Влияние коррозии на легкие стальные тонкостенные конструкции
Автор: Ананина Мария Владимировна, Бересенева Н.А., Шуровкина Л.Л.
Журнал: Строительство уникальных зданий и сооружений @unistroy
Рубрика: Строительные материалы
Статья в выпуске: 7 (22), 2014 года.
Бесплатный доступ
Технология строительства на основе легких стальных тонкостенных конструкций (ЛСТК) - это каркасная технология, позволяющая возводить дома в короткие сроки. Суть этой технологии заключается в использовании панелей из легких стальных оцинкованных перфорированных и неперфорированных профилей, которые образуют металлический каркас здания. Существует много различных характеристик ЛСТК, которые изучались и исследуются по сегодняшний день. Одной из таких характеристик является устойчивость к коррозии. Защита от коррозии легких стальных конструкций, выполненных из холодногнутыхпрофилей, чрезвычайно важна. Недостаточно защищенные тонкостенные конструкции могут разрушиться в короткий срок. Для строительных стальных конструкций существенное значение имеет электрохимическая коррозия. Она вызывается водными растворами электролитов и является результатом действия микроэлементов, образующихся на поверхности металла, соприкасающегося с электролитом. Роль, которую механические напряжения играют в явлениях коррозии металла, очень сложна и еще не полностью выяснена. По единодушному мнению многих исследователей, коррозионное растрескивание происходит под действием растягивающих напряжений, которые вызывают расширение возможных трещин. Сжимающие напряжения противодействуют растрескиванию, вызывая закрытие трещин. Напряжения от кручения дополнительно увеличивают скорость коррозионного растрескивания на несколько десятков процентов. Чем ниже содержание углерода, тем выше коррозионная стойкость нержавеющих сталей. Чем меньше размер зерна металла, тем больше его устойчивость против коррозионного растрескивания. При увеличении размера зерна уменьшается время до разрушения. Наиболее стойка перед коррозией решетка из труб. В случае со сплошной стенкой большую прочность имеет балка коробчатого сечения, чем двутавровая. В настоящее время наиболее распространенным способом защиты от коррозии является применение покрытий в виде слоя краски. Они уступают первенство, с точки зрения стойкости, гальваническим и металлическим покрытиям, но более доступны.
Коррозия, легкие стальные конструкции, низкоуглеродистая сталь, холодногутые профили, низколегированные стали, катод, анод, оксид
Короткий адрес: https://sciup.org/14322144
IDR: 14322144 | УДК: 69
Текст обзорной статьи Влияние коррозии на легкие стальные тонкостенные конструкции

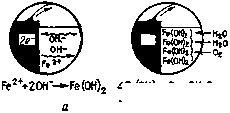
4Fe(0H)2»0,t2H^) — ^4Fe(OH)j a IFe^HjO^tZHjO
Construction technology based on the light steel gauge construction is a frame technology, allowing erecting houses in a short time. The essence of this technology is using panels of light steel galvanized perforated and nonperforated profile, which form the metal frame of the building. There are many characteristics of LSGF that have been explored for nowadays. One of the main such characteristics is corrosion resistance. Corrosion protection of light steel structures is extremely important. For the construction of steel structures, electrochemical corrosion has the main importance. Electrochemical corrosion processes take place in aqueous solutions. When the metal surface in contact with the electrolyte solution there is an interaction of the metal with charged particles of solution and the transition metal ions into the solution. It is known that stress corrosion cracking occurs under the action of tensile stresses, which cause dilation of cracks. Compressive stresses counteract cracking, causing the closure of cracks. Growth of carbon content in steel leads to growth of its resistance to stress corrosion including in environments of nitrides and hydroxides. Reducing the grain size of steel increases its resistance to cracking, which is associated with the increase of cracking of path and strength increase. In a high risk of corrosion, it is better to use solid, closed sections than lattice. Greater consumption of steel will pay off as a result of increasing physical strength of the structure. Currently, the most common method of corrosion protection is the application of coatings in a layer of paint. They concede superiority in terms of stability, plated and metal coatings, but more are available.
Contents
General55
Main body55
Corrosion of steel56
Voltage range56
Protection58
Choice of the form of steel structures59
Design of steel structures60
Covering of the layer of paint60
Conclusion61
General
Construction technology based on the light steel gauge construction is a frame technology, allowing erecting houses in a short time. The essence of this technology is using panels of light steel galvanized perforated and non-perforated profile, which form the metal frame of the building. This alternative frame construction technology with application of light steel gauge framing (LGSF) is the result of many years of close cooperation of civil engineers, architects, producers and contractors [3, 7, 66, 77]. The last years in Russia and abroad it is seen that construction used LGSF grow steady [1,15, 24, 27, 31, 39, 48, 57, 74, 75, 89]. LGSF have become widely spread construction decision of low-rise buildings, attics, cladding construction of multistories and small bridge structures [2, 38, 50, 52, 55, 76, 88]. One can identify several important advantages of the technology LGSF: effective energy-saving, low operating costs, the rapid and all-weather mounting, high productivity, wide architectural possibilities and fields of application, reliability and durability, the small weight of structures, fire resistance, ecological compatibility [1, 3, 4, 5, 28, 29, 64].
