could seriously go wrong.
Much less risk-averse M. Chumakov, on the contrary, defended the trials with confidence. In his letter to A. Sabin, M. Chumakov called the opponents of OPV use ‘cowards and pseudospecialists’, and expressed regret that OPV trials were postponed because of the interference of his opponents [15]. Only by the end of 1958 Chumakov succeeded – on December 19, 1958, he informed A. Sabin by telegram that he had received all permits and began mass OPV trials [14].
When the mass trials had shown the safety of the vaccine by the summer of 1959, the public health authorities faced one more risky decision – whether to terminate IPV production replacing it by OPV or to continue producing IPV until OPV use would reveal its consequences. Less risk-averse M. Chumakov believed that the production of IPV could be stopped and vacated production facilities could be used to manufacture OPV. Unlike M. Chumakov, O.G. Andzhaparidze, a virologist from the Research Institute of Poliomyelitis Drugs (a competitor of Chumakov’s institute) believed it was necessary to suspend mass vaccinations with OPV and to study the results of OPV use without terminating IPV manufacturing [25, l. 210]. Both options were risky. The decision taken on this issue minimized the risks: on the one hand, it was decided to focus on the production of OPV; on the other hand, they decided not to abandon the production of IPV completely. The Poliomyelitis Vaccination Committee decided that Chumakov’s institute should produce 80 million doses of OPV, whereas its competitors, Research Institute of Poliomyelitis Drugs, must manufacture 10 million doses of OPV and 20 liters of IPV [25, l. 215].
OPV trials organizers took one more risk – they had started mass vaccination before the trials were over. Instead of vaccinating 100,000 people according to the plan [25, l. 82], during the first 4 months more than 500, 000 people got the vaccine in Estonia, Lithuania and Kazakhstan [25, l. 129]. More than 2,000,000 got OPV in the Baltic republics, Ukraine, Belarus, Moldova, Kazakhstan and Uzbekistan by May 1959 [25, l. 165, 168].
Interestingly, during the discussions of the OPV trials virologists and pediatricians did not raise an issue of long-term health consequences of using an ‘untested’ vaccine; we failed to see in the archival documents any evidence of society’s resistance to the idea of vaccination, despite the fact that it was about the health of children – one of the most precious categories of Soviet citizens. This lack of resistance was perhaps the result of the successful fight against infectious diseases through vaccination in the first half of the 20th century. Advances in medicine, trust in American science, successful initial small-scale trials were the reasons why the scientists and health care organizers were ready to take risks associated with OPV trials.
Mikhail Chumakov and the Ministry of Health of the RSFSR: from conflict to distrust. There was though no unity in the system of health care about OPV trial risks. In particular, the idea of OPV trials was not favorably received by Russian officials from the Ministry of Health. On December 22, 1958, minister of health of the RSFSR S. Kurashov wrote a letter to the Minister of Health of the USSR M. Kovrigina with a message that he did not consider it possible to conduct OPV trials before testing OPV for safety [25, l. 109]. It had past half a year since the successful small-scale trial in Leningrad with participation of 1,200 children. Why did the Russian minister raise a question of OPV safety? Was he concerned with quality of vaccine produced by Chumakov’s institute? Was this violation of subordination principle the result of expectation that the days of M. Kovrigina in office were numbered (her replacement by S. Kurashov would follow in the first half of 1959)? Did any problems emerge during the trials?
On January 2, 1959, M. Chumakov wrote a letter to M. Kovrigina complaining that S. Kurashov instructed the head of the Moscow City Health Department, N.S. Lapchenko, to withdraw his consent to vaccinations in Moscow [25, l. 112].
Almost a year later, in October 1959, problems in relations between vaccine trial organizers and the RSFSR Ministry of Health remain unresolved: M. Chumakov and A. Smorodintsev wrote a letter to S. Kurashov, who by then had become the Minister of Health of the USSR. In this letter they complained, that the head of the Main Sanitary and Epidemiological Directorate Titkov had sent to Sanitary and epidemiological stations of the RSFSR a letter forbidding OPV use on the grounds that OPV trials had not yet been completed. Titkov’s letter, according to Chumakov, led to rumors about alleged failure of OPV trials [25, l. 191].
