Влияние различных классов танинов на метаногенез у жвачных животных (обзор)
Автор: Колесник Н.С., Боголюбова Н.В., Зеленченкова А.А.
Журнал: Сельскохозяйственная биология @agrobiology
Рубрика: Обзоры, проблемы
Статья в выпуске: 2 т.59, 2024 года.
Бесплатный доступ
К источникам парниковых газов часто относят жвачных животных, что становится актуальной экологической проблемой. Среди газов, с которыми связывают глобальное потепление, наибольшим потенциалом обладает метан (R.A. Muller с соавт., 2017). Сокращение эмиссии CН4 с кишечными газами поможет повысить эффективность использования энергии и снизить нагрузку на окружающую среду от сельского хозяйства (И.В. Петрунина с соавт., 2022). Существует несколько стратегий по снижению выбросов парниковых газов от жвачных животных, в частности через изменение рационов и использование различных кормовых добавок (Н.В. Боголюбова с соавт., 2022), включая жировые. Ненасыщенные жирные кислоты подавляют развитие метаногенов и простейших и снижают соотношение ацетат:пропионат в рубце, что приводит к уменьшению выработки метана (J.O. Zeitz с соавт., 2013). Другая многообещающая стратегией для сокращения выбросов метана жвачными - использование в качестве кормовой добавки вторичных метаболитов растений, в частности танинов, обладающих антиметаногенным потенциалом, выраженными антиоксидантными, антимикробными свойствами, а также способностью образовывать комплексы преимущественно с белками и некоторыми микроэлементами благодаря наличию большого количества фенольных гидроксильных групп (A.I. Roca-Fernández с соавт., 2020, P.R. Lima с соавт., 2019). Это высокомолекулярные полифенольные соединения, которые можно разделить на две группы - конденсированные и гидролизуемые танины (A.K. Patra с соавт., 2010). Их биологическая активность во многом зависит от химической структуры и дозировки. Гидролизуемые танины в отличие от конденсированных при высоких концентрациях обладают токсическим эффектом. Механизм их действия до конца не изучен. Одна из гипотез заключается в том, что танины воздействуют непосредственно на метаногены в рубце, изменяя проницаемость мембран некоторых микроорганизмов рубца и ингибируя их ферментативную активность (M.И. Caetano с соавт., 2019). Другая гипотеза предполагает ингибирование за счет уменьшения доступности питательных веществ для микроорганизмов рубца, что впоследствии снижает усвояемость субстрата и косвенно ингибирует микробные популяции (H.D. Naumann с соавт., 2017). Согласно еще одной гипотезе о том, как танины, а именно конденсированные танины, ингибируют CH4, они действуют как поглотитель протонов (H.D. Naumann с соавт., 2017). В настоящее время изучается влияние различных классов танинов на рубцовую микробиоту и процесс метаногенеза. Многочисленные исследования in vitro и in vivo показывают, что включение танинов в рацион жвачных животных (с растениями или в виде растительных экстрактов) приводит к снижению выделения CH4 (F. Hassanat с соавт., 2013, H.M. El-Zaiat с соавт., 2020). В некоторых работах также оценивается влияние смеси конденсированных и гидролизуемых танинов на характер ферментации в рубце (C.J. Marshall с соавт., 2022).
Гидролизуемые танины, конденсированные танины, метаногенез, микробиом рубца, жвачные животные
Короткий адрес: https://sciup.org/142242442
IDR: 142242442 | УДК: 636.2/.3:636.085.22 | DOI: 10.15389/agrobiology.2024.2.221rus
Текст обзорной статьи Влияние различных классов танинов на метаногенез у жвачных животных (обзор)
В настоящее время серьезную проблему представляет изменение климата, вызванное антропогенными выбросами парниковых газов (ПГ) (13). Агропродовольственные отрасли, такие как животноводство, — одни из ведущих антропогенных источников парниковых газов (4). Отмечается, что примерно две трети прямых выбросов ПГ связаны с животноводством (5). Во всем мире на долю животноводства приходится около 14-15 % от общего объема антропогенных выбросов ПГ, основной источник которых — кишечный метан (CН 4 ) (около 40 %) (6, 7). Более того, в европейских молочных стадах заводчики часто используют рационы, в которых содержание белка превышает реальные потребности животных. Избыток протеина в рационе увеличивает отток аммиака (NH 3 ) из рубца, который превращается в мочевину в печени и выделяется в основном с мочой (8). При хранении навоза мочевина быстро гидролизуется до NH 3 . Аммиак можно нитрифицировать до нитрата, который, в свою очередь, частично превращается в
Работа выполнена в рамках государственного задания FGGN-2022-0009.
закись азота (N 2 O) во время денитрификации (9). N 2 O является третьим по значимости парниковым газом с очень высокой степенью воздействия на глобальное потепление (8, 10, 11).
По сравнению с СО 2 атмосферы метан гораздо сильнее влияет на глобальное потепление (степень воздействия в 23 раза выше) при коротком жизненном цикле (период полураспада 8,6 года) (12), что делает метан важной мишенью в борьбе с глобальным потеплением (13-15). Также стоит отметить, что выделение CН 4 представляет собой потерю до 12 % энергии, поступающей с пищей в рубец (16-18). Сокращение выбросов кишечного CН 4 поможет повысить эффективность использования энергии организмом и снизить экологическую нагрузку на окружающую среду от сельского хозяйства (19, 20).
Во многих работах особое внимание уделяется факторам, влияющим на метаногенез в рубце, и потенциальным стратегиям снижения выбросов метана от жвачных (21-23). В последние годы достигнуты значительные успехи в понимании механизмов синтеза CH 4 в рубце, подробно описаны основные биохимические и микробиологические процессы, включая ферментацию и образование CH 4 у жвачных (24-26). На сегодняшний день опубликовано множество статей, затрагивающих темы от секвенирования генома метаногенов рубца до возможных подходов и методов, которые могут быть приняты для уменьшения выбросов ПГ (27-29).
Одна из наиболее популярных стратегий по снижению выбросов ПГ — контроль рационов и использование различных кормовых добавок (30-32). Изучено влияние ионофоров на метаногенез. Ионофоры — это высоколипофильные вещества с молекулярной массой от 500 до 2000 Да, которые способны закрепляться на липидном бислое клеточных мембран и переносить протоны (H+) и ионы металлов через мембрану в виде неспецифических ионных потоков, что в конечном итоге приводит к гибели микробной клетки (33). Однако на молочных коровах было показано, что применение ионофоров, таких как монезин, не может считаться эффективной стратегией снижения выбросов метана (34, 35). В Европейском союзе для борьбы с устойчивостью к антибиотикам в 2006 году введен запрет на использование ионофоров в качестве стимуляторов роста, что повысило спрос на альтернативные кормовые добавки, повышающие продуктивность животных и потенциально снижающие эмиссию метана (36-38).
Для сокращения выделения метана активно применяются разнообразные жировые добавки. Ненасыщенные жирные кислоты (НЖК) подавляют рост метаногенов и простейших и снижают соотношение ацетат:про-пионат в рубце, что приводит к уменьшению выработки метана (39, 40). Показано, что добавление масел (то есть полиненасыщенных жирных кислот и насыщенных жирных кислот со средней длиной цепи) в рационы жвачных снижает количество продуцентов H 2 — простейших, а также некоторых метаногенов (39, 41, 42). Кроме того, масляные добавки могут действовать как акцепторы H+ за счет биогидрирования жирных кислот, хотя этот эффект невелик (43).
В нескольких обзорах сообщается, что использование пищевых жирных кислот (ЖК), в частности ЖК из льняного семени, может стать одной из перспективных стратегий по снижению выбросов CH4 жвачными животными (44, 45). M. Doreau с соавт. (46) указывают, что добавление льняного семени в рационы дойных коров ведет к улучшению питательной ценности молока за счет небольшого увеличения количества линоленовой кислоты. Степень влияния добавок на основе льняного семени на эмиссию метана варьируется и зависит от концентрации. Например, при содержании ЖК менее 2 % у дойных коров не наблюдается снижения выделения метана (47). C. Martin с соавт. (48) показали дозозависимый эффект добавки экструдированного льняного семени на выделение метана. Наблюдалось линейное снижение содержания CH4 на 1 кг потребления сухого вещества (СВ) (от -7 до -37 % по сравнению с контрольным рационом) с увеличением количества льняного масла в качестве добавки (48).