There are many characteristics of LSGF that have been explored for nowadays [68]. One of the main such characteristics is corrosion resistance [8].
Goal: learn influence temperature and moisture on the condition resistance from corrosion of the light steel gauge framing.
Main body
Corrosion protection of light steel structures made of cold-formed profiles, are extremely important. Insufficiently protected thin-walled structures can be destroyed in a short period [23, 25, 30, 41, 47, 56, 65].
The following types of corrosion are chemical and electrochemical [13, 43, 90].
For the construction of steel structures electrochemical corrosion has the main importance.
Galvanic corrosion - it is the interaction of the metal with a corrosive environment (electrolyte solution) in which the ionization of the metal atoms and restoring the oxidative part of corrosion medium do not occur in a single event and their speed depend on the electrode potential [43, 44].
It is caused by an aqueous electrolyte solution it is result of the action of trace formed on the metal surface in contact with the electrolyte.
On the basis of the theoretical data it can be concluded:
-
1. Reaction of metals can be determined more accurately than because of their interaction with acids. If two metals are electrically connected with each other at one end, and their other two ends are immersed in a conventional electrolyte, the electrons will flow from a reactive metal to a less reactive. Force controlling the flow of electrons can be measured by a voltmeter: the higher the voltage, the greater the difference between the reactivity of the two metals. This combination, known as electrochemical cell, both metals are called electrodes, one of them, which loses electrons is called the anode, and acquires them - cathode.
-
2. Since the loss of electrons means turning metal into its compounds, it follows from this that the anode corrodes and is destroyed while the cathode remains intact.
-
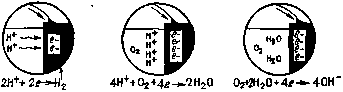
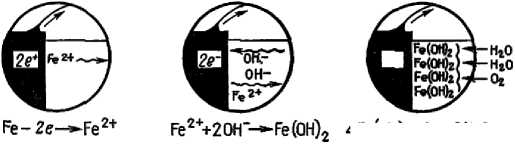
3. Step corrosion process is shown in detail in figure 1. Iron in the anode zone loses electrons. These electrons flow on metal and react with water and oxygen on the cathode with the formation of hydroxyl ions. The hydroxyl ions formed at the cathode react with the bivalent iron ions formed at the anode, the product of which is ferrous hydroxide. Further, in presence of oxygen iron hydroxide is oxidized with the formation of hydrated iron oxide that is rust [60].
-
4. As the accumulation of corrosion products at the anode and cathode, voltage is reduced. Potentials (in volts) of the anode and cathode approach each other and the corrosion rate decreases. The potential change is called polarization. The polarization of the cathode is usually greater than the anode. Hydrogen gas can isolate the cathode surface slowing even stopping the corrosion at the anode until the surface of the cathode will not be released [13, 43, 61, 63, 90].
4Fe(0H)2*02+2H50 —►
-4Fe(0H)j и 2Ре10^(Нг0)г*2Нг0
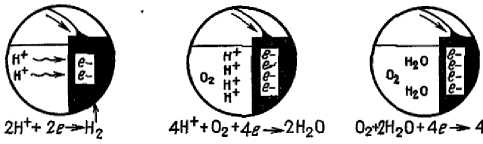
Figure 1 Process of corrosion. a - anode process, b- cathode process [60]
Corrosion of steel
The steel in normal climatic conditions corrode even without being connected electrically to another metal. Steel is heterogeneous and contains sections somewhat different in composition. At crystallite boundaries, unequal electrical potentials exist and some portions being anodic with respect to the other corrode and protect the latter, as in the case of two different metals [12, 20, 45, 60, 82].
Voltage range
Stability of the metal exposed to the corrosive factors depends on their potential difference. The farther two metals removed from each other in range and more metal potential difference between them, the stronger the electric current occurs when drop them into electrolyte, and the more corrode metal with a lower potential [61, 90]. The potential difference for some metals is given in Table 1.
Table 1 Potential difference of some elements [60]
|
Ion |
Normal potential |
Ion |
Normal potential |
|
Cu++ |
+0.34 |
Fe++ |
-0.44 |
|
Sn++ |
-0.14 |
Zn++ |
-0.76 |
|
Ne++ |
-0.24 |
Mn++ |
-1.04 |
|
Al+++ |
-1.66 |
Electrochemical corrosion processes take place in aqueous solutions. When the metal surface in contact with the electrolyte solution there is an interaction of the metal with charged particles of solution and the transition metal ions into the solution. According to the ideas of Frumkin A.N. and his school, in this case take place two coupled processes[36, 60]:
-
- transition of ions from metal into the solution with the formation a sulfated (in aqueous solution -hydrated) ions (anodic or oxidation process):
Me + mHO = Me " + ■ mHO + ne (1)
-
- the transition of these ions from the solution with their separation on the metal surface in the form of neutral atoms being th epart of the metal lattice structure (or reducing cathodic process) [3, 35, 63].