What is the reason for RSFSR officials’ distrust of M. Chumakov and his institute? There is a probability that this distrust is the result of the previous experience of interaction between M. Chumakov and the Ministry of Health of the RSFSR regarding the production of IPV by his institute.
At the beginning of 1958, M. Chumakov had a conflict with S.I. Didenko, the director of the State Control Institute of Vaccines and Serums (SCI), the body that controlled the quality of vaccines produced in the USSR. In his letter on January 13, 1958, Didenko wrote to M. Chumakov that his institute used the wrong type of filters -different from the one mentioned in the instructions on producing IPV [26, p. 5].
In particular, the institute had a serious issue with batch 5 of IPV. Although an expert of SCI had doubts about the vaccine quality, in January 1958 IPV was sent to the Moscow Regional Sanitary and Epidemiological Station for vaccination. During the additional test at SCI, a live poliovirus was found in it (the virus in IPV must be inactivated, i.e. killed; otherwise, the vaccine can cause disease). According to SCI, when monkeys and mice got the 5th batch of IPV, they developed paralytic poliomyelitis. Employees of the control institute wrote a letter to the Ministry of Health with a request to stop the use of the corresponding batch of IPV [26, p. 23, 24].
Later, in February 1958, a special commission of the USSR Ministry of Health studied the work of Institute for the Study of Poliomyelitis and found out the problems and breaches in vaccine manufacturing at the institute. Infectious material was transported across all Moscow – between the two bases of the institute (Vnukovo and Sokolinaya Gora), there was no constant supply of electricity and water to the Vnukovo base of the institute. Medical utensils were washed with cold water, distilled water was stored in the room where ‘the infectious material was killed’ [26, pp. 69-74]. Taking into consideration the poor conditions for the vaccines production at Chumakov’s institute, the commission members recommended shutting down the production facilities at the Institute for the Poliomyelitis Study of the USSR Academy of Medical Sciences and merging it with Poliomyelitis Drugs Research Institute of the Ministry of Health of the RSFSR [26, p. 76].
It is possible that this story of breaches of protocol in the course of IPV production was an example of the usual interdepartmental struggle for influence between the USSR Academy of Medical Sciences and Ministry of Health of the RSFSR, to which the corresponding institute belonged. Probably, someone wanted to gain control over Chumakov’s institute, taking into account that the expensive imported equipment was installed in it [26, p. 72].
The evidence in favor of an interdepartmental competition scenario is the fact that Chumakov began his response to the commission’s conclusion precisely by comparing the work of his institute and its competitor. He tried to prove that the competitors, who worked inefficiently, were producing less vaccine than they could, given the available resources (a large number of employees, monkeys, imported reagents [26, p. 43]). In the second part of the letter, he claimed that his institute’s vaccine was safe and that the controlling institute had made a gross mistake [26, p. 49].
In the end, M. Chumakov managed to win the fight for his institute and for IPV batch rejected by SCI. The following arguments were used in order to support the renewal of the vaccine use: repeated checks did not reveal any problems, Chumakov’s own children were vaccinated with IPV from the batch in question, rumors reached the government that a good drug was not used for vaccination [26, pp. 111-116]. Ministry of health of RSFSR had to get the batches of IPV in April [26, p. 83], but the relationship between M. Chumakov and this ministry remained complicated, which affected the subsequent OPV trial and led to the ministry’s distrust in Chumakov.
Trials and Politics. Just like now, there were attempts to use the vaccine trials for political purposes. In the 1950s, cold war narratives predetermined Soviet media’s construction of ‘rotting capitalism’ style messages in presenting information on vaccination abroad; in their turn, some western scholars and state organizations did not trust the Soviet scientists and the results of their trials [35, pp. 63, 64].
In the USSR by the summer of 1959, there had been several million Soviet people who got OPV, its success became obvious at least in terms of safety and the ability to produce antibodies as a result of vaccination. Soviet propaganda could not miss the chance to take advantage of this success of Soviet science and public health organizers. On June 17, 1959, Izvestia published an article by E. Arenin “Blow on a Dangerous Virus”, which stated that the vested interests of American companies and doctors, as well as “wolf laws of capitalism” prevented OPV trials in the United States [26, p. 189].