Источниками жира в рационах жвачных животных могут быть продукты с различным жирно-кислотным составом (32). Так, G. Fiorentini с соавт. (49) оценили влияние липидов с разным профилем жирных кислот на потребление, производительность и выделение кишечного СН 4 бычками породы Nellore. Выделение кишечного метана (г/кг СВ) снижалось в среднем на 30 % при использовании рационов, содержащих соевые бобы, льняное и пальмовое масло (p < 0,05), при этом энергетические потери (в виде метана) также были ниже, чем при использовании рационов без добавок жира и с добавлением защищенного жира (3,3 против 4,7 %) (49).
В аналогичном исследовании на бычках Nellore в условиях выпаса, получавших дополнительно льняное масло, выбросы CH 4 снижались на 38 %, причем источники липидов не влияли на прибавку массы животных (50). Также сообщается, что льняное семя в сочетании с нитратами может оказывать долгосрочный эффект снижения содержания CH 4 у молочных коров: - 47 % (г/сут); - 30 % (г/кг сухого вещества); - 33 % (г/кг молока, с поправкой на содержание жира и белка), при этом молочные продукты от коров, получающих нитраты, по содержанию остаточных нитратов и нитритов безопасны (51). В нескольких исследованиях показано, что соевые липиды эффективно снижают выбросы метана как в условиях откормочных станций, так и на пастбище (52-54).
Еще одна перспективная стратегия снижения выбросов ПГ жвачными животными — использование добавок на основе вторичных метаболитов растений (эфирные масла, танины, сапонины, флавоноиды и др.), которые обладают антиметаногенным потенциалом (55). Эти метаболиты в основном характеризуются как модуляторы состава и функциональной активности микробных популяций, изменяющие процесс ферментации в рубце и биогидрогенизацию жирных кислот (56-58). Показано, что различные эфирные масла (например, полученные из чеснока, тимьяна, эвкалипта, орегано, корицы и ревеня) снижают выработку CH 4 in vitro, но очень немногие соединения обладают долгосрочным антиметаногенным действием in vivo (59). Помимо антиметаногенного эффекта такие соединения, как танины и сапонины, защищают организм жвачных животных от окислительного стресса, что способствует улучшению продуктивности и качества мясной продукции (60, 61). Защищая кормовой белок от чрезмерного брожения в рубце, танины увеличивают показатели роста и надои молока, повышают фертильность и устойчивость животных к некоторым кишечным паразитам (62). Дополнительные преимущества танинов — предотвращение вздутия живота за счет способности снижать устойчивость пены, которая улавливает газы брожения в рубце, и противопаразитарное действие (62-64).
Мы провели анализ сообщений о влиянии вторичных метаболитов растений на метаногенез у жвачных животных, уделив внимание танинам и механизму их действия. В настоящий обзор вошли публикации за последние 10 лет. Приведены результаты исследований in vitro и in vivo по влиянию конденсированных и гидролизуемых танинов, а также их совместного действия на метанообразование в организме крупного рогатого скота и мелких жвачных животных.
Химия танинов. Танины представляют собой сложные водорас- творимые полифенолы растительного происхождения с относительно высокой молекулярной массой (от 500 до 20000 Да) (65-67). Эти соединения обладают выраженными антиоксидантными, антимикробными свойствами, а также способностью образовывать комплексы преимущественно с белками и некоторыми микроэлементами благодаря наличию большого числа фенольных гидроксильных групп (68, 69). Танины встречаются во многих важ- ных кормовых деревьях, кустарниках и бобовых, фруктах, злаках и зерне.
Танины можно разделить на две группы: гидролизуемые таннины (ГТ) и конденсированные танины (КТ) (рис. 1) (70-72).
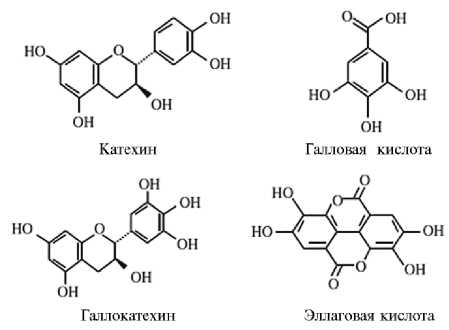
Рис. 1. Мономерные звенья конденсированных (катехин и галлокатехин) и гидролизуемых дубильных веществ (галловая и эллаговая кислоты) (70) .
Гидролизуемые танины представляют собой сложные молекулы с полиолом в качестве центрального ядра, обычно глюкозой, но роль центральной молекулы также могут играть глюцитол, хамамелоза, шикимовая или хинная кислоты, которые частично или полностью этерифицированы галловой кислотой (70, 73, 74). Структура ГТ может значительно усложняться за счет добавления следующих галлоильных групп, внутримолекулярного окислительного свя- зывания галлоильных групп замещенного полиольного ядра, раскрытия кольца глюкозного ядра и оли- гомеризации полученных соединений посредством межмолекулярного окислительного связывания (73). ГТ гидролизуются кислотами, основаниями или эстеразами (например, танназой) с образованием полиола и составляющих его фенольных кислот (70, 75).
На основании структурных особенностей молекул, которые обра- зуются при этих химических превращениях, ГТ можно разделить на три основных подкласса: галлоглюкозы, галлотанины и эллагитанины. Гал-логлюкозы представляют собой молекулы глюкозы, в которых по крайней мере одна гидроксильная группа глюкозы этерифицирована галловой кислотой. Распространенным представителем этого подкласса является 1,2,3,4,6-пентагаллоглюкоза (ПГГ). Звенья галловой кислоты могут быть
Рис. 2. Структурная формула галлотанина (75) . добавлены к существующим галлоильным группам галлоглюкозы, что приводит к образованию галлотанинов. Классический пример галлотанина — дубильная кислота (рис. 2) (73, 75).
При внутримолекулярном окислительном связывании происходит димеризация заместителей галловой кислоты с образованием фрагментов эллаговой кислоты.
Конденсированные танины, также называемые проантоцианиди-нами, представляют собой олигомеры или полимеры, образующиеся в результате конденсации двух или более звеньев 2-фенил-3,4-дигидро-2Н-хро-мен-3-ола (флаван-3-ола). Субъединицы флаван-3-ола, обнаруживаемые в КТ, различны, но обычно встречаются катехин, эпикатехин, галлокатехин и эпигаллокатехин (74, 76). Структурно проантоцианидины могут отличаться друг от друга по числу и положению гидроксильных групп, связанных с ароматическими кольцами (или B-кольцом); стереохимии гетероцикла флавонола (или C-кольца); типу связи между различными звеньями (рис. 3).
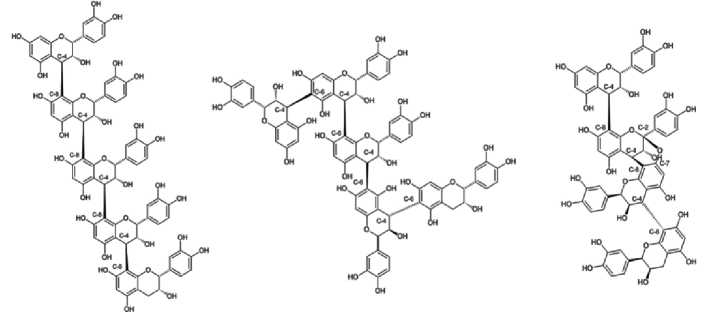
Рис. 3. Пример различных типов межфлавановых связей в конденсированных таниновых олигомерах и полимерах (73) .
В соответствии с числом и положением гидроксильных групп можно выделить три основных типа проантоцианидинов: пропеларгони-дины (имеют только одну гидроксильную группу), процианидины (с двумя гидроксильными группами) и продельфинидины (с тремя гидроксильными группами). В кислой среде они гидролизуются с образованием соответствующих антоцианов (76, 77). Стоит также отметить, что высокомолекулярные КТ нерастворимы.
Хотя у жвачных танины, особенно гидролизуемые, могут вызывать токсическую реакцию при чрезмерном употреблении, в низких или умеренных дозах они оказывают благоприятное воздействие. Для жвачных животных конденсированные танины обычно нетоксичны, поскольку не всасываются, но они могут необратимо связывать часть питательных веществ, делая их недоступными (78).
Механизм действия. Известно множество факторов, влияющих на активность и эффективность танинов, что затрудняет однозначный вывод об их практическом применении. Сложная химическая структура танинов обусловливает огромную вариабельность биологической активности даже для молекул одной и той же группы — гидролизуемых или конденсированных танинов. Часто сообщается о дозозависимом влиянии танинов на метаболизм рубца. Кроме того, эффект кормовых танинов может варьироваться в зависимости от основного рациона животных.
Механизм, с помощью которого танины влияют на метаногенез и снижают выработку метана у жвачных животных, недостаточно изучен. Существует множество гипотез о том, как эти вещества ингибируют процесс метаногенеза, но ни одна из них не доказана окончательно (73). Известно, что действие танинов на рубцовую микробиоту изменяется в зависимости от типа танина и видов микроорганизмов, при этом гидролизуемые танины считаются более подверженными микробному гидролизу (66, 79).
