Uchinchi avlod tutqanoqqa qarshi vositalar: farmakodinamikasi, farmakokinetikasi va qo‘llanilishi
Автор: Raximboev Suxrob Davlater ko'mirlar, Sanoev Zafar Isomiddinovich
Журнал: Re-health journal @re-health
Статья в выпуске: 3 (23), 2024 года.
Бесплатный доступ
Tahliliy maqolada yangi avlod epilepsiyaga qarshi vositalar: eslikarbazepin, lakosamid, retigabin, perampanel, everolimus, brivarasetam va zonisamidning farmakologik xususiyatliri ko‘rib chiqiladi. Farmakokinetikasi, ta’sir mexanizmlari, dorilar bilan o‘zaro ta’sirlari, qo‘llashga ko‘rsatmalar va nojo‘ya ta’sirlari haqida ma’lumotlar keltiriladi. Ushbu preparatlar avvalgi qo‘llanilgan birinchi va ikkinchi avlod tutqanoqqa qarshi vositalaridan samaraliroq va xavfsiziroq deb tan olingan. Yangi ochilgan epilepsiyaga qarshi vositalarning ko‘pchiligi fokal hurujlarni, shuningdek spesifik epileptik sindromlar (Lennoks-Gasto sindromi, Dreve sindromi) hamda tuberoz sklerozlarni davolashda ishlatiladi. Preparatlarning ta’sir mexanizmi, farmakokinetik xususiyatlari, klinik samaradorligi va nojo‘ya ta’sirlari bemorlarni davolashda individual yondashuvni talab qiladi. To‘g‘ri tanlangan davo sxemasi epileptik hurujlarni nazoratga olish hamda asoratlar rivojlanishini oldini olishga imkon beradi. Epilepsiyaga qarshi vositalarni tayinlashda ularning farmakokinetikasi va farmakodinamikasini hisobga olish lozim.
Epilepsiyaga qarshi vositalar, tutqanoqqa qarshi vositalar, tarqalgan epilepsiya, fokal epilepsiya, farmakokinetika, eslikarbazepin, lakosamid, retigabin, perampanel, everolimus, brivarasetam, zonisamid
Короткий адрес: https://sciup.org/14130770
IDR: 14130770
Текст научной статьи Uchinchi avlod tutqanoqqa qarshi vositalar: farmakodinamikasi, farmakokinetikasi va qo‘llanilishi
Epilepsiya keng tarqalgan va nogironlikka olib keluvchi bosh miyaning surunkali polietiologik kasalligidir. Bosh miya kasalliklari ichida uchrashi bo‘yicha uchinchi o‘rinni egallaydi [1]. Dunyo bo‘ylab epilepsiyaning bolalarda uchrash ko‘rsatkichi turli manbalarda turlicha va taxminan 4-5%, kattalarda 1-2% ni tashkil qiladi. Epilepsiya hurujlari bosh miya faoliyatiga, jumladan, kognitiv, psixik hamda vegetativ funksiyalariga salbiy ta’sir etadi va sog‘liqni saqlash sohasidagi asosiy muammolardan biri bo‘lib qolmoqda [2-3]. Epilepsiya rivojlanishini tushunish, medikamentoz davolashni optimizatsiyasi, adekvat dozalash ishlari yaxshi yo‘lga qo‘yilganiga qaramay taxminan 30% bemorlarda davolashga nisbatan rezistentlik rivojlanadi [4]. Yuqoridagi sanab o‘tilgan va boshqa sabablar tufayli neyrofarmakologiya sohasida yangi epilepsiyaga qarshi birikmalarni izlash va amaliyotga tadbiq etish dolzarbligicha qolmoqda.
1990 yillardan beri 20 dan ortiq samarali epilepsiyaga qarshi vositalar ishlab chiqilib amaliyotga yo‘naltirilgan. Bugungi kunda ularning ko‘pchiligi (m: karbamazepin, valproy kislota unumlari) birinchi avlod epilepsiyaga qarshi vositalardan farqli o‘laroq monoterapiyada qo‘llanadi. Ular farmakokinetik ko‘rsatkichlari, nojo‘ya ta’sirlari kamligi hamda tutqanoqqa qarshi ta’sirining selektivligi kabi qator afzalliklarga ega [5]. Ikkinchi avlod epilepsiyaga qarshi vositalar refrakter epilepsiyalarda samarador, kognitiv funksiyalarga va emotsional sferaga ijobiy ta’sir etadi [6]. Ko‘pchilik uchinchi avlod epilepsiyaga qarshi vositalar ikkinchi avlod preparatlarining hosilalari yoki metabolitlari bo‘lishiga qaramasdan yaxshiroq farmakologik ko‘rsatkichlarga ega, ta’sir mexanizmlari bo‘yicha farqlanadi ayrimlari bir nechta ta’sir nishonlariga ega (1-jadval) [7].