The American media reprinted this article, putting A. Sabin in extremely unpleasant position from the perspective of American public [30]. Americans might think that he was criticizing the USA in conversations with his Soviet colleagues.
Nobody, of course, can guarantee that he didn’t. As for his public statements, A. Sabin mentioned the following reason as preventing vaccine trials in the United States: the virus in the vaccinated stool was more virulent than the virus in the vaccine; extensive tests were needed in order to understand how dangerous it was for the vaccinated and those around them. That was difficult to do under conditions of widespread and successful use of IPV in the United States [29, pp. 422, 423]. Outside the US, the considerably lower share of the population was covered by IPV vaccines and therefore trials would be more valid there.
The American public might think that it was A. Sabin who talked about “wolf capitalism” in conversations with Soviet virologists. Taking into account Sabin’s origin (he was born in the Russian Empire) and the continuing cold war (the USSR had just tested intercontinental ballistic missiles), Sabin’s position was difficult. One more unpleasant fact was that the reader of Arenin’s article could think that the vaccine was created not by an American virologist A.Sabin, but rather by the Soviet scholar A. Smorodintsev.
A. Sabin immediately sent telegrams to M. Chumakov and A. Smorodintsev with a request to make a public statement correcting this article [25, p. 189]. On June 21, Chumakov responded with a telegram in which he supported A. Sabin, mentioned that he had made a statement to the media and expressed hope that it would be published along with Smorodintsev’s statement [5]. The Soviet delegation at the WHO session made a public apology on this occasion [35, pp. 63, 64].
Perhaps it is this story to which Chumakov’s letter to A. Sabin referred: ‘Smorodintsev, they say, got his lumps from our presidium of the Academy of Medical Sciences for his dishonest behavior towards you. And probably you have already received some sort of apology’ [32].
Unfortunately, this story was not the last case when A. Sabin’s work on the vaccine was ignored. Academy member B. Petrovsky published a brochure ‘Health care in the USSR’ in 1967 [28]. He proudly stated in it that Soviet Biology was at a high level, scientists had the necessary equipment, that the USSR almost completely eradicated poliomyelitis using OPV made according to the method developed by
M. Chumakov and A. Smorodintsev. Sabin’s name was not mentioned at all, which was, of course, serious violation of academic ethics. A. Sabin addressed the USSR Academy of Medical Sciences about this. The President of the USSR Academy of Medical Sciences V.D. Timakov gave some illogical reply to A. Sabin, stating that the problem was the result of an inaccurate translation (the brochure was published in English). He also argued that Sabin’s name was not mentioned because the book was about the health care of the USSR [22].
States use scientific achievements of their citizens to enhance state prestige. Apparently, publishing the brochure, the Soviet scholars hoped that incorrect information would not be noticed by the American side. This violation of scientific ethics was unnecessary. The USSR’s contribution to the fight against poliomyelitis was huge: one might name the audacity of trial organizers, participation of Soviet citizens in the trials (in the first year alone, 15 million people received the vaccine), the resources that the state invested in the trials. But open recognition of these contributions by Soviet propaganda would lead to unwanted image of Soviet citizens used as Guinea pigs in risky experiments and conspiracy theories. The image of Soviet scientists, well supported by the Soviet state, who managed to advance vaccinology was much less challenging in terms of unwanted associations.
Ethical issues of OPV trials and vaccine production. While ethical issues of using primates in vaccine production and OPV safety testing are currently on the agenda, in the late 1950s the need to exterminate vast numbers of monkeys was more of a commercial than bioethical issue. Justifying the necessity of switching from IPV to OPV, OPV proponents pointed out that the kidneys of one monkey were needed in order to produce 1,000 doses of IPV, while OPV production required significantly fewer monkeys [25, l. 11]. OPV proponents stated that three doses of OPV cost 10 kopecks, while the price of 3 doses of the IPV vaccine was 4 rubles [25, l. 137].
Monkeys died not only due to slaughtering for vaccine production. The authors of memoirs about M. Chumakov and his institute wrote with sympathy that only a third of the purchased monkeys survived the journey from China by railway. The climate of Moscow region did not suit the monkeys; they died from pneumonia and diseases of the digestive system [35, pp. 154-156].
Over time, scientists found ways to reduce the number of monkeys slaughtered for the production and testing of the vaccine. Scientists began using Vero cells: cells that replicated many times.