Одна из гипотез заключается в том, что танины воздействуют непосредственно на метаногены в рубце (73, 80). В работе F. Ng с соавт. (81) сообщалось о существовании адгезина, вероятно, расположенного на кончиках фимбрий, которые способствуют симбиозу метаногена и простейших. Части клеточной оболочки, включая клеточную мембрану, стенку и глико-каликс, также содержат этот белок. Возможно, что КТ связываются с этим адгезином или частями клеточной оболочки, препятствуя образованию комплексов метаногенов с простейшими и уменьшая межвидовой перенос H 2 (81). Подавление таких симбиотических отношений может негативно сказаться на популяции ресничных простейших — инфузорий. R. Bhatta с со-авт. (82) определили, что обилие ресничных простейших в рубце уменьшалось, когда корм содержал 26 и 13,8 % КТ соответственно из Ficus bengal-ensis L. и Azardirachta indica A. Juss. В исследовании in vivo на овцах было сделано предположение, что КТ непосредственно ингибируют некоторые грамположительные фибролитические бактерии рубца ( Fibrobacter succinogenes , Ruminococcus albus , Ruminococcus flavefaciens , Butyrivrio proteoclasticus ) (83).
S.A. Salami с соавт. (79) включали 4 % КT ( Mimosa pudica L., Uncaria gambir W.Hunter) или ГT ( Castanea sp., Caesalpinia spinosa Molina, Kuntze) в рацион ягнят и не наблюдали разницы в абсолютном числе бактерий и грибов, в то время как численность метаногенов уменьшалась на 12 % одинаково для обоих типов танинов. В недавнем исследовании in vitro при использовании танинов каштана (ГT) наблюдалось снижение эмиссии метана на 12,5 % по сравнению с контролем, а продукция ацетата увеличилась (84). В работе F. Mannelli с соавт. (85) продемонстрирована важность химической структуры танинов и синергического эффекта всех компонентов для прямого ингибирования метаногенов наряду с активностью других микроорганизмов рубца.
Еще одна гипотеза состоит в том, что танины уменьшают доступность питательных веществ для микроорганизмов рубца, что снижает усвояемость субстрата и косвенно ингибирует микробные популяции рубца (73). КТ связываются с катионами металлов и органическими молекулами (белки, углеводы, липиды), и эти комплексы, возможно, становятся недоступными в качестве субстрата для рубцовой микробиоты (86, 87).
Высокая молекулярная масса и полифенольная природа танинов также приводят к образованию комплексов с микробными ферментами. Проявляемая активность может вызывать ингибирование целлюлолитических или протеолитических бактерий или метаногенов. Было показано, что конденсированные танины сильнее связываются с питательными веществами, чем гидролизованные, поскольку имеют более высокую степень полимеризации, что затрудняет их разложение в рубцовой среде (1). Напротив, сообщалось, что ГТ обладают большей способностью к осаждению белка, что связано с более высокой биологической активностью по сравнению с КТ. Кроме того, активность ГТ может быть усилена прямой токсической метаногенной активностью, проявляемой фракциями ГТ, которые образуются в результате разложения ГТ ферментом микроорганизмов рубца — танназой (1, 78). Конденсированные танины оказывают прямое ингибирующее действие на гемицеллюлазы, эндоглюканазы и протеолитические ферменты некоторых микроорганизмов рубца, таких как Fibrobacter succinogenes, Butyrivibrio, Fibrisolvens, Ruminobacter amylophilus и Streptococcus bovis. И наоборот, Prevotella ruminicola может противодействовать негативному влиянию танинов благодаря синтезу защитного внеклеточного мате- риала. Более того, в случае ГТ некоторые микроорганизмы, относящиеся как к бактериям, так и к грибам, способны полностью разлагать галловую кислоту (основной компонент ГТ) до ацетата и бутирата (75).
Также высказывается предположение, что конденсированные танины ингибируют продукцию CH 4 , действуя как поглотители протонов. Сообщалось о снижении выработки метана в жидкой среде рубца in vitro, которое происходит линейно с добавлением флаван-3-ол катехина. В этом эксперименте на молекулу катехина приходилось до шести захваченных атомов водорода, а выработка CH 4 снижалась со скоростью 1,2 моль CH 4 /моль катехина. Авторы охарактеризовали свойства продуктов метаболизма катехина, действующего как акцептор протонов (73).
Таким образом, действие танинов на биоту рубца может быть как негативным, так и благоприятным в зависимости от состава рациона, вида животных, источника танинов и их дозировки (88-90). Танины могут оказывать токсическое действие на некоторые микроорганизмы рубца, изменяя проницаемость мембран, а также ингибировать ферментативную активность. Однако токсический эффект сильно зависит от дозы, природы танинов и вида бактерий.
Влияние танинов на метаногенез в организме жвачных. В разных исследованиях in vitro было показано, что включение танинов в рацион жвачных животных (в виде растений или растительных экстрактов) приводит к снижению содержания CH 4 . F. Hassanat с соавт. (91) продемонстрировали, что при добавлении экстрактов ГТ из каштана (37,8 г/кг СВ) или валонеи (35,6 г/кг СВ) снижение эмиссии CH 4 in vitro достигает 40 %. A. Jayanegara с соавт. (78), изучая влияние очищенных ГТ каштана и сумаха и КТ мимозы и квебрачо на выработку метана и ферментацию в рубце, а также структуру микробной популяции, обнаружили, что все танины уменьшали концентрацию CH 4 в рубце, снижение описывалось линейной или квадратичной зависимостью, причем его величина была больше для ГТ, чем для КТ, и коррелировала с их способностью к осаждению белка. В другой работе (55) было продемонстрировано, что разные концентрации КТ значительно влияли на ферментацию в рубце in vitro и снижение выработки метана у коров, получавших в составе рационов траву и бобовые культуры в соотношении 50:50. Однако авторы уточняют, что использование растений с высоким содержанием танинов чревато снижением усвояемости кормов (55).
В исследованиях in vitro достаточно постоянный и повторяемый эффект снижения эмиссии CH 4 показали листья лещины ( Corylus avellana L.), которые богаты различными полифенолами из группы конденсированных танинов (КТ) (71, 92). В дальнейшем M. Terranova с соавт. (93) оценили влияние добавки листьев лещины на эмиссию парниковых газов и молочную продуктивность коров. Авторы отобрали 12 бурых швицких коров и 8 голштинских коров массой 711±50 кг (2-7-я лактации, удой 23,4±4,7 кг/сут). В этом эксперименте выбросы CH 4 существенно снижались (p < 0,001) с увеличением доли лещины в рационе (93). В целом схожие результаты были получены S. Wang с соавт. (94) в эксперименте на овцах с аналогичной долей лещины в рационе. Однако неясно, вызвано ли наблюдаемое снижение количества CH 4 эффектом танинов из листьев лещины, одревесневшим волокном или и тем, и другим.
C.J. Marshall с соавт. (66) оценивали влияние кормления телок смесью конденсированных и гидролизуемых танинов на характер ферментации в рубце, содержание азота мочевины крови (АМК) и аминокислотный профиль. В перекрестном эксперименте на четырех телках (голштино-фризская порода ½ джерсейская порода, средняя живая масса 319,5±8,38 кг) смесь танинов снижала выработку неглюкогенных летучих жирных кислот (ЛЖК, на 2 %), соотношение ацетата и пропионата (на 13 %) и долю специфичных метаногенов — архей Methanobrevibacter и Methanosarcina.
H.M. El-Zaiat с соавт. (95) в исследовании in vitro на овцах установили, что добавление в рацион Leucaena leucocephala Lam., Atriplex halimus L. или Acacia saligna Labill. (50:50), богатых танинами, снижало выделение CH 4 почти на 23 % по сравнению с контрольной группой. В условиях in vivo у овец наблюдалось снижение продукции CH 4 на 11,45 % (95). J.C. Ku-Vera с соавт. (96) подтвердили, что скармливание L. leucocephala (до 30-35 % сухого вещества) способствует уменьшению выделения CH 4 . A.T. Piceiro-Vazques с соавт. (97) оценили скармливание L. leucocephala телкам и установили снижение образования CH 4 на 61 % при дозе добавки 800 г/кг сухого вещества. Схожие результаты получили M.D. Montoya-Flores с соавт. (98).