1-jadval. Ayrim uchinchi avlod epilepsiyaga qarshi vositlar ta’sir nishonlari
|
Preparat |
Ta’sir mexanizmi |
|
Eslikarbazepin, lakosamid, zonisamid |
Potensialga bog‘liq Na+- kanallarini blokadasi |
|
Brivaratsetam |
Presinaptik neyron vezikulalari membranasidagi SV2A oqsilining modulyatsiyasi |
|
Retigabin |
GAMK A – retseptori aktivatsiyasi |
|
Vigabatrin |
GAMK-transaminazaning selektiv qaytmas ingibirlanishi |
|
Perampanel |
AMPA - glutamat retseptorlarining blokadasi |
|
Zonisamid, lakosamid, everlimus |
Multinishon ta’sirlashish |
Eslatma. SV2A (synaptic vesicle protein 2A) – sinaptik vezikulalar 2A oqsili
GAMK – gammaaminomoy kislota, AMPA – alfa-amino-3-gidroksi-5-metil-4-izoksazolpropion kislotasi.
Evrolimus va kannabidol tuberoz skleroz bilan bog’liq epilepsiyalar, shuningdek Lennoks-Gasto sindromi, Drave sindromlari patogenetik nishon terapiyasida qo’llaniladi [8].
Uchinchi avlod epilepsiyaga qarshi vositalar
Perampanel
Perampanel (Fycompa ® ) - preparat birinchi marta 2012 yilda FDA (Food and Drug Administration, AQSH) tomonidan klinik amaliyotda parsial tutqanoq shakllarini davolashda qo’llashga ruxsat etilgan. Keyinchalik 2015 yilda EMA (European Medicines Agency) tomonidan Yevropa Ittifoqida qo‘llashga ruxsat berilgan. Perampanel postsinaptik AMPA - glutamat retseptorlarining selektiv nokonkurent antagonisti hisoblanadi, preparat neokorteks va gippokamp neyronlarida qo‘zg‘alish jarayonlarining susayishiga olib keladi [9-10]. Glutamat - bosh miyadagi asosiy qo‘zg‘atuvchi neyromediator hisoblanadi, shu sababli neyronlarning giperqo‘zg‘aluvchanlligi sababli kelib chiquvchi qator nevrologik kasalliklar patogenezida muhim o‘rin tutadi. Glutamat neyromediatorining tezkor qo‘zg‘atuvchi ta’siri yuzaga chiqishida AMPA-retseptorlari asosiy rol o'ynaydi deb qaraladi. In vitro tadqiqotlarda perampanel AMPA-indutsirlangan hujayra ichi Ca2+ -ionlari oshishini to'xtatgan. Bunda NMDA-indutsirlangan hujayra ichi Ca2+- ionlari oshishiga ta’sir qilmagan. In vivo sharoitda AMPA-indutsirlangan tutqanoq modellarida latent davrini sezilarli uzaytiradi (1-rasm). Dastlab preparat epilesiyadan tashqari bir qator nevrologik kasalliklarni davolashda sinovdan o'tkazilgan va qo'llashga ruxsat etilgan [11]. Keyinchalik bu yo'nalishdagi ishlar to'xtatilgan.
1-rasm. Perampanel kimyoviy strukturasi
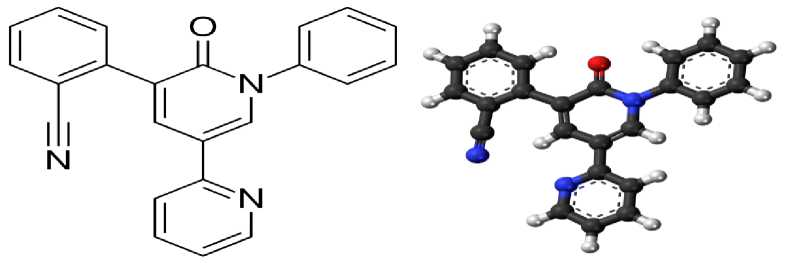
2016 yildan migren, tarqalgan skleroz, neyropatik og'riqlarda va Parkinson kasalligida qo'llash to'xtatildi [12]. Perampanel katta dozalarda ketaminga o'xshab eyforiya chaqiradi. Doza oshishiga qarab agressivlik, psixik qo‘zg‘alishlar va delirioz holatlar rivojlanishi mumkin. Asosiy ustunligi terapevtik dozalarda kognitiv funksiyalarga ta’sir etmasligi, peroral qabul qilinishi, sutkalik dozani bir martada qabul qilinishi va b. [13].
Perampanelga chiziqli farmakokinetika xos. Peroral qabul qilingandan keyin tez va to'liq so'riladi. Zardob oqsillari bilan bog'lanish darajasi juda yuqori bo‘lib 95% gacha yetadi. Jigarda biotransformatsiyaga uchraydi. Metabolik transformatsiya jarayonlari sitoxrom P 450 3A4 (CYP3A4) izoformasi yoradmida amalga oshadi. Keyinchalik glyukuron kislota bilan konyugatsiyalanib peshob orqali chiqariladi. Preparat ko‘p miqdorda axlat orqali ham chiqariladi. T 1/2 - davri 105 soatni tashkil qiladi. CYP3A4 induktorlari (karbamazepin, fenobarbital) perampanel metabolizmini telashtirib preparatning qon plazmasidagi miqdorini kamaytiradi [14].
Eslikarbazepin
Eslikarbazepin asetat (Exalief®) - epilepsiyaga qarshi vosita, karboksamid unumi. Likarbazepin preparatining S-enantiomeri hisoblanadi. Likarbazepin potensialga bog'liq Na+ -kanallari blokatori, samarali antikonvulsant va timoleptik okskarbazepinning faol metabolitidir (2-rasm).