One more ethical issue of OPV trials is the selection of vulnerable people. In the 1950s, there were apparently no bans on using orphans in trials. Al least, A. Smorodintsev was able to obtain permission to OPV on babies in closed institutions [25, l. 1, 4].
Apparently, in the 1950s the ethical issues of drug trials on vulnerable people were not studied well enough by ethicists. The organizers of OPV studies, for instance, had the idea to conduct trials on people with intellectual disability. In March 1957, the President of the Academy of Medical Sciences A. Bakulev sent a letter to the Minister of Health of the USSR with a request to provide “500–1000 healthy” people with intellectual disabilities for tests conducted by the Institute of Experimental Medicine in Leningrad [25, l. 1]. However, subsequently, A. Smorodintsev, the organizer of the OPV trials in Leningrad, said that there was no point in conducting tests on adults: the intestines of an adult are less susceptible to polio than those of a child and the results would not be reliable because of “neurological status” of people with intellectual disability [25, l. 2]. We don’t know for sure whether the tests were eventually conducted on people with mental disability.
It looks like in the West in the 1950s ethical problems of medicine did not have solutions supported by everyone. For example, OPV was originally tested on 30 volunteer prisoners in the United States at Chillicothe Prison [23], which is also contrary to modern regulations about trials on vulnerable people. It is interesting that they were paid a fee for volunteering and the term of their imprisonment was slightly reduced in return for their help to science [20].
During coronavirus vaccine trials, Association of Clinical Research Organizations expressed outrage [27] that the head of the Gamaleya Center (the organization that had developed Sputnik V vaccine) tested the vaccine on himself, his granddaughter and employees of his research institute. The history of polio vaccine trials knows similar examples: A. Smorodintsev’s granddaughter, M. Chumakov’s children, employees of the corresponding research institutes were vaccinated with OPV before or during the early period of the trials. A. Sabin tested OPV on himself and his family members. Now testing medications on institute employees is called into question, since they are not totally independent people.
Results. Soviet OPV trial organizers worked under conditions of the Cold War, that gave a special character to their cooperation with foreign colleagues. The confrontation between states can both prevent scientific contacts and, paradoxically, become a showcase of cooperation, whereas everything else beyond which is a competition or rivalry.
Scientific cooperation project related to polio vaccine trials became a successful field of Soviet-American relations. Several stakeholders were interested in this project. For both states, this cooperation became an opportunity to improve their image for external and internal audiences, as well as an example demonstrating that collaboration with the “main adversary” was possible and fruitful in principle. This project was important for citizens of both countries because it saved their lives and health; finally, the scientists themselves received experience of interaction with foreign colleagues, established personal contacts, got new knowledge, technologies, and an incentive to continue research. Cooperation in times of political confrontation made scientists to obtain approval from state organizations on various aspects of international cooperation, as well as invest additional emotional resources and determination in cooperation, separate themselves from states participating in the conflict, work to the limit of their abilities, actualize the best in themselves both as scientists and people. The nature of the problems resolved (thousands of children who could be saved from death and disability) must also have made the Soviet and American researchers and their relationship emotional.
The success of OPV trials was preceded by troubles with obtaining permission for mass trials within the USSR. Chumakov’s determination, his ability to establish connections and convince those in power, good knowledge of principles according to which state machine operates – all these factors made the success possible.
Stories with attributing the authorship of OPV first to A. Smorodintsev (Soviet newspaper), and then to A. Smorodintsev and M. Chumakov (brochure by Academy member B. Petrovsky) cast a shadow on relations between Soviet and American virologists. It is quite possible that in the case of the newspaper article, it was not even A. Smorodintsev’s desire to attribute the authorship of OPV to himself, but rather the bias of Soviet media. Soviet media tasks included promotion of Soviet scientists’ scientific achievements, which is why the issue of OPV trials was so much politicized. Soviet media promoted the narrative of the state’s concern for the material and technical provision of science that predetermined success of Soviet science, which meant the effectiveness of the socialist system. The journalist Arenin compared Soviet readiness for trials with an allegedly different attitude to the issue in the USA, attributing it to American “wolf laws of capitalism.” The latter supposedly prevented trials. Unlike a journalist who could get confused about the authorship (which sometimes happens to media representatives), the author of the brochure on Soviet medicine, Academy member B. Petrovsky, knew exactly who had actually developed OPV tested in the USSR. In Petrovsky’s work, the name of A. Sabin was not mentioned at all, which gave readers the impression that OPV had been developed by the Soviet virologists M. Chumakov and A. Smorodintsev. Unethical attitude to the issue of OPV authorship undermined trust in Soviet science and organizers of health care in the USSR, making conditions for future collaborations less favorable.