The rates of corrosion depending on the atmospheric conditions are given in Table 2.
Construction of Unique Buildings and Structures, 2014, №7 (22)
Table 2 The rate of corrosion depending on the atmospheric conditions [13]
|
Air ambient the construction |
The rate of corrosion of unprotected steel profiles, mm/year |
|
Country |
0.004 |
|
City |
0.03-0.06 |
|
Industrial |
0.04-0.106 |
|
Sea |
0.064-0.16 |
The electrolyte is the atmospheric moisture containing CO 2 , but more often other chemical compounds. Water condenses on the metal, which contains a number of salts. Small layer of electrolyte solution creates conditions conducive to electrochemical corrosion [17, 71].
In a temperate coastal climate, there are several mechanisms that lead to moisture collection in the exterior sheathing.
-
1. Rain penetration past the cladding system can wet the sheathing directly.
-
2. The combination of moisture absorbed or held in a cladding and solar radiation on the cladding can lead to high inward vapor pressure differences that can drive moisture across the weather resistive barrier into the sheathing.
-
3. In winter, humidity from the indoor environment can enter the cavity by diffusion or air movement (the dominant mechanism). This moisture will tend to collect on the materials on the cold side of the cavity—that is, the exterior sheathing.
-
4. Any water that enters the inside of the cavity by bypassing the cladding system, weather resistive barrier, and exterior sheathing (such as leaks at window penetrations) also tends to be driven to the materials on the cold side of the cavity [51,83, 85].
The chemical composition of the alloy and metallurgical methods used to obtain it also belong to the essential factors that have a major impact on the course of corrosion damage [45, 46, 72].
The requirement for lightness of structures necessitates the use of more thin-walled cells operating at high voltages. If the metal can withstand these stresses, so the protective shell of oxides formed on its surface and characterized by other mechanical and physical properties than metal, often receives local damage that can independently "heal" only with a particularly favorable conditions [30, 73].
Shell of the oxide, which, in a complete seal protects the metal from a chemical reaction with the environment when local damage becomes a factor in accelerating and focusing action in the areas of damage. As a result, despite the fact that most of the metal element remains almost intact by corrosion can quickly destroy construction due to the occurrence of deep pitting in some places.
The natural oxide shell protects the metal from the corrosive effects of the environment the better the much closer its crystal structure ii to the structure of the metal base. Corrosive centers more often appear at locations on the metal surface, where the metal structure has defects such as contamination, grain boundaries or dislocations [71].
The role that mechanical stress plays in the phenomena of corrosion of the metal is very complex and not yet fully understood. According to the unanimous opinion of many researchers, stress corrosion cracking occurs under the action of tensile stresses, which cause dilation of cracks. Compressive stresses counteract cracking, causing the closure of cracks. Voltage of torsion additionally increase the rate of corrosion cracking by several tens percent. Stress corrosion cracking moves into the material in a direction perpendicular to the direction of the tensile stress, and it may extend along the grain boundaries (more often observed in industrial environments) or inside the grains, or even be mixed [15, 50].
Growth of carbon content in steel leads to growth of its resistance to stress corrosion including in environments of nitrides and hydroxides. Reducing the grain size of steel increases its resistance to cracking, which is associated with the increase of cracking of path and strength increase [72, 73].
Mild steel or low alloy steel deoxidized with aluminum are characterized by greater resistance to stress corrosion cracking than the non-deoxidized steel, semi-killed steel or deoxidized by silicon steel. The presence of aluminum in the steel causes light steel surface passivation, a passive layer is characterized by considerable stability to mechanical damage [16, 20, 34].
Low alloy welding steel used in construction, due to the property of deoxidation, and the presence of alloying additions fine grain are more resistant to stress corrosion cracking than carbon steel [49].
Wolberg [84] obtained interesting results by conducting research in the area of the effect of atmospheric corrosion on the fracture behavior of structural steels. Investigations were carried out on the basis of impact test, static bending of samples and eccentric stretching of flat samples with a sharp change in cross-section [80].
Based on the findings we can make the following conclusion:
-
- Corrosion of the steel exerts influence on the character of damage increasing danger of appearance of cracking not only during dynamic but also during statics efforts. Except for the increase of critical temperatures of fragility, influence of corrosion causes also diminution of plastic characteristics of steel during all temperature trials.
-
- Action of the corrosion has much stronger influence on the steel of such sort that more sensible to
concentration of the efforts.
-
- The increase of critical temperature of fragility, which has been discovered on the patterns with
different changes of the section, proves that during equal disposition of atmospheric corrosion appear abrupt changes of the section.
-
- When estimation of inclination steel to cracking it is necessary to consider the character of the environment in which the construction works [84].