FDA va EMA tomonidan 2017 yilda 4 yoshdan oshgan bolalarda va kattalarda parsial tutqanoq hurujlari va ikkilamchi tarqalgan tutqanoq hurujlarini davolashda qo'llash uchun ruxsat etilgan. Preparat monoterapiya va boshqa epilpsiyaga qarshi vositalar bilan kominatsiyada qo’llanilishi mumkin. Davolashda okskarbazepindan eslikarbazepinga o‘tish xurujlar sonini kamaytirib, kasallik kechishini stabillashtirgan. Kognitiv va psixik funksiyalarga ta’siri kam. Preparat peroral qo'llaniladi. Yarim ajralish davri uzoqligi uchun sutkada bir marta qabul qilinadi.
2-rasm. Likarbazepinning R- va S- enantiomerlarining kimyoviy strukturasi
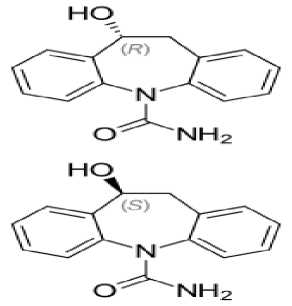
Zaharlilik ko‘rsatkichlari ancha past, boshqa preparatlar bilan o‘zaro ta’siri yaqqol ifodalanmagan [15].
Ta’sir mexanizmi neyron membranasidagi potensialga bog‘liq Na+ - ion kanallariga ta’siri bilan bog'liq. Potensialga bog'liq Na + - ion kanallari ishining fazasiga qarab uch xil funksional holatda bo'lishi mumkin. Birinchi holat tinchlik holati - kanal yopiq - depolyarizatsiyalovchi stimulga javob beradi. Ikkinchi holat depolyarizatsiya holati - kanal ochiq. Uchinchi holat inaktiv holat - kanal yopiq - depolyarizatsiyadan keyingi holat bo‘lib kanal ta’surotlarga javob bermaydi. Eslikarbazepin potensialga bog‘liq Na+ - ion kanallarini davomli inaktiv holatda ushlab turilishiga yordam beradi [16]. Shuningdek potensialga bog'liq Ca2+ - kanallariga bloklovchi ta’siri bor deb qaraladi.
Eslikarbazepin asetat prodori hisoblanadi. Peroral qabul qilingandan keyin portal vena orqali jigarga tushib u yerda gidrolizlanib faol metabolit S-likarbazepinga aylanadi. Preraratning plazmadagi pik konsentratsiyasi 1-4 soatni tashkil etadi. Plazma oqsillari bilan bog'lanish darajasi yuqori emas va taxminan 30% ni tashkil qiladi. Eslikarbazepin jigar mikrosomal fermentlarining CYP3A4 izofermentining induktori hisoblanadi. Biroq bu faollik karbamazepinga nisbatan kuchsiz ifodalangan. Topiramat bilan birgalikda qo'llash topiramat biokirishuvchanligini 18% ga kamaytiradi, levetiratsetam va valproatlar bilan o'zaro ta’siri kuchsiz ifodalangan [17]. T 1/2 -davri 17 soatni tashkil qiladi, barqaror konsentratsiyaga peroral qo'llash boshlangandan keyin 4-5 kunda erishiladi. Eslikarbazepin metabolitlari asosan glyukuron kislota konyugatlari shaklida, kam miqdorda o'zgarmagan holda buyraklar orqali chiqariladi. Farmakokinetikasi chiziqli dozaga bog'liq [18].
Nojo'ya ta’sirlari dozaga bog'liq bo'lib, uyquchanlik, bosh og‘rig‘i, bosh aylanishi, ko'ngil aynishi, diplopiya shaklida namoyon bo'lishi mumkin. Giponatriyemiya okskarbazepindan kamroq 0,5-1,5% bemorlarda uchrashi mumkin [19].
Lakosamid
Lakosamid (Vimpat ® ) - faol moddasi 2 - asetamido - N - benzil - 3 -metoksipropionamidning R-enantiomeridir. Bu birikma L-serinning hosilasidir. Preparatni 20082009 yillarda AQSH va Yevropa Ittifoqida parsial tutqanoq hurujlarini davolash uchun qo'llashga ruxsat etilgan. Lakosamid 4 yoshdan kattalarda parsial tutqanoq hurujlarini hamda ikkilamchi tarqalgan tutqanoq hurujlarini davolashda monoterapiya yoki qo'shimcha sifatida qo'llaniladi [20].
Preparatning ustunligi samaradorlik, xavfsizlik, yaxshi o‘zlashtirilishi, dorilar bilan o‘zaro ta’siri deyarli yo'qligi, enteral va parenteral qo'llash mumkinligidadir (3-rasm). Lakosamid pediatriya amaliyotida o'zining muhim o'rnini topib ulgurgan [21].
3-rasm. Lakosamidning kimyoviy tuzilishi
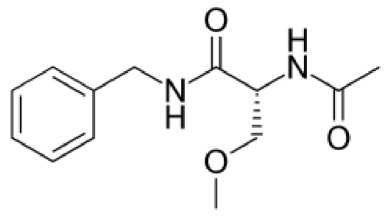
Lakosamid neyronlarning patologik giperqo‘zg‘aluvchanligini modulyatsiyalaydi. Ta’sir mexanizmida bir nechta komponent mavjud. Birinchi holatda potensialga bog‘liq Na+ - kanallarining inaktivatsiya davrini uzaytiradi, ikkinchi holatda NMDA-retseptorlari bilan antagonizm namoyon qiladi. Shuningdek neyronlar aksoni o‘sishi va differensirovkasida ahamiyatli bo‘lgan kollapsin oqsili (collapsin response mediator protein, CRMP-2) ga ta’sir qilib uning faolligini pasaytiradi. Bu bilan epileptik o‘choqda neyronlarning “qayta qurilishi” ni to‘xtatadi [22].
