Внутриопухолевая гетерогенность паренхимы и стромы рака мочевого пузыря
Автор: Жестков И.А., Олейникова Н.А., Охоботов Д.А., Камалов Д.М., Дзитиев В.К., Варенцов М.Г., Нестерова О.Ю., Тивтикян А.С., Мальков П.Г., Камалов А.А.
Журнал: Экспериментальная и клиническая урология @ecuro
Рубрика: Онкоурология
Статья в выпуске: 3 т.17, 2024 года.
Бесплатный доступ
Введение. Взаимодействие стромы и паренхимы опухоли с эпителиальными клетками рака мочевого пузыря (РМП) играет важную роль в течении и прогрессировании заболевания. Однако в настоящее время отсутствуют четко определенные маркеры, отражающие окружение опухоли, что затрудняет оценку их влияния на течение РМП.
Рак мочевого пузыря, молекулярные подтипы, опухолевая гетерогенность, опухоль-ассоциированные фибробласты, cafs, фибробласты, микроокружение опухоли, уротелиальная карцинома, немышечно-инвазивный рак мочевого пузыря, нмирмп, мышечно-инвазивный рак мочевого пузыря, мирмп
Короткий адрес: https://sciup.org/142243275
IDR: 142243275 | DOI: 10.29188/2222-8543-2024-17-3-52-61
Текст обзорной статьи Внутриопухолевая гетерогенность паренхимы и стромы рака мочевого пузыря
онкоурология экспериментальная и клиническая урология № 3 2024
По данным Международного агентства по изучению рака (МАИР), в 2020 году рак мочевого пузыря (РМП) занял 10-е место по заболеваемости в мире среди всех злокачественных заболеваний во всех возрастных группах. При этом, по информации благотворительной организации Cancer Research UK, с 1970 по 2010 год были достигнуты минимальные успехи в улучшении показателей выживаемости пациентов с диагнозом РМП так 5-летняя выживаемость после радикальной цистэктомии или комбинированной химиолучевой терапии сохраняется на уровне 50% [1].
При первичной диагностике РМП до 80% образований приходится на уротелиальную карциному [2]. На основании степени инвазии в мышечный слой опухоль классифицируется на немышечно-инвазивный рак мочевого пузыря (НМИРМП), ограниченный слизистым и подслизистым слоем, и мышечно-инвазивный рак мочевого пузыря (МИРМП), характеризующийся прорастанием в мышечную оболочку мочевого пузыря. При первичной диагностике РМП в 75% случаев выявляют немышечно-инвазивные формы, характеризующиеся благоприятным прогнозом [3].
Хирургическое лечение МИРМП высокотравматично, связано с высоким риском послеоперационных осложнений и рецидива заболевания. Неоадъювантная химиотерапия позволяет улучшить результаты оперативного лечения, а в 20-40% случаев и добиться полного патоморфологического ответа до радикального оперативного вмешательства [4]. Успехи неоадъювантной терапии подтолкнули к использованию тримодальной терапии, включающей трансуретральную резекцию мочевого пузыря (ТУР МП) с последующей химиотерапией и лучевой терапией.
При лечении НМИРМП основной проблемой остается высокая частота рецидивов. В течение первого года после изолированной ТУР МП (основного метода лечения НМИРМП, Та-Т1 стадии) у 30-80% пациентов происходит рецидив заболевания [5]. Для профилактики развития рецидива Европейской организацией по исследованию и лечению рака (European Organisation for Research and Treatment of Cancer, EORTC) и Испанской группой по онкоурологии (CUETA) на основании клинических признаков опухоли предлагалось разделить НМИРМП на группы риска [6]. Группы риска позволяют выработать персонифицированную тактику лечения и спрогнозировать течение заболевания. В настоящее время признано, что обе прогностические модели переоценивают риск рецидива и прогрессии НМИРМП и не позволяют предсказать ответ на внутрипузырную иммунотерапию [7]. Так, до 40% опухолей оказываются резистентны к внутрипузырной химио- и БЦЖ терапии и требуют цистэктомии [8]. Предложено два механизма уклонения от иммуного ответа: внутриклеточный, по- средством активности P-гликопротеида, и внеклеточный, благодаря микроокружению опухоли [9, 10].
Недостаточная предиктивная ценность существующих прогностических моделей, поиск новых точек воздействия лекарственных средств,основанных на классических клинико-морфологических критериях может быть следствием внутриопухолевой гетерогенности, что делает перспективным определение молекулярно-генетических изменений МИРМП и НМИРМП.