Protection
Steel structures protection from corrosion is achieved by the following methods:
-
- selection of appropriate alloying elements in steel (e.g., nickel, chromium, copper), whereby it acquires a property not rust;
-
- giving profile and construction the proper form;
-
- isolation of the metal surface from the corrosive agent;
-
- by full treatment as a preparatory operations for the formation of a continuous oxide film;
-
- pre-preparation, including, if required, the surfaces that are inside the gaps and clearances;
-
- providing access contained in the working environment of oxygen for the formation and maintain a
robust, protective surface film [18, 32, 54].
In our conditions, stainless steels for steel structures are used rarely because of their high cost. The designer has a great opportunity in the formation of structures, thus reducing the risk of corrosion.
Separation of the metal surface from corroding agent can be achieved by:
-
- the right choice of steel structures shape
-
- steel coating with a thin layer of protective of the metal which is resistant to the action of corrosive agents by immersion elements
-
- construction a molten metal plating, carburizing or sputtering;
-
- changing of the surface layer of the metal by chemical means,
-
- formation of oxide layer on the metal surface by the oxidation, phosphate, etc.;
-
- covering the metal surface by layer of fat or oil;
-
- covering of the metal protective sheath paint, varnish or synthetic material;
-
- covering the metal by bitumen compounds;
-
- application a protective concrete layer of 15 mm [14, 54, 62, 67, 69, 70, 87].
Let us consider the most important of these problems.
Choice of the form of steel structures
It can be said that the losses caused by corrosion is significantly greater than losses due to mechanical factors. By choosing the type of construction and designing the individual elements, the designer is guided by the terms of strength, stiffness and stability without taking into account the problems normally associated with the appropriate selection of shapes of structures and its elements that minimize the impact of corrosion [4, 5, 19, 81].
The greatest impact of corrosion is observed regardless of the environment in the construction of the coating (trusses, purlins, and connections), which usually consist of elements with thin walls.
Crane rainway beams and support, typically consisting of elements with thicker walls susceptible to corrosion with much less degree. High resistance to corrosion of supports is also due to their vertical position, hindering the deposition of dust on the design [34, 65].
The main objective when designing structural steel profiles is a selection of profile in the structure in which it is possible to be less exposed to corrosion. Therefore, an important task is to study the influence of the shape of structural elements on the corrosion resistance.
Figures showing the effects of corrosion on different types of steel sections with designation of control sample weight loss in grams on the different intervals of elements are given in figure 2.
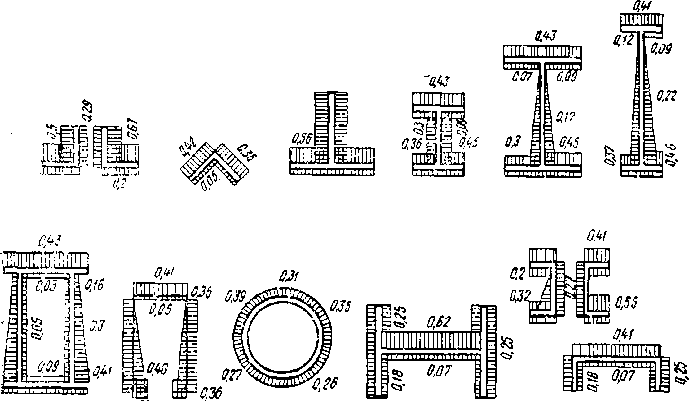
Figure 2 Effects of corrosion on different types of steel sections [13]
The experiments show that:
-
- corrosion rate depends on the form of profile;
-
- the amount of corrosion in different parts of profile is different ;
-
- he best profiles are enclosed and streamlined , with no areas that hold moisture, that is tubular profiles, two-stage box-shaped profiles or profiles with angled walls;
-
- the worst profiles are composed of two angled sections, I-beams with wide flanges, etc. [10, 34, 58].
The elements consist of paired angular profiles have very uncomfortable gap, difficult during application anticorrosion coatings, so they do not work well in harsh environments [37, 81].
In a high risk of corrosion, it is better to use solid, closed sections than lattice. Greater consumption of steel will pay off as a result of increasing physical strength of the structure.
Design of steel structures
In the project should be provided a method of corrosion protection (process instruction) depending on the corrosive environment (class) type section (open or closed), the planned durability of the project, its purpose, working conditions and the location (ease of access to the structure.) Depending on the factors mentioned above it is selected method of corrosion protection.
It should be emphasized that the number of methods of protection and its resources is growing rapidly, so the determination of the appropriate method of protection requires an appropriate expertise [19].
Covering of the layer of paint
Currently, the most common method of corrosion protection is the application of coatings in a layer of paint. They concede superiority in terms of stability, plated and metal coatings, but more are available [33, 42, 49, 62, 67].
Before covering with a protective layer, structures must be thoroughly cleaned of rust and scale, moisture and technical fats. The duration of the existence of these coatings depends on the proper preparation of the surface of the structural elements prior to application of the protective layer on them [15, 21].
Recently it is started using polyvinyl paint that is applied by spraying. This two-layer coating: base layer and a surface layer.
Steel, which are made of thin-walled profiles, must be protected from mechanical damage even during transportation and storage in a covered warehouse. Before starting making the material, it should be thoroughly cleaned of rust and other contaminants [53, 69].