Lakosamid peroral qabul qilinganda tez va to‘liq so‘riladi. Biokirishuvchanligi 98% dan yuqori. Plazma oqsillari bilan bog‘lanishi 15% dan yuqori emas. T 1/2 – davri 13 soat. Qondagi stabil konsentratsiyaga 3-4 kunlik qabuldan keyin erishiladi. Yarim ajralish davri qisqa bo‘lganligi uchun kuniga ikki mahal qabul qilinadi. Preparat jigarda CYP2C9 fermenti yordamida metabolizmga uchraydi, taxminan 40% i o‘zgarmagan holda buyraklar orqali chiqariladi. Buyrak va jigar yetishmovchiligida preparatning plazmadagi miqdori oshishi mumkin. Preparatning neyrotoksik ta’sirlari depressiya, quloqlarda shovqin, og‘iz qurishi, mushak uchishlari, rang ajratishning buzilishi kabi holatlar bo‘lishi mumkin [23].
Lakosamidning boshqa epilepsiyaga qarshi vositalar bilan o‘zaro ta’siri minimal darajada bo‘lib bu uni boshqa epilepsiyaga qarshi vositalar bilan kombinatsiyada qo‘llashga imkon beradi [24].
Retigabin
Retigabin (Trobalt®) – karbonat kislotaning etil efiri bo‘lib boshqa epilepsiyaga qarshi vositalarga o‘xshamaydigan kimyoviy tuzilishga ega. Retigabin unikal ta’sir mexanizmiga ega preparatdir. Preparat (shuningdek ezogabin) 2011 yildan beri AQSH va Yevropa mamlakatlarida yoshi katta bemorlarda fokal tutqanoq hurujlari va ikkilamchi tarqalgan tutqanoq hurujlarini davolashda qo‘llaniladi. Retigabin rezerv preparat bo‘lib boshqa epilepsiyaga qarshi vositalar samarasizligida yoki o‘zlashtirish bilan muammolar bo‘lganda qo‘llaniladi. Preparatning “xavf/foyda” munosabati boshqa epilepsiyaga qarshi vositalar bilan bir xil bo‘lishiga qaramasdan pediatriya amaliyotida qo‘llashga hozircha ruxsat yo‘q. Tadqiqotchilar retigabinni halqali XX xromosoma sindromi (epileptik K+ -kanalopatiya)ni davolashda qo‘llash ustida ishlamoqda [25].
Retigabin ta’sir mexanizmi neyronlardagi KCNQ2 va KCNQ3 – kaliy kanallarining aktivatsiyasi va ochilishi bilan bog‘liq. Bu esa o‘z navbatida K+ - ionlarining sitoplazmadan hujayralararo muhitga chiqishi va neyronlarning giperpolyarizatsiyalanishiga olib keladi (4-rasm). Natijada elektr qo‘zg‘alishning bo‘sag‘a potensiali oshadi va epileptik impulslar hosil bo‘lishi bloklanadi [26].
4-rasm. Retigabinning kimyoviy tuzilishi
Preparat chiziqli farmakokinetikaga ega. Peroral qo‘llanilganda meda ichak traktidan tez so‘riladi. Biokirishuvchanligi 60% ni tashkil qiladi, qondagi maksimal konsentratsiyaga taxninan 1,52 soatlarda erishiladi. Plazma oqsillari bilan bog‘lanish darajasi 80%. Biotransformatsiyasi jigarda amalga oshadi. Metabolitlari buyraklar orqali ajratiladi. T 1/2 – davri taxminan 9 soat [20].
Retigabinning nojo‘ya ta’sirlari orasida peshob tutilishi, bosh aylanishi, uyquchanlik, afaziya, tremor, qabziyat, muvozanatni uylay olmaslik va boshqalar uchrab turadi [27].
Retigabin dorilar bilan minimal o‘zaro ta’sirga ega. Fenitoin va karbamazepin retigabinning klirensini biroz oshiradi. preparat lamotridjinning metabolizmini kuchaytiradi. Oral kontraseptiv vositalar bilan o‘zaro ta’sirga ega emas [ 20 ].
Everolimus
Everolimus (Afinitor®) – epilepsiyaga qarshi vosita bo‘lib immunodepressant va antiblastom vosita sirolimusning hosilasidir. 2010 yilda FDA tomonidan tuberoz skleroz bilan assotsirlangan gigant hujayrali astrotsitomani davolash maqsadida qo‘llashga ruhsat berilgan. Bugungi kunda butun dunyo bo‘ylab 2 yoshdan kattalarda tuberoz skleroz bilan assotsirlangan epilepsiyani davolashda qo‘llanilmoqda (5-rasm).