T.M. Denninger с соавт. (99) изучали влияние конденсированных танинов из акации Acacia mearnsi (De Wild.) на эмиссию метана и молочную продуктивность КРС. Двадцать лактирующих бурых швицких молочных коров были разделены на две группы по 10 гол. с высокой и низкой эмиссией метана (средняя разница — 0,10 от общего количества). Животных помещали в дыхательные камеры на 4 сут. Сначала (0-е сут) их кормили контрольным рационом, дополненным травяными гранулами и концентратами. Затем травяные гранулы заменили на гранулы, содержащие 141 г акации на 1 кг, что обеспечило 30 г акации/кг сухого вещества рациона (1-е-3-и сут). Выбросы метана измеряли каждые 10 мин, для анализа некоторых жирных кислот в ежесуточных пробах молока использовали газовую хроматографию. Было обнаружено, что выработка метана значительно снизилась через 20 мин после начала приема добавок, при этом продукция метана (г/сут) и выход метана (г/кг потребления сухого вещества) уменьшались линейно с 0-х по 3-и сут. Кроме того, изменения пропорций различных жирных кислот молока произошли в течение 3 сут скармливания рациона с 30 г ака-ции/кг сухого вещества (99).
K. Yang с соавт. (100) изучали влияние гидролизуемых танинов (на примере дубильной кислоты) на эмиссию метана у мясного скота. Добавление дубильной кислоты в рацион в количестве 6,5; 13,0 или 26,0 г/кг СВ снизило (p < 0,01) выработку CH 4 (л/кг потребления СВ) соответственно на 11,1; 14,7 и 33,6 %. Добавление ГТ в количестве 13,0 или 26,0 г/кг СВ снижало соотношение ацетата и пропионата, а также ацетата и аммонийного азота (N-NH 3 ) (p < 0,05), отмечалась тенденция к снижению общей концентрации летучих жирных кислот (ЛЖК) в рубцовой жидкости (p = 0,07). Также показано, что добавка дубильной кислоты в количестве 26,0 г/кг СВ снижала обилие простейших, метаногенов и Ruminococcus albus относительно общей микробной численности (по общей бактериальной 16S рДНК рубца) у мясного скота (p < 0,05) (100).
-
H. Liu с соавт. (101) исследовали влияние гидролизуемых танинов каштана (ТК) и кокосового масла (КМ) на показатели роста, выделение метана, ферментацию в рубце и микробные популяции у овец. В эксперименте 48 овец Rideau Arcott (средняя масса 31,5±1,97 кг, возраст 16 нед) были случайным образом распределены на 6 групп (8 гол. в группе) по факторному плану 3½2, с ТК и КМ в качестве добавок к рациону. Схема кормления включала контрольный рацион, 10 или 30 г ТК/кг рациона (ТК10 и ТК30), 25 г КМ/кг концентрата (КМ25) и 10 или 30 г ТК/кг рациона + 25 г КМ/кг концентрата (ТК10КМ25 и ТК30КМ25). После пробного кормления 228
(60 сут) всех овец переводили в дыхательные камеры для измерения эмиссии CH 4 . Добавление танинов не оказало существенного влияния на показатели роста овец, но снизило выброс CH 4 и значительно уменьшило популяции метаногенов и простейших.
Для определения эффективности комбинаций с различным содержанием танина на мелких жвачных провели эксперимент на 36 ягнятах породы Malpura на откорме. Животных разделили на три группы (Т1, Т2 и Т3) по 12 гол. в каждой и скармливали вволю полноценные рационы, состоящие из 65, 30 и 5 частей соответственно концентрата, грубых кормов и патоки. В качестве грубых кормов добавляли 15 частей сена Vigna sinensis L. и 15 частей листьев Ziziphus nummularia Burm. f. в T1, 15 частей сена V. sinensis и 15 частей листьев Acacia nilotica L. в T2, 15 частей листьев Z. nummularia и 15 частей листьев A. nilotica в T3. Содержание общего танина в группах Т1, Т2 и Т3 составило соответственно 0,78; 2,37 и 2,9 г/100 г СВ. Потребление сухого вещества снижалось (p < 0,05) при увеличении дозы танинов в рационе. Усвояемость питательных веществ и баланс азота были лучше у ягнят из группы Т2 по сравнению с Т3. Однако общая продукция летучих жирных кислот и численность популяций метаногенов и простейших были ниже в группах Т2 и Т3 по сравнению с группой Т1. Потеря метаболической энергии через метан была на 3,1 и 1,8 % ниже в группах Т2 и Т3, чем в группе Т1. Среднесуточный прирост и коэффициент конверсии корма значительно улучшились в группах Т2 и Т3 по сравнению с Т1 (102). Однако, исходя из данных R.N.S. Torres с соавт. (103), из-за отрицательного влияния на массу туши танины не следует скармливать ягнятам до 3-месячного возраста.
S.L. Ibrahim с соавт. (104) оценили влияние добавок, инкапсулированных и не инкапсулированных танинов мимозы ( Acacia mearnsii De Wild.) на показатели роста, усвояемость кормов, эмиссию CH 4 и ферментацию в рубце у южноафриканских мериносовых баранов. A. mearnsii , (мимоза, или черная акация) служит богатым источником конденсированных и гидролизуемых танинов. Показано, что неинкапсулированный танин и танин, инкапсулированный в подсолнечном масле, в сочетании с увеличением потребления сухого вещества корма (на 20 г/кг) можно использовать для снижения выбросов метана CH 4 . Однако инкапсулированный танин, не влияя на усвояемость корма, уменьшал выделение CH 4 в большей степени, чем такая же доза танина без инкапсуляции (104).
Необычное исследование провели F.E.F. Febres с соавт. (105). Его цель состояла в оценке влияния экстрактов танина из коры каштана ( Castanea spp.) на селективность, потребление органических веществ и сухого вещества, увеличение массы и эмиссию метана у лам. В эксперименте использовали 20 молодых самок лам (возраст 4,1±0,34 года) со средней живой массой 99,2±5,21 кг в начале опыта. Животных разделили на контрольную и тестовую группы. В тестовой группе животные получали экстракт коры каштана (доза танина 12 г/сут) перорально перед выпасом. Селективность рационов в обеих группах была одинаковой (p > 0,05) при потреблении 91,5 % трав и злаковых, 84,5 % листьев и стеблей и 61,5 % зеленой массы. Потребление корма в контрольной группе составляло 1614,64 г/сут, в группе, получавшей танин, — 1624,70 г/ сут (p > 0,05). Прибавка живой массы на 31-е сут оказалась одинаковой в обеих группах (соответственно 5,7 и 5,2 кг в контроле и опыте), выбросы метана составили 27,35±1,573 и 19,32±1,057 г/сут (p < 0,001) (105).
Итак, использование танинов в кормлении — это перспективная стратегия снижения выбросов метана как от крупного рогатого скота, так и от мелких жвачных животных. Танины — обширный класс высокомолекулярных полифенольных соединений растительного происхождения. И конденсированные, и гидролизуемые танины обладают выраженными ан-тиметаногенными свойствами, но их биологическая активность зависит от химической структуры, размеров молекулы, а также дозировки и вида микроорганизмов. Механизм действия танинов в настоящее время недостаточно изучен. Предполагается, что они изменяют проницаемость мембран и могут ингибировать ферментативную активность микроорганизмов рубца. Конденсированные танины также могут действовать как поглотители протонов. Считается, что гидролизуемые танины обладают более высокой биологической активностью, хотя их токсичность и подверженность микробному гидролизу выше. Из-за низкой токсичности конденсированных танинов их чаще используют в качестве кормовой добавки, снижающей выделение метана жвачными, но есть данные о влиянии как гидролизуемых, так и конденсированных танинов на метаногенез. Более того, в ряде исследований отмечается синергизм танинов разной природы. Однако объем накопленных знаний все же недостаточен для разработки рекомендаций по практическому применению танинов в животноводстве. Требуется уточнение механизма действия танинов различной природы на рубцовую микробиоту, необходимо изучить эффект синергизма конденсированных и гидролизуемых танинов, определить дозировки действующих веществ. Влияние танинов на метаногенез следует оценивать методами in vivo.
Список литературы Влияние различных классов танинов на метаногенез у жвачных животных (обзор)
- Cardoso-Gutierrez E., Aranda-Aguirre E., Robles-Jimenez L.E., Castelán-Ortega O.A., Chay-Canul A.J., Foggi G., Angeles-Hernandez J.C., Vargas-Bello-Pérez E., González-Ronquillo M. Effect of tannins from tropical plants on methane production from ruminants: a systematic review. Veterinary and Animal Science, 2021, 14: 100214 (doi: 10.1016/j.vas.2021.100214).
- Cardona-Iglesias J.L., Mahecha-Ledesma L., Angulo-Arizala J. Arbustivas forrajeras y ácidos grasos: estrategias para disminuir la producción de metano entérico en bovinos. Agronomía Mesoamericana, 2017, 28(1): 273-288 (doi: 10.15517/am.v28i1.21466).