In the formation of thin-walled elements, it is necessary to ensure that do not create places difficult to paint. It is impossible to construct profiles in the form of tanks, hold water. Should avoid the grooves and crevices, and the welds may be used more tightly. After making the profile at the plant, it is applied anti-corrosion protective film.
Remember that thin steel elements cannot touch building materials comprising gypsum, magnesium chloride, coal or coke ash, slag and coke, because they cause rapid corrosion of the steel. Do not put xylolite on the overlapping without proper protection of light steel structures [9, 22].
Light steel structure of hot rolled profiles protect from corrosion by painting twice [53].
Coloring should be done at suitable weather conditions: in dry and not frost period [33, 40, 70, 86].
Coating of metal layer. The best protection though the most expensive are the metallic coatings, especially hot dip galvanized.
Practice of recent years has shown that coatings formed by spraying, is not worse than coatings which made by electroplating or hot surfaces methods.
Spraying process in comparison with other techniques has the following advantages: the possibility of spraying on the finished object, adjusting the thickness of the coating and application of this method for application to elements of any shape and size, and significantly better fit of paint to the plane in the case color of spraying coating [21].
Sprayed layer contains pure zinc, thus has good anti-corrosion properties [26, 67, 70, 78]. We cannot get by hot way such a pure coatings. Atmospheric factors influence the zinc coating extremely slow.
Freshly sprayed zinc layer is quickly covered with a layer of oxides, resistant against corrosive agents. However, this layer of zinc compounds on the protective coating is quite fragile (e.g., rain washes it out). The thicker the zinc shell, the more effective it is [6, 22, 87].
The thickness of zinc coatings depending on the environment and should be equal to:
-
- 0.05 mm at low relative humidity (particularly when the protective paint coating is provided);
-
- 0.1 mm with high humidity;
-
- 0.2 mm with air pollution from industrial gases and elements, that is immersed in water.
Resistance zinc coating depends largely on the temperature. At temperatures up to 50 ° C, this resistance is quite good. at a temperature above 50 ° C decreases, and at a temperature of 60 - 65 ° C reaches a minimum. With further temperature rises resistance increases, and at 100 ° C is only slightly different from resistance at 50 ° C.
The effectiveness of the protective action of sprayed coatings significantly increases due to the damage of the protective layer of paint. Such coatings are expensive because of the additional coating and high flow rate of paint (100% more than the non-metallized surface coloration) but it pays off, as they provide a solid protection operation for many years [11, 19, 78, 79].
Covered with synthetic material . Recently, wide steel strips coated with synthetic materials are widely used. After coating, the bands are cut and profiles manufacture from them.
Covering strips with synthetic material is produced by coating them with liquid or semisolid thermoplastic or thermo-reactive materials and the finished film by gluing. The shell thickness may be from 20 to 400 microns. a protective layer is additionally superimposed on the finished coatings to protect them from damage during transportation.
Due to perfect bonding of synthetic material, as well as a good protective effect of zinc, and soil layer is no need of special protection of edges. With thicker sheet metal edges can be protected by a thick varnish and drying of pastes [32, 67].
Conclusion
The rapid growth living standards in the industrialized world changes economic situation reduced. Savings of industrial raw materials and produced energy are necessary for social reasons, and so we simply cannot afford the rising costs of dismantling and replacement of old and rusty equipment with new, especially if such a replacement can be avoided or if it is not necessary from the point of view of maintaining the desired performance [59, 66].
We live in the age of technology. Therefore, uncontrolled corrosion effect is not limited to the state of the corrosive object such corrode also has a significant impact on people, their economic and social well-being.
Список литературы Влияние коррозии на легкие стальные тонкостенные конструкции
- Aktuganov A.N., Aktuganov O.A. Protection of metals against corrosion by coating with zinc//Практика противокоррозионной защиты. 1998. № 2. С. 28-32.
- Amundarain A., Torero J., Usmani A., Al-Remal A. Light Steel Framing: Improving the Integral Design [electronic resource] System Requirements: AdobeAcrobatReader. URI: http://hdl.handle.net/1842/1409 (Issue Date: 11.09.2006)
- Basaglia C., Camotim D., Silvestre N. Post-buckling analysis of thin-walled steel frames using generalized beam theory (GBT)//Thin-Walled Structures. 2013. V. 62, Pp. 229-242
- Cheng Y., Schafer B.W. Simulation of cold-formed steel beams in local and distortional buckling with applications to the direct strength method//Journal of construction steel reaserch. Vol. 63. Issue 5. Pp.
- Chou S. M., Rhodes J. Review and compilation of experimental results on thin-walled structures//Computers & Structures. 1997. Volume 65. Issue 1. Pp. 47-67.
- Cui Yongqi, Study on hygrothermal performance of the slotted light steel-framed composite walls in cold area, Master's thesis, Harbin Institute of Technology, 2006, 90p.