5-rasm. Sirolimus va everolimus preparatlarining kimyoviy tuzilishi
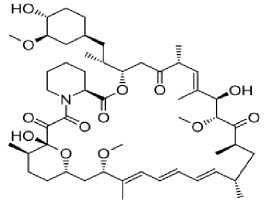
sirolimus
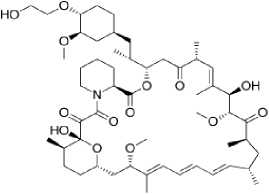
everolimus
Ta’sir mexanizmi immunosupressor, proliferativ signalning ingibitori. T-hujayralarning antigen-aktivlashgan proliferatsiyasini ingibirlash orqali ta’sir etadi. T-hujayralarning o‘sish omili sababli boshlangan hujayralar proliferatsiyasini ichki signal yo‘llarini bloklash orqali (p70 S6 kinazaning fosforillanishini ingibirlaydi) to‘xtatadi. Natijada hujayralar bo‘linishi G 1 fazasida to‘xtaydi. Kinaza hujayralar proliferatsiyasi va o‘sishida asosiy omildir. Kinazaning yuqori faolligi neyronlar o‘lchamining kattalashishiga, mielinizatsiyaning kamayishiga, akson va dendritlar o‘rtasida abberant (noodatiy) aloqalar kelib chiqib sinaptik disfunksiyaga olib keladi. Tuberoz skleroz bilan bog‘liq epilepsiyalarda preparat bosh miya hujayralarining tezlashgan proliferatsiyasini, anomal o‘lchamlargacha o‘sishini, anomal shakllarga kirishini to‘xtatib neyronlar faoliyatini va sinaptik plastiklikni yaxshilaydi [28].
Qondagi pik konsentratsiyasiga peroral kiritilgandan keyin 1-2 soatda erishiladi. Peroral qabul qilinganda biokirishuvchanligi 16% dan oshmaydi. Zardob oqsillari bilan bog‘lanish darajasi 74%. Metabolizmi jigarda CYP3A4 vositasida amalga oshadi. Faol metabolitlari mavjud emas. T 1/2 – davri 18-35 soat. Asosiy miqdori axlat bilan (80%) kam miqdorda peshob bilan (2-5%) ajraladi [29].
Nojo‘ya ta’sirlaridan eng ko‘p uchraydiganlariga stomatit, diareya, nazofaringit, yuqori nafas yo‘llari yallig‘lanishi, shilliq qavat kandidozlari va isitma kiradi [30].
Fokal hurujlarni davolashda qo‘llanuvchi ba’zi vositalar, jumladan, karbamazepin, okskarbazepin, fenobarbital, fenitoin va topiramat CYP3A4 – izofermentini indutsirlashi hisobiga everolimus biotransformatsiyasini tezlashtirishi mumkin. Shuningdek metoklopramid va boshqa prokinetik ta’sirli vositalar preparat so‘rilishini kamaytiradi [31].
Brivaratsetam
Brivaratsetam (Briviak®) – ratsetamlar guruhiga kiruvchi epilepsiyaga qarshi vosita bo‘lib parsial tutqanoq hurujlari va ikkilamchi tarqalgan tutqanoq hurujlarini davolashda qo‘shimcha vosita sifatida ishlatiladi. 2016 yidan boshlab Yevropa Ittifoqida 16 yoshdan kattalarda qo‘llashga ruxsat berilgan (6-rasm). Preparat levetiratsetamga har taraflama o‘xshash, biroq qator afzalliklarga ega.
Jumladan, kam zaharlik, yuqori samaradorlik, enteral va parenteral qo‘llash imkoniyati. Brivaratsetam levetiratsetamdan farqli o‘laroq kognitiv va motor funksiyalarga ta’sir qilmaydi [32].
6-rasm. Brivaratsetam molekulyar formulasi
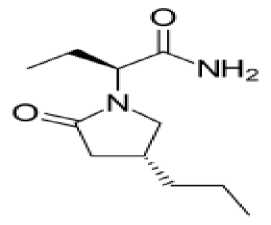
Ta’sir mexanizimi presinaptik neyronlar oxiridagi sinaptik vezikulalar oqsili SV2A (synaptic vesicle protein 2A) bilan bog‘lanib uni ingibirlashi bilan bog‘liq. Brivaratsetaming ushbu oqsilga yaqinligi levetiratsetamga qaraganda 15 marta yuqoridir. SV2A oqsili qo‘zg‘atuvchi neyrotransmitterlar (glutamat, aspartat) ajralishida muhim ahamiyatga ega. Oqsilning bloklanishi natijasida qo‘zg‘atuvchi impuls uzatilmaydi va tutqanoq hurujlari soni kamayadi. Ba’zi ma’nbalarda braviratsetamning potensialga bog‘liq Na+ -kanallariga ingibirlovchi ta’sirga ham egaligi haqida ma’lumotlar bor [33].
Brivaratsetam peroral qabul qilinganda tez va deyarli to‘liq so‘riladi. Plazmadagi maksimal konsentratsiyaga 2 soatdan keyin erishiladi. Peroral qabul qilinganda biokirishuvchanlik 90% dan oshadi. Yog‘li ovqatlar preparat so‘rilishini sekinlashtirishi mumkin. Brivaratsetam lipofil birikma bo‘lganligi uchun passiv diffuziya yo‘li bilan gematoensefalik baryerdan tez o‘tadi. Biotransformatsiyasi jigarda amalga oshadi, uchta nofaol metabolitga parchalanadi. Konyugatlar shaklida buyraklar orqali (95%) chiqariladi. Plazma oqsillari bilan birikish darajasi 20%dan oshmaydi. T 1/2 -davri 7-8 soatni tashkil qiladi [34].