It seemed to us important to pay tribute to A. Smorodintsev. Despite the scandal with OPV authorship, A. Smorodintsev was indeed the first in the USSR to obtain permits for the trial of Sabin’s OPV and conducted it in Leningrad. Later, his relationship with A. Sabin became complicated because of an article in the media that attributed the authorship of the vaccine to the Soviet virologist. His role in this story remains unclear: whether he really told the journalist that he had developed the vaccine or he was used in propaganda against his will. After the first trial in Leningrad, A. Smorodintsev and M. Chumakov, organized mass OPV trials in the republics of the USSR, for which both subsequently received the Lenin Prize. Paying tribute to Mikhail Chumakov, we must point at his determination, charisma, outstanding communication and organizational skills, despite disability (his hearing was impaired). He was very good at concentrating material resources under his control, which allowed his institute to become a major manufacturer of vaccines. Looks like Chumakov’s personal charisma and the scale of his personality created a larger place for Chumakov than for Smorodintsev in history of medicine. More research into Smorodintsev’s work is needed to assess the relevance of the narratives constructed by now.
Paradoxically, even what looks like Chumakov’s complicated relationship with A. Smorodintsev actually created an incentive for more intensive work. Their institutes competed in terms of receiving state orders to arrange OPV trials in certain USSR republics. Ultimately, this competition increased the speed of immunization of Soviet children, as well as the speed with which the world learned about the effectiveness and safety of OPV.
M. Chumakov faced a lot of issues while organizing the momentous trials, one of the issues was the burden of previous experience of relations between his institute and health ministry of the RSFSR. Because of the story with compromised IPV vaccine from the Institute for the Study of Poliomyelitis, the officials of RSFSR Ministry of Health for a certain period refused to give the green light to OPV trials in the territory of the RSFSR, apparently being unsure about the quality of OPV produced by Chumakov’s research institute. This story demonstrates the extreme importance of prior experience for the implementation of projects in health care. The issue of children’s health was so sensitive that RSFSR officials even challenged the hierarchy of the state, refusing to obey orders from the top.
The narrative of the OPV trials presented in the media and textbooks is a simplified and glamorized version of reality. Now, in the era of the coronavirus, some authors want to present Soviet vaccine trials as something 100% safe. Meanwhile, virologists of the past faced almost the same challenges as vaccine trial organizers face now. Virologists of the past also operated under conditions of uncertainty and enormous risks. The risks included using a vaccine capable of immunizing the environment of the vaccinated. Reversion also was on the agenda, meaning that a weakened live poliovirus could, as a result of a transformation, become dangerous to the health of vaccinated people and those in contact with them. Mass vaccination with OPV before trials was completed was another risk. The long-term consequences of OPV for the health of Soviet population were unpredictable as well. The USSR took all these risks. This approach was a success.
We failed to find any discussions concerning ethical aspects of vaccine research in the archival documents we studied. The scientists considered their duty to save the lives and health of people around the globe from polio; for the researchers courage and determination rather than caution were the expressions of benevolence. This approach, though far from ideal ethically (at first, trials were carried out on vulnerable people – orphans in the USSR and prisoners in the USA, children and grandchildren of virologists), worked to achieve the goal of eradicating poliomyelitis. The USA (since 1979), Russia (since 2002), the African continent (since 2020) have the status of polio-free territories. However, the cases of vaccine-associated poliomyelitis became the price of this victory.
Of course, history of OPV trials was the success story for American and Soviet virologists and the corresponding states. Nevertheless, while constructing historical memory of these events, it is important to talk about real problems faced by the trial organizers. This search for the complex picture is important for both science and practice: if the picture of the past is too smooth, it will be more difficult for modern society to accept the fact that vaccine trials are impossible without risks and herd immunity comes with a price.