- Kobus J. Corrosion protection of steel structures by protective paint systems according to EU standards//Проблемы машиностроения и автоматизации. 2007. № 4. С. 125-130
- Kosov V.P. Application of periodic current with reverse pulse in the study of the basic parameters of electrochemical shape formation//Surface Engineering and Applied Electrochemistry. 2003. № 3, Pp. 7-12
- Lawton Mark, Lessons to be learned from Performance Failures of Framed walls in High-Rise buildings, Building IX, 10p.
- Niemenen Jyri, Salonvaara Mikael, Hydrothermal performance of light steel-framed walls. Espoo 2000. Technical Research Centre of Finland, VTT Tiedotteita -Medelanden -Research Notes 2070, 26p.
- Pavlov A.B., Ayrumian E.L., Kamynin S.V., Kamenschikov N.I. Bystrovozvodimye malojetazhnye zhilye zdanija s primeneniem legkikh stalnykh tonkostennykh konstruktsij//Promyshlennoe I grazhdanskoe stroitelstvo. 2006. № 9. Pp. 51-53.
- Robert J. Light Gauge Steel Framing: Interiors//Construction Dimensions. 1990. February. Pp. 13-17
- Sedlacek G., Bild, J. Ungermann, D. On the buckling of plates -Some recent developments in light weight structures//4th international conference on aluminium weldments, Tokyo. 1988. Pp.10-17.
- Talbot, David, Corrosion science and technology, 298 p. cm.
- Werner M. Building with steel//Buildings. 2000. №2. 30p.
- Wu S., Zhang S.M., Yang H. Experimental research on thermal behavior of light gauge steel-framed composite walls with slotted studs//Harbin gongye daxue xuebao/journal of harbin institute of technology. 2010. №4. Pp. 550-555
- Zhaojun Wang, Sumei Zhang, and Yongqi Cui Study on the heat and mass transfer of the slotted light steel-framed composite wall at minimum temperature in Harbin, 6p.
- Zhaojun Wang, Yongqi Cui, Sumei Zhang Study on mass transfer of light steel-framed composite walls in cold area, Journal of Harbin Institute of Technology (Supplement), 2007. 67-71.
- Айрумян Э.Л. Особенности расчёта стальных конструкций из тонкостенных гнутых профилей//Монтажные и специальные работы в строительстве. 2008. № 3. С. 2-7.
- Айрумян Э.Л., Белый Г.И. Исследования работы стальной фермы из холодногнутых профилей с учетом их местной и общей устойчивости//Промышленное и гражданское строительство. 2010. № 5. С. 41-44.
- Айрумян Э.Л., Каменщиков Н.И. Рамные конструкции стального каркаса из оцинкованных гнутых профилей для одноэтажных зданий различного назначения//Мир строительства и недвижимости. 2006. №36. С. 9-11.
- Актуганов А.Н. Долговечность легких стальных конструкций покрытий зданий цветной металлургии. Диссертация на соиск. учен. степ. к.т.н., Спец. ВАК 05.23.01.
- Актуганов А.Н. Долговечность стальных листов покрытий зданий промышленных предприятий//Коррозия строительных конструкций и оборудования. Методы их защиты. 1982. С. 61-65.
- Альхименко А.И., Ватин Н.И., Рыбаков В.А. Технология легких стальных тонкостенных конструкций. СПб.: Изд-во СПбГПУ, 2008. 27с.
- Баенкович В. В. Лакокрасочные материалы для защиты от коррозии//Пром. окраска: Технологии. Материалы. Оборудование. 2006. № 2. С. 4-7
- Блам У., Бреннер А. Цинковое покрытие на стали//Коррозия металлов. М.-Л.: Государственное научное издательство химической литературы. 1952. С. 857-873
- Броуде З.И. Учет коррозии стали при проектировании новых и перерасчете эксплуатируемых стальных конструкций//Материалы по стальным конструкциям. № 3. 1958. С. 164-184.
- Брудка Я., Лубиньски М. Легкие стальные конструкции. М.: Стройиздат, 1974, 342 с.
- Свинарёв В.В. Коррозия. Способы борьбы с коррозией//Уфа, Уфимский гос. нефтяной технический унт Препринт. 2009. № 1
- Ватин Н.И., Жмарин Е.Н., Куражова В.Г., Усанова К.Ю. Конструирование зданий и сооружений. Лёгкие стальные тонкостенные конструкции. Изд-во Политехн. Ун-та. 2012.
- Ватин Н.И., Рыбаков В.А. Расчет металлоконструкций: седьмая степень свободы//CтройПРОФИль. 2007. №2(56). С. 60-63
- Ватин Н.И., Синельников А.С. Большепролетные надземные пешеходные переходы из легкого холодногнутого стального профиля//Строительство уникальных зданий и сооружений. 2012. № 1. С. 47-52.
- Ватин Н.И., Синельников А.С. Холодногнутый стальной профиль в малых мостовых конструкциях//Строительство уникальных зданий и сооружений. 2012. №3.
- Ведяков И.И., Одесский П.Д. Защита от коррозии стальных строительных конструкций цинкованием//Вестник Волгоградского государственного архитектурно-строительного университета. Серия: Строительство и архитектура. 2013. № 31-2 (50). С. 20-23.