Nojo‘ya ta’sirlaridan bosh aylanishi, bosh og‘riqlari, mushak uchishlari, apatiya, uyquchanlik, depressiya va boshqalarni ko‘rsatib o‘tish mumkin. Ba’zida anoreksiya, ko‘ngil aynish va qusish kabi nojo‘ya ta’sirlar chaqirishi mumkin [35].
Preparat boshqa dori vositalari bilan juda sust o‘zaro ta’sirga ega. Boshqa epilepsiyaga qarshi vositalar bilan birgalikda qo‘llanishi mumkin. Mikrosomal ferment ingibitorlari preparatning plazmadagi konsentratsiyasini kamaytirishi mumkin, biroq bu konsentratsiyani korreksiya qilishga zaruriyat tug‘dirmaydi [36].
Zonisamid
Zonisamid (Zonegran®) – benzisoksazolning sulfonamid hosilasidir. 1989 yilda Yaponiyada 6 yoshdan kattalarda fokal va tarqalgan tutqanoq hurujlarini davolashda qo‘llashga ruxsat etilgan (7-rasm). Xalqaro epilepsiyaga qarshi liga (ILAE) zonisamidning parsial tutqanoq hurujlarida samaradorligini a darajasida etirof etgan va boshlang‘ich bosqichlarda monoterapiya uchun tavsiya etgan [37].
7-rasm. Zonisamid molekulasining tuzilishi
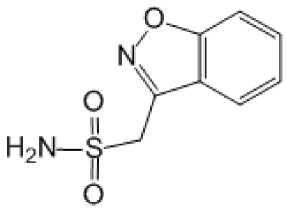
Ta’sir mexanizmi to‘liq o‘rganilmagan. Multinishon ta’sirlarga ega deb qaraladi. Ta’sir mexanizimida asosiy o‘rinda potensialga bog‘liq Na+ - kanallarini inaktiv fazasini uzaytirish turadi.
Shuningdek T-tip Ca2+- kanallarini bloklash (bu holat absans hurujlarda samaradorligini tushuntiradi) va GAMK ajralishini rag‘batlantirish kabi ta’sirlarga ega deb qaraladi. Zonisamid asetilxolin, dofamin va seratonin metabolizmini biroz sekinlashtiradi, karbongidrazani ingibirlaydi [38].
Eng ko‘p uchraydigan nojo‘ya ta’sirlariga uyquchanlik, apatiya, anoreksiya, xotira pasayishi, psixik reaksiyalarning sekinlashishi, paresteziyalarni keltiriah mumkin. Preparat ba’zida hazm sistemasida dispeptik o‘zgarishlar chaqirishi mumkin. Preparat tana massasini kamaytirishi mumkin
Zonisamid lamotridjin, oral kontraseptivlar kabi preparatlar bilan klinik ahamiyatli o‘zaro ta’sirlarga ega emas. CYP3A4 induktorlari yoki ingibitorlari zonisamidning plazmadagi konsentratsiyasiga sezilarli ta’sir qilishi mumkin, bunda dozani korrektirovka qilish lozim bo‘ladi
Xulosa. Tahliliy maqolada hozirgi kunda klinik amaliyotda qo‘llanilayotgan eng muhim uchinchi avlod epilepsiyaga qarshi vositalarning ta’sir mexanizmlari, qo‘llanilishi, farmakokinetikasi, nojo‘ya va dorilar bilan o‘zaro ta’sirlari kabi farmakologik xususiyatlari haqida umumlashtirilgan ma’lumotlar berildi. Qo‘llashdagi ba’zi nyuanslar ko‘rsatib o‘tildi. Epilepsiya patogenezini chuqurroq o‘rganish natijasida preparatlarni monoterapiya yoki birgalikda qo‘llashning foyda/zarar koeffisiyentini yaxshilashga imkon beradi. Maqolada preparatlarni tanlash va qo‘llashda ushbu koeffisiyentni yaxshilash haqida xorijiy mualliflarning bir qancha takliflari keltirib o‘tildi.
Yoritib o‘tilgan epilepsiyaga qarshi vositalar turli xil ta’sir mexanizmlariga ega bo‘lib, epilepsiya va epileptik sindromlarni davolashda keng imkoniyatlar ochib beradi. Uchinchi avlod epilepsiyaga qarshi vositalar dastlabki avlod vakillariga qaraganda yaxshi o‘zlashtirish, qaramlik chaqirmasligi, kamroq o‘zaro ta’sir, kam zaharlilik, yaxshiroq farmakokinetik ko‘rsatkichlarga ega. Yangi avlod epilepsiyaga qarshi preparatlari haqida ma’lumotlarga ega bo‘lish shu yo‘nalishda ish olib borayotgan farmakolog, klinik-farmakolog va nevrologlar ilmiy faoliyatida yordam beradi.
ADABIYOTLAR
6 th ed. Philadelphia, PA: Elsevier/Saunders; 2013: 1153 pp.
RHJ-3-2024
Список литературы Uchinchi avlod tutqanoqqa qarshi vositalar: farmakodinamikasi, farmakokinetikasi va qo‘llanilishi
- Карлов В.А. Эпилепсия у детей и взрослых женщин и мужчин. Руководство для врачей. 2-е изд. М.: Бином; 2019: 896 с.