- Eugène M., Klumpp K., Sauvant D. Methane mitigating options with forages fed to ruminants. Grass and Forage Science, 2021, 76(2): 196-204 (doi: 10.1111/gfs.12540).
- Audsley E., Wilkinson M. What is the potential for reducing national greenhouse gas emissions from crop and livestock production systems? Journal of Cleaner Production, 2014, 73: 263-268 (doi: 10.1016/j.jclepro.2014.01.066).
- Slade E.M., Riutta T., Roslin T., Tuomisto H.L. The role of dung beetles in reducing greenhouse gas emissions from cattle farming. Scientific Reports, 2016, 6(1): 1-9 (doi: 10.1038/srep18140).
- Gerber P.J., Hristov A.N., Henderson B., Makkar H., Oh J., Lee C., Meinen R., Montes F., Ott T., Firkins J., Rotz A., Dell C., Adesogan A.T., Yang W. Z., Tricarico J.M., Kebreab E., Waghorn G., Dijkstra J., Oosting S. Technical options for the mitigation of direct methane and nitrous oxide emissions from livestock: a review. Animal, 2013, 7(s2): 220-234 (doi: 10.1017/S1751731113000876).
- Vargas J., Ungerfeld E., Muñoz C., DiLorenzo N. Feeding strategies to mitigate enteric methane emission from ruminants in grassland systems. Animals, 2022, 12(9): 1132 (doi: 10.3390/ani12091132).
- Focant M., Froidmont E., Archambeau Q., Van Q.D., Larondelle Y. The effect of oak tannin (Quercus robur) and hops (Humulus lupulus) on dietary nitrogen efficiency, methane emission, and milk fatty acid composition of dairy cows fed a low-protein diet including linseed. Journal of Dairy Science, 2019, 102(2): 1144-1159 (doi: 10.3168/jds.2018-15479).
- Eckard R.J., Grainger C., de Klein C.A.M. Options for the abatement of methane and nitrous oxide from ruminant production: a review. Livestock Science, 2010, 130(1-3): 47-56 (doi: 10.1016/j.livsci.2010.02.010).
- Dijkstra J., Oenema O., Van Groenigen J.W., Spek J.W., van Vuuren A.M., Bannink A. Diet effects on urine composition of cattle and N2O emissions. Animal, 2013, 7(s2): 292-302 (doi: 10.1017/S1751731113000578).
- Федоров Ю.А., Сухоруков В.В., Трубник Р.Г. Аналитический обзор: эмиссия и поглощение парниковых газов почвами. Экологические проблемы. Антропогенная трансформация природной среды, 2021, 7(1): 6-34 (doi: 10.17072/2410-8553-2021-1-6-34).
- Muller R.A., Muller E.A. Fugitive methane and the role of atmospheric half-life. Geoinformatics & Geostatistics: An Overview, 2017, 5(3): 1-7 (doi: 10.4172/2327-4581.1000162).
- Ugbogu E.A., Elghandour M.M.M.Y., Ikpeazu V.O., Buendía G.R., Molina O.M., Arunsi U.O., Okezie E., Salem A.Z.M. The potential impacts of dietary plant natural products on the sustainable mitigation of methane emission from livestock farming. Journal of Cleaner Production, 2019, 213: 915-925 (doi: 10.1016/j.jclepro.2018.12.233).
- Елисеев А.В. Глобальный цикл метана: обзор. Фундаментальная и прикладная климатология, 2018, 1: 52-70 (doi: 10.21513/2410-8758-2018-1-52-70).
- Rira M., Chentli A., Boufenera S., Bousseboua H. Effects of plants containing secondary metabolites on ruminal methanogenesis of sheep in vitro. Energy Procedia, 2015, 74: 15-24 (doi: 10.1016/j.egypro.2015.07.513).
- Dong L.F., Ferris C.P., McDowell D.A., Yan T. Effects of diet forage proportion on maintenance energy requirement and the efficiency of metabolizable energy use for lactation by lactating dairy cows. Journal of Dairy Science, 2015, 98(12): 8846-8855 (doi: 10.3168/jds.2015-9465).
- Hristov A.N., Oh J., Giallongo F., Frederick T.W., Harper M.T., Weeks H.L., Branco A.F., Moate P.J., Deighton M.H., Williams S.R.O., Kindermann M., Duval S. An inhibitor persistently decreased enteric methane emission from dairy cows with no negative effect on milk production. Proceedings of the National Academy of Sciences, 2015, 112(34): 10663-10668 (doi: 10.1073/pnas.1504124112).
- Bhatta R., Uyeno Y., Tajima K., Takenaka A., Yabumoto Y., Nonaka I., Enishi O., Kurihara M. Difference in the nature of tannins on in vitro ruminal methane and volatile fatty acid production and on methanogenic archaea and protozoal populations. Journal of Dairy Science, 2009, 92(11): 5512-5522 (doi: 10.3168/jds.2008-1441).
- Dong L., Li B., Diao Q. Effects of dietary forage proportion on feed intake, growth performance, nutrient digestibility, and enteric methane emissions of Holstein heifers at various growth stages. Animals, 2019, 9(10): 725 (doi: 10.3390/ani9100725).
- Петрунина И.В., Горбунова Н.А. Системные меры по снижению выбросов парниковых газов в животноводческих хозяйствах. Обзор. Пищевые системы, 2022, 5(3): 202-211 (doi: 10.21323/2618-9771-2022-5-3-202-211).
- Henderson G., Cox F., Ganesh S., Jonker A., Young W., Janssen P.H. Rumen microbial community composition varies with diet and host, but a core microbiome is found across a wide geographical range. Scientific Reports, 2015, 5(1): 14567 (doi: 10.1038/srep14567).
- Henderson G., Cook G.M., Ronimus R.S. Enzyme- and gene-based approaches for developing methanogen-specific compounds to control ruminant methane emissions: a review. Animal Production Science, 2016, 58(6): 1017-1026 (doi: 10.1071/AN15757).
- Seshadri R., Leahy S.C., Attwood G.T., Teh K.H., Lambie S.C., Cookson A.L, Eloe-Fadrosh E.A., Pavlopoulos G.A., Hadjithomas M., Varghese N.J., Paez-Espino D., Hungate1000 project collaborators, Perry R., Henderson G., Creevey C.J., Terrapon N., Lapebie P., Drula E., Lombard V., Rubin E., Kyrpides N.C., Henrissat B., Woyke T., Ivanova N.N., Kelly W.J. Cultivation and sequencing of rumen microbiome members from the Hungate1000 Collection. Nature biotechnology, 2018, 36(4): 359-367 (doi: 10.1038/nbt.4110).
- Ungerfeld E.M. Metabolic hydrogen flows in rumen fermentation: principles and possibilities of interventions. Frontiers in Microbiology, 2020, 11: 589 (doi: 10.3389/fmicb.2020.00589).
- Morgavi D.P., Forano E., Martin C., Newbold C.J. Microbial ecosystem and methanogenesis in ruminants. Animal, 2010, 4(7): 1024-1036 (doi: 10.1017/S1751731110000546).
- Beauchemin K.A., Ungerfeld E.M., Eckard R.J., Wang M. Review: Fifty years of research on rumen methanogenesis: Lessons learned and future challenges for mitigation. Animal, 2020, 14(S1): s2-s16 (doi: 10.1017/S1751731119003100).
- Eckard R.J., Clark H. Potential solutions to the major greenhouse-gas issues facing Australasian dairy farming. Animal Production Science, 2018, 60(1): 10-16 (doi: 10.1071/AN18574).
- Arndt C., Hristov A.N., Price W.J., McClelland S.C., Pelaez A.M., Cueva S.F., Dijkstra J., Bannink A., Bayat A.R., Crompton L.A., Eugène M.A., Enahoro D., Kebreab E., Kreuzer M., McGee M., Martin C., Newbold C.J., Reynolds C.K., Schwarm A., Shingfield K.J., Veneman J.B., Yáñez-Ruiz D.R., Yu Z. Full adoption of the most effective strategies to mitigate methane emissions by ruminants can help meet the 1.5 C target by 2030 but not 2050. Proceedings of the National Academy of Sciences, 2022, 119(20): e2111294119 (doi: 10.1073/pnas.2111294119).
- Hristov A.N., Oh J., Firkins J.L., Dijkstra J., Kebreab E., Waghorn G., Makkar H.P.S., Adesogan A.T., Yang W., Lee C., Gerber P.J., Henderson B., Tricarico J.M. Special topics—Mitigation of methane and nitrous oxide emissions from animal operations: I. A review of enteric methane mitigation options. Journal of Animal Science, 2013, 91(11): 5045-5069 (doi: 10.2527/jas.2013-6583).