Список литературы Trial risks, scientific competition and politicization: the thorny way of live vaccine against poliomyelitis in the USSR in 1950-1960s
- Istoriya sozdaniya [Creation History]. Sayt NIIgrippa imeni A.A. Smorodintseva [Website of the Smorodintsev Research Institute of Influenza]. URL: https://www.influenza.spb.ru/institute/history/
- Clements C.J., ed. Supplementary Information on Vaccine Safety. Part 2: Background Rates of Adverse Events Following Immunization. Geneva, WHO, 2000. 112 p. URL: https://apps.who.int/iris/ bitstream/handle/10665/66675/WHO_V-B_00.3 6_ eng.pdf? sequence=1&isAllowed=y
- Boiko V.M. Epidemiologija i puti likvidacii poliomielita v Uzbekskoj SSR [Epidemiology and Paths to Eradication of Poliomyelitis in the Uzbek SSR]. Tashkent, Medicine Publ., 1965. 118 p.
- Chumakov M.P., Prisman I.M., Zacepin T.S. Poliomijelit. Detskij spinno-mozgovoj paralich [Children's Spinal Paralysis]. Moscow, Medgiz Publ., 1953. 480 p.
- Chumakov's Telegram to Sabin A. 1959.06.20. Sabin Collection Fair Use Policy. URL: https:// drc.libraries.uc.edu/bitstream/handle/2374.UC/669967/ sovietunion_1959-68_021.pdf?sequence= 1
- Drozdov S.G., Ivanova O.E. Poliomielit [Poliomyelitis]. Problemy Virusologii [Problems of Virology], 2012, no. 1, p. 82. URL: https://cyberleninka.ru /article/n/poliomielit
- Drozdov S.G. Poliomielit i egoprofilaktika v razlichnyh stranah mira [Poliomyelitis and Its Prevention in Various Countries of the World]. Moscow, Medicine Publ., 1967. 87 p.
- Evdoshenko V.G. Problema likvidacii zabolevaemosti poliomielitom v Kirgizskoj SSR: avtoref. dis. ... d-ra med. nauk [The Problem of Eradication of Poliomyelitis in Kyrgiz SSR. Dr. med. sci. abs. diss.]. Moscow, 1968. 39 p.
- Horstmann D.M. The Sabin Live Poliovirus Vaccination Trials in the USSR, 1959. Yale Journal of Biology and Medicine, 1991, Sept.-Oct., no. 64 (5), pp. 499-512. URL: https://www.ncbi.nlm.nih.gov/pmc/ articles/PMC2589490/?page= 1
- Lashkevich V.A. Istorija sozdanija v 1959 g. zhivoj vakciny iz attenuirovannyh shtammov A. Seibina i ideja iskorenenija poliomielita [The History of the Creation in 1959 of a Live Vaccine from Attenuated Strains of A. Sabin and the Idea of Eradicating Poliomyelitis]. Voprosy virusologii [Problems of Virology], 2013, no. 1, vol. 58, p. 5. URL: https ://cyberleninka. ru/article/n/istoriya-sozdaniya-v-1959-g-zhivoy-vaktsiny-iz-attenuirovannyh-shtammov-a-sebina-i-ideya-iskoreneniya-poliomielita/ viewer
- Letter from Babione R.W. to Sabin Albert B. 1956.06.07. Sabin Collection Fair Use Policy. URL: http://hdl.handle.net/2374.UC/689825
- Letter from Bayne-Jones S. to Sabin Albert B. 1956.02.20. Sabin Collection Fair Use Policy. URL: http://hdl.handle.net/2374.UC/690912
- Letter from Chumakov M. to Sabin A. 1957.11.27. Sabin Collection Fair Use Policy. URL: https://drc.libraries.uc.edu/handle/2374.UC/679111
- Letter from Chumakov M. to Sabin A. 1958.12.19. Sabin Collection Fair Use Policy. URL: http://hdl.handle.net/2374.UC/679118
- Letter from Chumakov M. to Sabin A. 1958.12.26. Sabin Collection Fair Use Policy. URL: http://hdl.handle.net/2374.UC/679103
- Letter from Dick G.W.A. to Sabin A.1958.07.24. Sabin Collection Fair Use Policy. URL: http:// hdl.handle.net/2374.UC/673520
- Letter from H.Van Zile Hyde to Sabin A. 1956.01.09. Sabin Collection Fair Use Policy. URL: http://hdl.handle.net/2374.UC/690625
- Letter from Rudolph Walter M. to Sabin A. 1956.06.04. Sabin Collection Fair Use Policy. URL: http://hdl.handle.net/2374.UC/689824
- Letter from Sabin A. to Chumakov M.1957.03.15. Sabin Collection Fair Use Policy. URL: http:// hdl.handle.net/2374.UC/679115
- Letter from Sabin A. to MacKay D.C. 1956.05.04. Sabin Collection Fair Use Policy. URL: http://hdl.handle.net/2374.UC/701501
- Letter from Sabin A. to Ramos-Alvarez M. 1957.04.08. Sabin Collection Fair Use Policy. URL: http://hdl.handle.net/2374.UC/673666
- Letter from Timakov V.D. to Sabin A. URL: https://drc.libraries.uc.edu/bitstream/handle/2374.UC/ 669997/sovietunion_1959-68_051.pdf?sequence=1
- Live Polio Vaccine May Immunize 40 Years. Evening Star, 1955, May 4, p. A-15.