- Devinsky O., Vezzani A., O’Brien T.J., et al. Epilepsy. Nat Rev Dis Primers. 2018; 4: 18024. https://doi.org/10.1038/nrdp.2018.24.
- Sanoev Z.I., Ismailova D.S., Rakhimboev S.D., Tolibovich T.T., Elmuradov B.J., Abdinazarov I.T., Rashidov S.Z. (2023). Synthesis And Research Anticonvulsant Activity Of Annulated Triazolothiadiazine Derivative In Laboratory Animals. Biomedical & Pharmacology Journal. Vol. 16(4), p. 2457-2467.
- Janmohamed M., Brodie M.J., Kwan P. Pharmacoresistance – epidemiology, mechanisms, and impact on epilepsy treatment. Neuropharmacology. 2020; 168: 107790. https://doi.org/10.1016/j.neuropharm.2019.107790.
- Nakken K.O., Brodtkorb E. Are new anti-epileptic drugs any better than their predecessors? Tidsskr Nor Laegeforen. 2020;140 (17). https://doi.org/10.4045/tidsskr.20.0657.
- Trinka E., Ben-Menachem E., Kowacs P.A., et al. Efficacy and safety of eslicarbazepine acetate versus controlled-release carbamazepine monotherapy in newly diagnosed epilepsy: a phase III double-blind, randomized, parallel-group, multicenter study. Epilepsy. 2018; 59 (2): 479–91. https://doi.org/10.1111/epi.13993.
- Sills G.J., Rogawski M.A. Mechanisms of action of currently used antiseizure drugs. Neuropharmacology. 2020; 168: 107966. https://doi.org/10.1016/j.neuropharm.2020.107966.
- Curatolo P., Napolioni V., Moavero R. Autism spectrum disorders in tuberous sclerosis: pathogenetic pathways and implications for treatment. J Child Neurol. 2010; 25 (7): 873–80. https://doi. org/10.1177/0883073810361789.
- Rektor I., Krauss G.L., Bar M., et al. Perampanel Study 207: long-term open-label evaluation in patients with epilepsy. Acta Neurol Scand. 2012; 126 (4): 263–9. https://doi.org/10.1111/ane.12001.
- Sanoyev Z. I. Psixotrop vositalar [Matn]: oʻquv qoʻllanma / – Toshkent: “Barkamol Fayz Media”, 2022. – 192 b.
- Chong DJ, Lerman AM (April 2016). "Practice Update: Review of Anticonvulsant Therapy". Current Neurology and Neuroscience Reports. 16 (4): 39. doi:10.1007/s11910-016-0640-y. PMID 26984292. S2CID 9090302
- Besag FM, Patsalos PN (2016). "Clinical efficacy of perampanel for partial-onset and primary generalized tonic-clonic seizures". Neuropsychiatric Disease and Treatment. 12: 1215–20. doi:10.2147/NDT.S83842. PMC 4876101. PMID 27274257. S2CID 14176469
- Patsalos P.N. The сlinical рharmacology рrofile of the new antiepileptic drug perampanel: а novel noncompetitive AMPA receptor аntagonist. Epilepsia. 2015; 56 (1): 12–27. https://doi.org/10.1111/epi.12865.
- Patsalos P.N. The сlinical рharmacology рrofile of the new antiepileptic drug perampanel: а novel noncompetitive AMPA receptor аntagonist. Epilepsia. 2015; 56 (1): 12–27. https://doi.org/10.1111/epi.12865.
- Trinka E., Ben-Menachem E., Kowacs P.A., et al. Efficacy and safety of eslicarbazepine acetate versus controlled-release carbamazepine monotherapy in newly diagnosed epilepsy: a phase III double-blind, randomized, parallel-group, multicenter study. Epilepsy. 2018; 59 (2):479–91. https://doi.org/10.1111/epi.13993.
- Parada A., Soares-da-Silva P. The novel anticonvulsant BIA 2-093 inhibits transmitter release during opening of voltage-gated sodium channels: a comparison with carbamazepine and oxcarbazepine. Neurochem Int. 2002; 40 (5): 435–40. https://doi.org/10.1016/S0197-0186(01)00101-2.
- Singh R.P., Asconapé J.J. A review of eslicarbazepine acetate for the adjunctive treatment of partial-onset epilepsy. J Cent Nerv Syst Dis. 2011; 3: 179–87. https://doi.org/10.4137/JCNSD.S4888.
- Mäkinen J., Rainesalo S., Peltola J. Transition from oxcarbazepine to eslicarbazepine acetate: a single center study. Brain Behav. 2017; 7 (3): e00634. https://doi.org/10.1002/brb3.634.
- Ben-Menachem E., Gabay A.A., Hufnagel A., et al. Eslicarbazepine acetate as an adjunct therapy in adult patients with partial epilepsy. Epilepsy Res. 2010; 89 (2–3): 278–85. https://doi.org/10.1016/j. eplepsyres.2010.01.014.
- Luzhczky J.J. Third-generation antiepileptic drugs: mechanisms of action, pharmacokinetics and interactions. Pharmacol Rep. 2009; 61 (2): 197–216. https://doi.org/10.1016/s1734-1140(09)70024-6.