- Боголюбова Н.В., Зеленченкова А.А., Колесник Н.С., Лахонин П.С. Метанообразование в рубце и методы его снижения с использованием алиментарных факторов (обзор). Сельскохозяйственная биология, 2022, 57(6): 1025-1054 (doi: 10.15389/agrobiology.2022.6.1025rus).
- Knapp J.R., Laur G.L., Vadas P.A., Weiss W.P., Tricarico J.M. Invited review: Enteric methane in dairy cattle production: quantifying the opportunities and impact of reducing emissions. Journal of Dairy Science, 2014, 97(6): 3231-3261 (doi: 10.3168/jds.2013-7234).
- Almeida A.K., Hegarty R.S., Cowie A. Meta-analysis quantifying the potential of dietary additives and rumen modifiers for methane mitigation in ruminant production systems. Animal Nutrition, 2021, 7(4): 1219-1230 (doi: 10.1016/j.aninu.2021.09.005).
- Chow J.M., Van Kessel J.A.S., Russell J.B. Binding of radiolabeled monensin and lasalocid to ruminal microorganisms and feed. Journal of Animal Science, 1994, 72(6): 1630-1635 (doi: 10.2527/1994.7261630x).
- Grainger C., Williams R., Eckard R.J., Hannah M.C. A high dose of monensin does not reduce methane emissions of dairy cows offered pasture supplemented with grain. Journal of Dairy Science, 2010, 93(11): 5300-5308 (doi: 10.3168/jds.2010-3154).
- Benchaar C. Diet supplementation with cinnamon oil, cinnamaldehyde, or monensin does not reduce enteric methane production of dairy cows. Animal, 2016, 10(3): 418-425 (doi: 10.1017/S175173111500230X).
- da Silva Marques R., Cooke R.F. Effects of ionophores on ruminal function of beef cattle. Animals, 2021, 11(10): 2871 (doi: 10.3390/ani11102871).
- Jouany J.-P., Morgavi D.P. Use of ‘natural’products as alternatives to antibiotic feed additives in ruminant production. Animal, 2007, 1(10): 1443-1466 (doi: 10.1017/S1751731107000742).
- Witzig M., Zeder M., Rodehutscord M. Effect of the ionophore monensin and tannin extracts supplemented to grass silage on populations of ruminal cellulolytics and methanogens in vitro. Anaerobe, 2018, 50: 44-54 (doi: 10.1016/j.anaerobe.2018.01.012).
- Mao H.-L., Wang J.-K., Zhou Y.-Y., Liu J.-X. Effects of addition of tea saponins and soybean oil on methane production, fermentation and microbial population in the rumen of growing lambs. Livestock Science, 2010, 129(1-3): 56-62 (doi: 10.1016/j.livsci.2009.12.011).
- Zeitz J.O., Bucher S., Zhou X., Meile L., Kreuzer M., Soliva C.R. Inhhibitory effects of saturated fatty acids on methane production by methanogenic Archaea. Journal of Animal and Feed Sciences, 2013, 22(1): 44-49 (doi: 10.22358/jafs/66015/2013).
- Guyader J., Eugène M., Doreau M., Morgavi D.P., Gérard C., Loncke C., Martin C. Nitrate but not tea saponin feed additives decreased enteric methane emissions in nonlactating cows. Journal of Animal Science, 2015, 93(11): 5367-5377 (doi: 10.2527/jas.2015-9367).
- Guyader J., Eugène M., Meunier B., Doreau M., Morgavi D.P., Silberberg M., Rochette Y., Gerard C., Loncke C., Martin C. Additive methane-mitigating effect between linseed oil and nitrate fed to cattle. Journal of Animal Science, 2015, 93(7): 3564-3577 (doi: 10.2527/jas.2014-8196).
- Ungerfeld E.M. Shifts in metabolic hydrogen sinks in the methanogenesis-inhibited ruminal fermentation: a meta-analysis. Frontiers in Microbiology, 2015, 6: 37 (doi: 10.3389/fmicb.2015.00037).
- Grainger C., Beauchemin K.A. Can enteric methane emissions from ruminants be lowered without lowering their production? Animal Feed Science and Technology, 2011, 166-167: 308-320 (doi: 10.1016/j.anifeedsci.2011.04.021).
- Martin C., Morgavi D.P., Doreau M. Methane mitigation in ruminants: from microbe to the farm scale. Animal, 2010, 4(3): 351-365 (doi: 10.1017/S1751731109990620).
- Doreau M., Bauchart D., Chilliard Y. Enhancing fatty acid composition of milk and meat through animal feeding. Animal Production Science, 2010, 51(1): 19-29 (doi: 10.1071/AN10043).
- Livingstone K.M., Humphries D.J., Kirton P., Kliem K.E., Givens D.I., Reynolds C.K. Effects of forage type and extruded linseed supplementation on methane production and milk fatty acid composition of lactating dairy cows. Journal of Dairy Science, 2015, 98(6): 4000-4011 (doi: 10.3168/jds.2014-8987).
- Martin C., Ferlay A., Mosoni P., Rochette Y., Chilliard Y., Doreau M. Increasing linseed supply in dairy cow diets based on hay or corn silage: Effect on enteric methane emission, rumen microbial fermentation, and digestion. Journal of Dairy Science, 2016, 99(5): 3445-3456 (doi: 10.3168/jds.2015-10110).
- Fiorentini G., Carvalho I.P.C., Messana J.D., Castagnino P.S., Berndt A., Canesin R.C., Frighetto R.T.S., Berchielli T.T. Effect of lipid sources with different fatty acid profiles on the intake, performance, and methane emissions of feedlot Nellore steers. Journal of Animal Science, 2014, 92(4): 1613-1620 (doi: 10.2527/jas.2013-6868).
- Carvalho I.P.C., Fiorentini G., Berndt A., Castagnino P.D.S., Messana J.D., Frighetto R.T.S., Reis R.A., Berchielli T.T. Performance and methane emissions of Nellore steers grazing tropical pasture supplemented with lipid sources. Revista Brasileira de Zootecnia, 2016, 45(12): 760-767 (doi: 10.1590/S1806-92902016001200005).
- Guyader J., Doreau M., Morgavi D.P., Gérard C., Loncke C., Martin C. Long-term effect of linseed plus nitrate fed to dairy cows on enteric methane emission and nitrate and nitrite residuals in milk. Animal, 2016, 10(7): 1173-1181 (doi: 10.1017/S1751731115002852).
- Jose Neto A., Messana J.D., Ribeiro A.F., Vito E.S., Rossi L.G., Berchielli T.T. Effect of starchbased supplementation level combined with oil on intake, performance, and methane emissions of growing Nellore bulls on pasture. Journal of Animal Science, 2015, 93(5): 2275-2284 (doi: 10.2527/jas.2014-8500).
- Jose Neto A., Messana J.D., Rossi L.G., Carvalho I.P.C., Berchielli T.T. Methane emissions from Nellore bulls on pasture fed two levels of starch-based supplement with or without a source of oil. Animal Production Science, 2018, 59(4): 654-663 (doi: 10.1071/AN16095).
- Silva R.A., Fiorentini G., Messana J.D., Lage J.F., Castagnino P.S., San Vito E., Carvalho I.P.C., Berchielli T.T. Effects of different forms of soybean lipids on enteric methane emission, perfor-mance and meat quality of feedlot Nellore. The Journal of Agricultural Science, 2018, 156(3): 427-436 (doi: 10.1017/S002185961800045X).
- Roca-Fernández A.I., Dillard S.L., Soder K.J. Ruminal fermentation and enteric methane pro-duction of legumes containing condensed tannins fed in continuous culture. Journal of Dairy Science, 2020, 103(8): 7028-7038 (doi: 10.3168/jds.2019-17627).
- Рязанов В.А., Шейда Е.В., Дускаев Г.К., Рахматуллин Ш.Г., Кван О.В. Оценка воздействия фитобиотических препаратов Salviae folia, Scutellaria baicalensis, Origanum vul-gare на обменные процессы в модели рубца. Аграрная наука, 2022, 1(7-8): 86-92 (doi: 10.32634/0869-8155-2022-361-7-8-86-92).
- Atlanderova K., Makaeva A., Rysaev A., Nurzhanov B., Duskaev G., Rayzanov V. The effect of medicinal extracts on microflora and enzymatic processes of calf rumen. Journal of Animal Sci-ence, 2020, 98(Suppl_4): 258 (doi: 10.1093/jas/skaa278.466).
- Kumar R., Kumar B.A. New claims in folk veterinary medicines from Uttar Pradesh, India. J. Ethnopharmacol, 2013, 146(2): 581-593 (doi: 10.1016/j.jep.2013.01.030).