МАТЕРИАЛЫ И МЕТОДЫ
Проведен поиск публикаций в базе данных MEDLINE на платформе PubMed, в научной электронной библиотеке eLibrary.ru и в поисковой системе Google Schоilar. Поисковые запросы включали следующие комбинации слов: «рак мочевого пузыря», «опухолевая гетерогенность», «микроокружение опухоли» («bladder cancer», «tumor heterogeneity», «microenvironment»). Всего было проанализировано 159 публикаций, из которых для настоящего обзора отобраны 55 публикаций. Были исключены тезисы конференций, короткие сообщения, дублирующиеся публикации.
РЕЗУЛЬТАТЫ
Молекулярные подтипы рака мочевого пузыря
Развитие методов секвенирования, иммуногистохимии (ИГХ) и полимеразной цепной реакции (ПЦР) позволило выявить внутриопухолевую биологическую и морфологическую гетерогенность клеток и таким образом,объяснить низкую эффективность лечения для образований различной локализации [11]. С целью выявления внутренних подтипов уротелиального РМП D. McC onkey и исследовательская группа MD Anderson (MDA) разработали собственную методику, основываясь на результатах исследований подтипов рака молочной железы,проведенных C.M. Perou и коллегами [12]. В результате было предложено несколько молекулярных классификаций МИРМП и НМИРМП.
J. Damrauer и соавт. из Университета Северной Каролины (University of North Carolina, UNC) разделили МИРМП на базальный и люминальный подтип. Базальный подтип характеризуется высокой генетической нестабильностью, мутациями в генах, контролирующих клеточный цикл и ДНК-репарацию, клинически характеризуется агрессивным течением и низкой опухоль-специфической выживаемостью (в среднем 14,9 месяцев), но демонстрирует хороший ответ на неоадъювантную химиотерапию.Люминаль-ный подтип характеризуется экспрессией генов, связанных с эпителиальной дифференцировкой и эстрогеновым рецептором, клинически характеризуется высокой опухоль-специфической выживаемостью (в среднем 65,6 месяцев) и низкой вероятностью рецидива [13].
В онкологическом центре Андерсона (MD Anderson, MDA), опираясь на предыдущие работы, разработали свой подход в классификации МИРМП,выделив в отдельную группу МИРМП на основании экспрессии гена p53 дикого типа [14]. Клинически подтип характеризовался отсутствием эффекта от неоадъювантной химиотерапии цисплатином. Эти данные подтвердили результаты исследования R. Millikan, в котором пациенты с неоадъювантной химиотерапией по схеме M-VAC (Метотрексат, Винбластин, Адриамицин и Цисплатин) так же были резистентны к проводимой терапии [15].
Проект атласа генома рака (The C ancer Genome Atlas Program, TCGA) на основании данных экспрессии стромальных и воспалительных сигнатур выявил 4 кластера МИРМП. Кластеры I и II имели высокий уровень экспрессии рецептора эпидермального фактора роста 2-го типа (human epidermal growth factor receptor 2, HER2), эстрогенового рецептора и по характеристикам были похожи на люминальный подтип РМП. Особенностью кластера I являлось сходство с папиллярной карциномой и наличием дисрегуляции в факторе роста фибробластов 3-го типа (Fibroblast Growth Factor-3, FGF-3). Кластер III показал высокий уровень экспрессии KRT14 и CD44. Кластер IV имел общие признаки с опухолями кластера III, но также отражал характеристики окружающей стромы и мышц [16].
В 2010 г. коллектив авторов из университета Лунд предпринял первую попытку по идентификации молекулярных подтипов РМП. Был выполнен анализ 144 образований, включавших МИРМП и НМИРМП. Удалось выявить два основных молекулярных подтипа (Molecular Subtype, MS). MS1 характеризовался 1-ой и 2-ой степенью дифференцировки, согласно классификации ВОЗ от 1999 г., и преимущественно Та стадией. MS2 характеризовался 3-ей степенью дифференцировки и преимущественно ≥Т2 стадиями. Стадии Т1 распределились поровну [17].