- Ведяков И.И., Одесский П.Д. Защита от коррозии стальных строительных конструкций цинкованием//Вестник Волгоградского государственного архитектурно-строительного университета. Серия: Строительство и архитектура. 2013. № 31-2 (50). С. 20-23.
- Вольберг Ю.Л., Коряков А.С. Влияние агрессивных сред на несущую способность строительных металлических конструкций//Долговечность строительных конструкций на Севере. Якутск, 1981. С. 21-
- Воронов Н.М. Влияние конструктивной формы узлов фермы строительных стальных конструкций на стойкость против атмосферной коррозии. Владимир, 1958. 50 с.
- Голубев А.И. Антикоррозионная защита металлических конструкций. М.: ЦНИИПСК. 1975. 38 с.
- Голубев К.В., Федотов К.А. Проблемы использования новых технологий малоэтажного домостроения//Вестник ПНИПУ. Урбанистика. 2013. № 3(11). С. 23-30
- Гордеева А.О., Ватин Н.И. Расчетная конечно-элементная модель холодногнутого перфорированного тонкостенного стержня в программно-вычислительном комплексе SCAD Office//Инженерно-строительный журнал. 2011. №3(21). С. 36-46.
- Горохов Е.В., Брудка Я., Лубиньски М. и др. Долговечность стальных конструкций в условиях реконструкции. М.: Стройиздат, 1994. С. 224-237.
- Жиглецова С.К., Родин В.Б., Расулова Г.Е., Поломина Г.М., Холоденко В.П. Способ защиты от коррозии холодногнутого стального профиля//Строительство уникальных зданий и сооружений. 2012. № 1. С. 47-52.
- Жмарин Е.Н. Международная ассоциация легкого стального строительства//Инженерно-строительный журнал. 2009. №6.
- Жмарин Е.Н. Международная ассоциация легкого стального строительства//Строительство уникальных зданий и сооружений. 2012. №2. С. 27-30.
- Жмарин Е. Н., Рыбаков В.А. ЛСТК -инструмент для реализации программы «Доступное и комфортное жилье»//CтройПРОФИль. 2007. №7(61). С. 118-119.
- Жук Н.П. Курс теории коррозии и защиты металлов. М.: Изд. «Металлургия», 1976.
- Каталонская М.А. ЛСТК -быстрое решение для строительства доступного жилья//Строительные материалы, оборудование, технологии ХХI века. 2009. №2. С. 15-16.
- Каталонская М.А. ЛСТК -быстрое решение для строительства доступного жилья//Строительные материалы, оборудование, технологии ХХI века. 2008. №10 С. 30-32.
- Кикин А. И., Васильев А.А. Повышение долговечности металлических конструкций промышленных зданий. М.: Стройиздат, 1984. 304 с.
- Кикоть А.А., Григорьев В.В. Влияние ширины пояса и параметров стенки на эффективность стального тонкостенного холодогнутого профиля сигма-образного сечения при работе на изгиб//Инженерно-строительный журнал. 2013. №1
- Косачев В. Б. Основные принципы защиты от наружной коррозии. Лакокрасочные покрытия, рекомендуемые для защиты от наружной коррозии. Опыт эксплуатации покрытий//Новости теплоснабжения. 2006. №2. С. 37-38.
- Косов В.П., Матюшенский А.С., Косова Т.С., Клаузер В.В. Новая технология и оборудование для электрохимической защиты металлов от коррозии//Stiinta Agricola. 2007. № 2. С. 68-74.
- Кошин И.И. Экспериментальное изучение влияния конструктивной формы элементов стальных конструкций на стойкость против атмосферной коррозии. Сб.: Стальные конструкции. Труды МИСИ им. В.В.Куйбышева. 1956. С. 21-28
- Кузьменко Д.В., Ватин Н.И. Ограждающая конструкция «нулевой толщины -термопанель//Инженерностроительный журнал. 2008. № 1. С. 13-21
- Куражова В. Г., Назмеева Т. В. Виды узловых соединений в легких стальных тонкостенных конструкциях//Инженерно-строительный журнал. 2011. № 3(21). С. 47-52.
- Лалин, В. В., Рыбаков, В.А. Конечные элементы для расчета ограждающих конструкций из тонкостенных профилей//Инженерно-строительный журнал. 2011. №8(26). С. 69-80.
- Ларионов В.И., Никитин А.И., Бугров Ю.М., Животиков Е.В., Ларионов Д.В. Композиция для антикоррозионного покрытия. Патент на изобретение RUS 2152415 13.02.1996
- Липкин Я.Н., Газизова Н.Я. Устройство для протекторной защиты. Патент на полезную модель RUS 65893 16.04.2007
- Люблинский Е.Я. Электрохимическая защита от коррозии. М.: Изд. «Металлургия». 1987. С. 43.
- Малахов А.И., Жуков А.П. Основы металловедения и теории коррозии: Учебник для машиностроительных техникумов. М.: Высшая школа, 1978. 192 с.