- Cobellis G., Trabalza-Marinucci M., Yu Z. Critical evaluation of essential oils as rumen modifiers in ruminant nutrition: a review. Science of the Total Environment, 2016, 545: 556-568 (doi: 10.1016/j.scitotenv.2015.12.103).
- Jacondino L.R., Poli C H.E.C., Tontini J.F., Corrêa G.F., Somacal S., Mello R.O., Leal M.L.R., Raimondo R.F.S., Riet-Correa B., Muir J.P. Acacia mearnsii tannin extract and -tocopherol sup-plementation in lamb diet: effects on growth performance, serum lipid peroxidation and meat quality. Animal Feed Science and Technology, 2022, 294: 115483 (doi: 10.1016/j.anifeedsci.2022.115483).
- Lobón S., Sanz A., Blanco M., Ripoll G., Joy M. The type of forage and condensed tannins in dams’ diet: Influence on meat shelf life of their suckling lambs. Small Ruminant Research, 2017, 154: 115-122 (doi: 10.1016/j.smallrumres.2017.08.005).
- Mueller-Harvey I., Bee G., Dohme-Meier F., Hoste H., Karonen M., Kölliker, R., Luscher A., Niderkorn V., Pellikaan W.F., Salminen J.P., Skot L., Smith L.M.J., Thamsborg S.M., Totter-dell P., Wilkinson I., Williams A.R., Azuhnwi B.N., Baert N., Brinkhaus A.G., Copani G., Desrues O., Drake C., Engstrom M., Fryganas C., Girard M., Huyen N.T., Kempf K., Ma-lisch C., Mora-Ortiz M., Quijada J., Ramsay A., Ropiak H.M., Waghorn G.C. Benefits of con-densed tannins in forage legumes fed to ruminants: importance of structure, concentration, and diet composition. Crop Science, 2019, 59(3): 861-885 (doi: 10.2135/cropsci2017.06.0369).
- Hoste H., Torres-Acosta J.F.J., Quijada J., Chan-Perez I., Dakheel M.M., Kommuru D.S., Mueller-Harvey I., Terrill T.H. Interactions between nutrition and infections with Haemonchus contortus and related gastrointestinal nematodes in small ruminants. Advances in Parasitology, 2016, 93: 239-351 (doi: 10.1016/bs.apar.2016.02.025).
- MacAdam J.W., Villalba J.J. Beneficial effects of temperate forage legumes that contain con-densed tannins. Agriculture, 2015, 5(3): 475-491 (doi: 10.3390/agriculture5030475).
- Junior F.P., Cassiano E.C.O., Martins M.F., Romero L.A., Zapata D.C.V., Pinedo L.A., Ma-rino C.T., Rodrigues P.H.M. Effect of tannins-rich extract from Acacia mearnsii or monensin as feed additives on ruminal fermentation efficiency in cattle. Livestock Science, 2017, 203, 21-29 (doi: 10.1016/j.livsci.2017.06.009).
- Marshall C.J., Beck M.R., Garrett K., Castillo A.R., Barrell G.K., Al-Marashdeh O., Gre-gorini P. The effect of feeding a mix of condensed and hydrolyzable tannins to heifers on rumen fermentation patterns, blood urea nitrogen, and amino acid profile. Livestock Science, 2022, 263: 105034 (doi: 10.1016/j.livsci.2022.105034).
- Makmur M., Zain M., Sholikin M.M., Suharlina, Jayanegara A. Modulatory effects of dietary tannins on polyunsaturated fatty acid biohydrogenation in the rumen: a meta-analysis. Heliyon, 2022, 8(7): e09828 (doi: 10.1016/j.heliyon.2022.e09828).
- Hagerman A.E. Fifty years of polyphenol—protein complexes. In: Recent Advances in Polyphenol Research /S. Quideau, V. Cheynier, P. Sarni-Manchado, S. Quideau (eds.). John Wiley & Sons, 2012, vol. 3: 71-97 (doi: 10.1002/9781118299753.ch3).
- Lima P.R., Apdini T., Freire A.S., Santana A.S., Moura L.M.L., Nascimento J.C.S., Ro-drigues R.T.S., Dijkstra J., Garcez Neto A.F., Queiroz M.A.Á., Menezes D.R. Dietary supple-mentation with tannin and soybean oil on intake, digestibility, feeding behavior, ruminal protozoa and methane emission in sheep. Animal Feed Science and Technology, 2019, 249: 10-17 (doi: 10.1016/j.anifeedsci.2019.01.017).
- Patra A.K., Saxena J. A new perspective on the use of plant secondary metabolites to inhibit methanogenesis in the rumen. Phytochemistry, 2010, 71(11-12): 1198-1222 (doi: 10.1016/j.phyto-chem.2010.05.010).
- Terranova M., Kreuzer M., Braun U., Schwarm A. In vitro screening of temperate climate forages from a variety of woody plants for their potential to mitigate ruminal methane and ammonia formation. The Journal of Agricultural Science, 2018, 156(7): 929-941 (doi: 10.1017/S0021859618000989).
- Rira M., Morgavi D.P., Popova M., Maxin G., Doreau, M. Microbial colonisation of tannin-rich tropical plants: interplay between degradability, methane production and tannin disappear-ance in the rumen. Animal, 2022, 16(8): 100589 (doi: 10.1016/j.animal.2022.100589).
- Naumann H.D., Tedeschi L.O., Zeller W.E., Huntley N.F. The role of condensed tannins in ruminant animal production: advances, limitations and future directions. Revista Brasileira de Zootecnia, 2017, 46(12): 929-949 (doi: 10.1590/S1806-92902017001200009).
- Díaz Carrasco J.M., Cabral C., Redondo L.M., Pin Viso N.D., Colombatto D., Farber M.D., Fernandez Miyakawa M.E. Impact of chestnut and quebracho tannins on rumen microbiota of bovines. BioMed Research International, 2017, 2017(3): 1-11 (doi: 10.1155/2017/9610810).
- Vasta V., Daghio M., Cappucci A., Buccioni A., Serra A., Viti C., Mele M. Invited review: Plant polyphenols and rumen microbiota responsible for fatty acid biohydrogenation, fiber digestion, and methane emission: experimental evidence and methodological approaches. Journal of Dairy Science, 2019, 102(5): 3781-3804 (doi: 10.3168/jds.2018-14985).
- Mannino G., Chinigò G., Serio G., Genova T., Gentile C., Munaron L., Bertea C.M. Proan-thocyanidins and where to find them: a meta-analytic approach to investigate their chemistry, biosynthesis, distribution, and effect on human health. Antioxidants, 2021, 10(8): 1229 (doi: 10.3390/antiox10081229).
- Mannino G., Gentile C., Ertani A., Serio G., Bertea C.M. Anthocyanins: biosynthesis, distribu-tion, ecological role, and use of biostimulants to increase their content in plant foods — a review. Agriculture, 2021, 11(3): 212 (doi: 10.3390/agriculture11030212).
- Jayanegara A., Goel G., Makkar H.P., Becker K. Divergence between purified hydrolysable and condensed tannin effects on methane emission, rumen fermentation and microbial population in vitro. Animal Feed Science and Technology, 2015, 209: 60-68 (doi: 10.1016/j.anifeedsci.2015.08.002).
- Salami S.A., Valenti B., Bella M., O'Grady M.N., Luciano G., Kerry J.P., Jones E., Priolo A., Newbold C.J. Characterisation of the ruminal fermentation and microbiome in lambs supple-mented with hydrolysable and condensed tannins. FEMS Microbiology Ecology, 2018, 94(5): fiy061 (doi: 10.1093/femsec/fiy061).
- Caetano M., Wilkes M.J., Pitchford W.S., Lee S.J., Hynd P.I. Effect of ensiled crimped grape marc on energy intake, performance and gas emissions of beef cattle. Animal Feed Science and Technology, 2019, 247: 166-172 (doi: 10.1016/j.anifeedsci.2018.10.007).
- Ng F., Kittelmann S., Patchett M.L., Attwood G.T., Janssen P.H., Rakonjac J., Gagic D. An adhesin from hydrogen‐utilizing rumen methanogen M ethanobrevibacter ruminantium M 1 binds a broad range of hydrogen‐producing microorganisms. Environmental Microbiology, 2016, 18(9): 3010-3021 (doi: 10.1111/1462-2920.13155).
- Bhatta R., Saravanan M., Baruah L., Prasad C.S. Effects of graded levels of tannin‐containing tropical tree leaves on in vitro rumen fermentation, total protozoa and methane production. Jour-nal of Applied Microbiology, 2015 118(3): 557-564 (doi: 10.1111/jam.12723).