- Ortiz de la Rosa J.S., Ladino L.D., Rodríguez L.D., et al. Efficacy of lacosamide in children and adolescents with drug-resistant epilepsy and refractory epileptic status: a systematic review. Seizure. 2018; 56: 34–40. https://doi.org/10.1016/j.seizure.2018.01.014.
- Rogawski M.A., Tofighi A., White H.S., et al. Current understanding of the mechanism of action of the antiepileptic drug lacosamide. Epilepsy Res. 2015; 110: 189–205. https://doi.org/10.1016/j. eplepsyres.2014.11.021.
- Rosenfeld V., Fountain N.B., Kaubrys G., et al. Safety and efficacy of adjunctive lacosamide among patients with partial-onset seizures in a long-term open-label extension trial of up to 8 years. Epilepsy Behav. 2014; 41: 164–70. https://doi.org/10.1016/j.yebeh.2014.09.074.
- Harris J.A., Murphy J.A. Lacosamide and еpilepsy. CNS Neurosci. Ther. 2011; 17 (6): 678–82. https://doi.org/10.1111/j.1755-5949.2010.00198.x.
- Walleigh D.J., Legido A., Valencia I. Ring chromosome 20: a pediatric potassium channelopathy responsive to treatment with ezogabine. Pediatr Neurol. 2013; 49 (5): 368–9. http://doi.org/10.1016/j.pediatrneurol.2013.06.005.
- Bialer M., Johannessen S.I., Kupferberg H.J., et al. Progress report on new antiepileptic drugs: a summary of the Seventh Eilat Conference (EILAT VII). Epilepsy Res. 2004; 61 (1–3): 1–48. http://doi.org/10.1016/j.eplepsyres.2004.07.010.
- Koltzenburg M., McMahon S.B., Tracey I., Turk D.C. (Eds.) Wall and Melzak's textbook of pain. 6 th ed. Philadelphia, PA: Elsevier/Saunders; 2013: 1153 pp.
- Li M., Zhou Y., Chen C., et al. Efficacy and safety of mTOR inhibitors (rapamycin and its analogues) for tuberous sclerosis complex: a metaanalysis. Orphanet J Rare Dis. 2019; 14 (1): 39. http://doi.org/10.1186/ s13023-019-1012-x
- MacKeigan J.P., Krueger D.A. Differentiating the mTOR inhibitors everolimus and sirolimus in the treatment of tuberous sclerosis complex. Neuro Oncol. 2015; 17 (12): 1550–9. http://doi.org/10.1093/ neuonc/nov152.
- French J.A., Lawson J.A., Yapichi Z., et al. Adjunctive everolimus therapy for treatment-resistant focal-onset seizures associated with tuberous sclerosis (EXIST-3): a phase 3, randomised, doubleblind, placebo-controlled study. Lancet. 2016; 388 (10056): 2153–63. http://doi.org/10.1016/S0140-6736(16)31419-2
- Franz D.N., Lawson J.A., Yapici Z., et al. Everolimus dosing recommendations for tuberous sclerosis complex-associated refractory seizures. Epilepsia. 2018; 59 (6): 1188–97. http://doi.org/10.1111/epi.14085.
- Liu E., Dilley D., McDonough B., et al. Safety and tolerability of adjunctive brivaracetam in pediatric patients < 16 years with epilepsy: an open-label trial. Pediatr Drugs. 2019; 21 (4): 291–301. http://doi.org/10.1007/s40272-019-00332-y.
- Klitgaard H., Matagne A., Nicolas J.M., et al. Brivaracetam: Rationale for discovery and preclinical profile of a selective SV2A ligand for epilepsy treatment. Epilepsia. 2016; 57 (4): 538–48. http://doi.org/10.1111/epi.13340.
- Strzelczyk A., Klein K.M., Willems L.M., et al. Brivaracetam in the treatment of focal and idiopathic generalized epilepsies and of status epilepticus. Expert Rev Clin Pharmacol. 2016; 9 (5): 637–45. http://doi.org/10.1586/17512433.2016.1156529.
- Dredge D.C. (Ed.) Handbook of pediatric epilepsy education. Springer; 2022: 447 pp.
- Rolan P., Sargentini-Maier M.L., Pigeolet E., Stockis A. The pharmacokinetics, CNS pharmacodynamics and adverse event profile ofbrivaracetam after multiple increasing oral doses in healthy men. Br J Clin Pharmacol. 2008; 66 (1): 71–5. http://doi.org/10.1111/j.1365-2125.2008.03158.x
- Kwan S.Y., Chuang Y.C., Huang C.W., et al. Zonisamide: review of recent clinical evidence for treatment of epilepsy. CNS Neurosci Ther. 2015; 21 (9): 683–91. http://doi.org/10.1111/cns.12418.
- Cornes S.B., Griffn Jr. E.A., Lowenstein D.H. Pharmacology of abnormal electrical neurotransmission in the central nervous system. In: Golan D.E., Armstrong E.J., Armstrong A.W. Principles of pharmacology: the pathophysiologic basis of drug therapy. 4th ed. Philadelphia: Wolters Kluwer; 2017: 249–64.
- Misty D.M., Metcalf C.S., Wilcox K.S. Pharmacotherapy of the epilepsies. In: Brunton L.L., Hilal-Dandan R., Knollmann B.C. (Eds.) Goodman and Gilman’s: the pharmacological basis of therapeutic. 13th ed. McGraw-Hill; 2018.