- History. Institut poliomielita im. M.P. Chumakova RAN [M.P. Chumakov Polio Institute of the Russian Academy of Sciences]. URL: http:// www.chumakovs.ru/history/#
- Materialy po ispolzovaniju zhivoj vakciny protiv poliomielita [Materials on Using Live Polio Vaccine]. GARF, f. 9226, inv. 1, d. 1894. 262 l.
- Materialy po razrabotke vakciny protiv poliomielita (stenogrammy, spravki, otchety, perepiska) [Materials on the Development of a Polio Vaccine (Records, Memos, Reports, Correspondence)]. GARF, f. 9226, inv. 1, d. 1632. 206 p.
- Otkrytoe obrashhenie Associacii organizacij po klinicheskim issledovanijam po povodu «neoficialnyh ispytanij» vakciny protiv COVID-19 sotrudnikami NICJeM im. N.F. Gamalei Minzdrava Rossii [An Open Letter by Members of Association of Clinical Research Organizations Regarding the "Informal Trials" of the COVID-19 Vaccine by the Staff of N. Gamaleya Rereach Center of the Russian Ministry of Health]. URL: http://acto-russia.org/index.php?option=com_content&task= view&id=400
- Petrovkij B.V. Public Health in the USSR. Moscow, Novosti Press Agency Publ. House, 1967. 76 p.
- Sabin A.B. Oral Poliovirus Vaccine: History of Its Development and Use and Current Challenge to Eliminate Poliomyelitis from the World. The Journal of Infectious Diseases, 1985, March, vol. 151, no. 3, pp. 422-423.
- Sabin's Cable to Chumakov. 1959.06.18. Sabin Collection Fair Use Policy. URL: https://drc.libraries.uc.edu/bitstream/handle/2374.UC/669970/ sovietunion_1959-68_024.pdf?sequence=1
- The Albert B. Sabin's Archives. URL: https://digital.libraries.uc.edu/collections/sabin/
- Translated Postscripts to Chumakov's Letter. 1960.12.29. Sabin Collection Fair Use Policy, p. 8. URL: http://hdl.handle.net/2374.UC/693561.
- Vargha D. Vaccination and the Communist State: Polio in Eastern Europe. Holmberg Ch., Blume S., Greenough P., eds. The Politics of Vaccination. A Global History. Manchester, Manchester University Press, 2017. 360 p.
- Velikanov V.I. Sudby ljudskie: (Semejnaja hronika) [Fates of People: Family Chronicle]. Moscow, s.n., 1998. 398 p.
- Drozdov S.G., ed. Vospominanija oMihaile Petroviche Chumakove [Memoirs About Mikhail Petrovich Chumakov]. Moscow, Institute of Poliomyelitis and Virus Encephalitis, Russian Academy of Sciences, 1999. 221 p.
- Smorodintsev A.A., ed. Zhivaja vakcina protiv poliomielita [Live Vaccine Against Poliomyelitis]. Collection of Works of Department of Virology. Leningrad, s.n., 1960. 299 p.