- Costa M., Alves S.P., Cabo Â., Guerreiro O., Stilwell G., Dentinho M.T., Bessa R.J. Modulation of in vitro rumen biohydrogenation by Cistus ladanifer tannins compared with other tannin sources. Journal of the Science of Food and Agriculture, 2018, 97(2): 629-635 (doi: 10.1002/jsfa.7777).
- Cappucci A., Mantino A., Buccioni A., Casarosa L., Conte G., Serra A., Mannelli F., Luci-ano G., Foggi G., Mele M. Diets supplemented with condensed and hydrolysable tannins af-fected rumen fatty acid profile and plasmalogen lipids, ammonia and methane production in an in vitro study. Italian Journal of Animal Science, 2021, 20(1): 935-946 (doi: 10.1080/1828051X.2021.1915189).
- Mannelli F., Daghio M., Alves S.P., Bessa R.J., Minieri S., Giovannetti L., Conte G., Mele M., Messini A., Rapaccini S., Viti C., Buccioni A. Effects of chestnut tannin extract, vescalagin and gallic acid on the dimethyl acetals profile and microbial community composition in rumen liquor: an in vitro study. Microorganisms, 2019, 7(7): 202 (doi: 10.3390/microorganisms7070202).
- Lavin S.R. Plant phenolics and their potential role in mitigating iron overload disorder in wild animals. Journal of Zoo and Wildlife Medicine, 2012, 43(3s): 74-82 (doi: 10.1638/2011-0132.1).
- Saminathan M., Tan H.Y., Sieo C.C., Abdullah N., Wong C.M.V.L., Abdulmalek E., Ho Y.W. Polymerization degrees, molecular weights and protein-binding affinities of condensed tannin fractions from a Leucaena leucocephala hybrid. Molecules, 2014, 19(6): 7990-8010 (doi: 10.3390/molecules19067990).
- Min B.R., Wright C., Ho P., Eun J.-S., Gurung N., Shange R. The effect of phytochemical tannins-containing diet on rumen fermentation characteristics and microbial diversity dynamics in goats using 16S rDNA amplicon pyrosequencing. Agriculture, Food and Analytical Bacteriology, 2014, 4(195-211): 141909.
- Gemeda B.S., Hassen A. Effect of tannin and species variation on in vitro digestibility, gas, and methane production of tropical browse plants. Asian-Australasian Journal of Animal Sciences, 2015, 28(2): 188-199 (doi: 10.5713/ajas.14.0325).
- Li Z., Wright A.-D.G., Liu H., Fan Z., Yang F., Zhang Z., Li G. Response of the rumen microbiota of sika deer (Cervus nippon) fed different concentrations of tannin rich plants. PLoS ONE, 2015, 10(5): e0123481 (doi: 10.1371/journal.pone.0123481).
- Hassanat F., Benchaar C. Assessment of the effect of condensed (acacia and quebracho) and hydrolysable (chestnut and valonea) tannins on rumen fermentation and methane production in vitro. Journal of the Science of Food and Agriculture, 2013, 93(2): 332-339 (doi: 10.1002/jsfa.5763).
- Terranova M., Wang S., Eggerschwiler L., Braun U., Kreuzer M., Schwarm A. Dose-response effects of woody and herbaceous forage plants on in vitro ruminal methane and ammonia for-mation, and their short-term palatability in lactating cows. Animal, 2020, 14(3): 538-548 (doi: 10.1017/S1751731119002076).
- Terranova M., Eggerschwiler L., Ortmann S., Clauss M., Kreuzer M., Schwarm A. Increasing the proportion of hazel leaves in the diet of dairy cows reduced methane yield and excretion of nitrogen in volatile form, but not milk yield. Animal Feed Science and Technology, 2021, 276: 114790 (doi: 10.1016/j.anifeedsci.2020.114790).
- Wang S., Terranova M., Kreuzer M., Marquardt S., Eggerschwiler L., Schwarm A. Supplemen-tation of pelleted hazel (Corylus avellana) leaves decreases methane and urinary nitrogen emissions by sheep at unchanged forage intake. Scientific Reports, 2018, 8(1): 5427 (doi: 10.1038/s41598-018-23572-3).
- El-Zaiat H.M., Kholif A.E., Moharam M.S., Attia M.F., Abdalla A.L., Sallam S.M.A. The ability of tanniniferous legumes to reduce methane production and enhance feed utilization in Barki rams: in vitro and in vivo evaluation. Small Ruminant Research, 2020, 193: 106259 (doi: 10.1016/j.smallrumres.2020.106259).
- Ku-Vera J.C., Jiménez-Ocampo R., Valencia-Salazar S.S., Montoya-Flores M.D., Molina-Bo-tero I.C., Arango J., Gómez-Bravo C.A., Aguilar-Pérez C.F., Solorio-Sánchez F.J. Role of sec-ondary plant metabolites on enteric methane mitigation in ruminants. Frontiers in Veterinary Sci-ence, 2020, 7: 584 (doi: 10.3389/fvets.2020.00584).
- Piñeiro-Vázquez A.T., Canul-Solis J.R., Jiménez-Ferrer G.O., Alayón-Gamboa J.A., Chay-Canul A.J., Ayala-Burgos A.J., Aguilar-Pérez C.F., Ku-Vera J.C. Effect of condensed tannins from Leucaena leucocephala on rumen fermentation, methane production and population of ru-men protozoa in heifers fed low-quality forage. Asian-Australasian Journal of Animal Sciences, 2018, 31(11): 1738 (doi: 10.5713/ajas.17.0192).
- Montoya-Flores M.D., Molina-Botero I.C., Arango J., Romano-Muñoz J.L., Solorio-Sánchez F.J., Aguilar-Pérez C.F., Ku-Vera J.C. Effect of dried leaves of Leucaena leucocephala on rumen fermentation, rumen microbial population, and enteric methane production in cross-bred heifers. Animals, 2020, 10(2): 300 (doi: 10.3390/ani10020300).
- Denninger T.M., Schwarm A., Birkinshaw A., Terranova M., Dohme-Meier F., Münger A., Eg-gerschwiler L., Bapst B., Wegmann S., Clauss M., Kreuzer M. Immediate effect of Acacia mearnsii tannins on methane emissions and milk fatty acid profiles of dairy cows. Animal Feed Science and Technology, 2020, 261: 114388 (doi: 10.1016/j.anifeedsci.2019.114388).
- Yang K., Wei C., Zhao G.Y., Xu Z.W., Lin S.X. Effects of dietary supplementing tannic acid in the ration of beef cattle on rumen fermentation, methane emission, microbial flora and nutrient digestibility. Journal of Animal Physiology and Animal Nutrition, 2017, 101(2): 302-310 (doi: 10.1111/jpn.12531).
- Liu H., Vaddella V., Zhou D. Effects of chestnut tannins and coconut oil on growth performance, methane emission, ruminal fermentation, and microbial populations in sheep. Journal of Dairy Science, 2011, 94(12): 6069-6077 (doi: 10.3168/jds.2011-4508).
- Bhatt R.S., Sarkar S., Sharma P., Soni L., Sahoo A. Comparing the efficacy of forage combina-tions with different hydrolysable and condensed tannin levels to improve production and lower methane emission in finisher lambs. Small Ruminant Research, 2023, 218: 106876 (doi: 10.1016/j.smallrumres.2022.106876).
- Torres R.N.S., Ghedini C.P., Paschoaloto J.R., da Silva D.A.V., Coelho L.M., Almeida Jun-ior G.A., Ezequiel J.M.B., Machado Neto O.R., Almeida M.T.C. Effects of tannins supplemen-tation to sheep diets on their performance, carcass parameters and meat fatty acid profile: a meta-analysis study. Small Ruminant Research, 2022, 206: 106585 (doi: 10.1016/j.smallrumres.2021.106585).
- Ibrahim S.L., Hassen A. Effect of non-encapsulated and encapsulated mimosa (Acacia mearnsii) tannins on growth performance, nutrient digestibility, methane and rumen fermentation of South African mutton Merino ram lambs. Animal Feed Science and Technology, 2022, 294: 115502 (doi: 10.1016/j.anifeedsci.2022.115502).
- Febres F.E.F., Terrazas L.A., Vasquez J.Ñ., Muñoz J.E.M., Howard F.S.M., Mariazza E.F. Ef-fects of chestnut bark (Castanea spp.) tannin extracts on selectivity, dry matter intake, weight gain, and enteric methane emission from llamas (Lama glama) under grazing conditions in the high Andean grasslands. Small Ruminant Research, 2021, 205: 106559 (doi: 10.1016/j.smallrum-res.2021.106559).