- Манапов А.З., Майстренко И.Ю. Оценка надежности конструкции с учетом коррозионного износа.//Известия КГАСУ. 2006. №1. С. 64-73.
- Манапов А.З., Маннанов И.И. Долговечность элементов стальных конструкций, подверженных коррозии. Оптимизация, расчет и испытание металлических конструкций: Межвуз.сб. Казань: КХТИ, 1984. С. 64-67.
- Марченко Т.В., Банников Д.О. Сопоставительный анализ форм потери устойчивости тонкостенных стержневых элементов//Металлические конструкции. 2009. №3. 166p. (rus)
- Мезенцева Е.А., Лушников С.Д. Быстровозводимые здания из легких стальных конструкций//Вестник МГСУ. 2009. Спецвып. № 1. С. 62-64.
- Назмеева Т.В. Обеспечение пространственной жесткости покрытия в зданиях из ЛСТК//Инженерностроительный журнал. 2009. №6
- Недвига П.Н., Рыбаков В.А. Эмпирические методы оценки несущей способности стальных тонкостенных просечно-перфорированных балок и балок со сплошной стенкой//Инженерно-строительный журнал. 2009. №8;
- Петров С.В. Сверхзвуковое напыление в защите от коррозии стальных конструкций//Практика противокоррозионной защиты. 2004. № 4. С. 39-45
- Плудек В. Защита от коррозии на стадии проектирования. Монография. М.: Мир, 1980. 440 с.
- Рыбаков В.А. Напряженно-деформированное состояние элементов каркасных сооружений из тонкостенных стержней//Строительство уникальных зданий и сооружений. 2013. №7 (12)
- Рыбаков В.А. Основы строительной механики легких стальных тонкостенных конструкций: учебное пособие. СПб.: Изд-во Политехн. ун-та, 2011. 207 с.
- Саламахин С. В., Синельников А. С. Моделирование узла винтового соединения тонкостенных стальных перфорированных профилей методом конечных элементов//Строительство уникальных зданий и сооружений. 2013. № 4. С. 53-63.
- Свистунова Т.В. Современные методы и технологии защиты от коррозии//Металлург. 2007. № 6. С. 80-82.
- Семенова И.В., Флорианович Г.М., Хорошилов А.В. Коррозия и защита от коррозии//Moscow. 2010. №
- Семенова И.В., Флорианович Г.М., Хорошилов А.В. Коррозия и защита от коррозии. М.: ФИЗМАТЛИТ, 2002. 336 с.
- Слугачёва Е.В. Лёгкие стальные тонкостенные конструкции//Приоритетные научные направления: от теории к практике. 2013. № 5. С. 6-9
- Смазнов Д.Н. Конечноэлементное моделирование работы жестких вставок тонкостенных холодноформованных стальных профилей//Научный журнал КубГАУ. 2011. №67(03). С. 1-13
- Смирнова Е. ЛСТК: новые возможности для сельского и загородного строительства//Строительные материалы, оборудование, технологии ХХI века. 2011. №3. С. 25-30
- Сокол И. Я., Ульянин Е. А., Фельдгандлер Э. Г. и др. Структура и коррозия металлов и сплавов. М.: Металлургия, 1989. 400 с.
- Солнцев С.С. Защитные технологические покрытия и тугоплавкие эмали. М.: Машиностроение, 1984. С. 256.
- Строительство универсальной спортивной арены в ганновере -ставка на стальные конструкции с покрытием//Черные металлы. 2006. № 10. С. 78.
- Томашов, Н.Д. Теория коррозии и коррозионностойкие сплавы. М.: Металлургия, 1986. С. 359
- Федонин О.Н. Технологическое обеспечение коррозионной стойкости деталей из конструкционных сталей в условиях электрохимической коррозии//Диссертация на соискание ученой степени доктора технических наук. Брянск, 2004
- Федосов С.В., Румянцева В.Е., Федосова Н.Л., Румянцева К.Е. Антикоррозионная защита металлов в строительстве: проблемы и пути их решения//Строительство и реконструкция. 2011. № 2. С. 97-103.
- Федотов С.Д., Улыбин А.В., Шабров Н.Н., О методике определения коррозионного износа стальных конструкций//Инженерно-строительный журнал. 2013. №1
- Филимонова В.А., Харчевникова Е.О. Защита металлов от коррозии//Вологдинские чтения. 2009. №76. С. 128-129.
- Хазанов Л. Цинкование -основной способ защиты стали от коррозии//Металлург. 2011. № 12. С. 99-101.
- Целиков В.К. Коррозиеустойчивость стали и отпускная хрупкость//Сталь. 1948. №8. С. 724
- Шкрибалов А. Как защитить ЛСТК от коррозии//Строительнаяя газета. 2011. 16 мая
- Шрайера Л. Л. Коррозия. Справочник. М.: Металлургия, 1981. С. 632
- Юрченко В.В. Проектирование каркасов зданий из тонкостенных холодногнутых профилей в среде SCAD Office//Инженерный строительный журнал. 2010. №08. С. 38-46.