На основании этой классификации в 2012 г. была создана первая версия молекулярной классификации Лунд. База пациентов была дополнена (116 больных – Та, 97 – Т1 и 93 ≥ Т2). На основании секвенирования и кластеризации были выделены семь статистически значимо различающихся подтипов MS1 a, M S1b MS2a1, MS2a2, MS2b1, MS2b2.1 и MS2b2.2. MS1 а и MS1b различаются профилем экспрессии генов раннего клеточного цикла (KRT 13, 15 и 17), белков клеточной адгезии, мутациями в генах, активирующими FGFR и ассоциированными с генами активаторами что говорит о сохраненной структурной организации. Данные подтипы были объеденены в Urothelial-like A или UroA подтип. MS2b1 кластер, преимущественно сформированный иммунными клетками, получил название инфильтративного (infiltrated group, Epi-inf). MS2a1 и MS2a2 подтипы отличались от UroA высокой экспрессией генов позднего клеточного цикла и ERBB2, низкой FGFR и мутациями в TP53. Данные подтипы были объеденены в кластер геномно-нестабильных (Genomically unstable, GU). MS2b2.1 и MS2b2.2 кластеры характеризовались мутациями в FGFR3 и генами, ассоциированными с мутациями в FGFR3, что сближало их с UroA. MS2b2.1 подтип получил название UroB. MS2b2.2 кластер демонстрировал высокую экспрессию кератина и плоскоклеточную дифференцировку. Данный подтип был идентифицирован как базально-плоскоклеточный (SCC-подобный; Ba/Sq) [18]. Для валидизации классификации использовались антитела CCND1, FGFR3, TP63, KRT20, ERBB2, CCNE1 CCNB1, KRT5, KRT14, KRT6 и EGFR. ИГХ выявила серьезные различия в топографической экспрессии генов. Так, в UroA подтипе KRT5 присутствовал только в клетках, примыкающих к строме, а в случае Ba/Sq подтипа окрашивались все клетки. GU, наоборот, плохо окрашивался KRT5, но хорошо ECAD и ERBB2, что стало предпосылкой для создания ИГХ классификации молекулярных подтипов. В результате из семи кластеров было сформировано 3 основных молекулярных подтипа уротелиального рака: GU, UroA и Ba/Sq [19].
В дальнейшем при изучении гетерогенности сигнатур в группе пациентов со стадией ≥ Т1 было выполнено секвенирование 307 опухолей с последующим ИГХ исследованием 27 антителами для валидизации результатов. В результате удалось выявить мелкокле-точный/нейроэндокринный подтип (small cell/neu-roendocrine-like, Sc/NE), экспрессирующий ENO2 CGHA и SYP. Подтип характеризовался неблагоприятным течением и плохим прогнозом,морфологиче-ски имел признаки нейроэндокринной опухоли при отсутствии уротелиальной дифференцировки [20].
Аналогичные классификации были разработаны для НМИРМП. Так в исследовании UROMOL выполнялся сравнительный транскрипционный анализ 460 образцов НМИРМП, что позволило разделить НМИРМП на три класса,различающихся профилем экспрессии генов-регуляторов клеточного цикла и дифференцировки опухоли. Опухоли 1-го класса характеризовались высокой экспрессией генов раннего периода клеточного цикла и уроплакинов, которые являются маркерами уротелиальной дифференциации. Опухоли 2-го класса экспрессировали гены позднего периода клеточного цикла и так же уроплакины. Опухоли 3-го класса проявляли высокую экспрессию KRT5 и KRT15, характерную для базальных клеток высокий уровень длинных некодирующих РНК (lncR-NAs). Для низкодифференцированных опухолей были характерны мутации в генах семейства APOBEC, что может служить причиной высокой мутационной нагрузки при РМП [21].
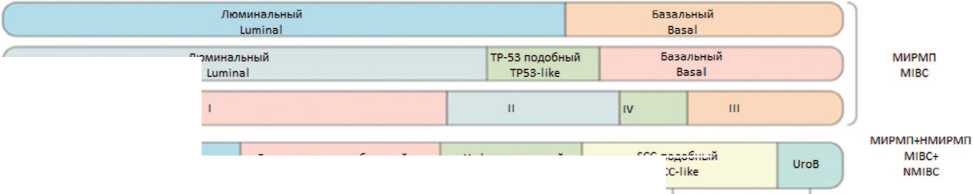
Множество молекулярно-генетических классификаций РМП с различными названиями подтипов и профилями экспрессии генов создают сложности для клинического использования классификации (рис. 1). Поэтому в 2020 г. коллективом экспертов EAU была предложена новая молекулярная классификация МИРМП, основанная на данных о 1750 пациентах с мышечно-инвазивным раком мочевого пузыря из 18 баз данных. Используя критерии и номенклатуру из существующих классификаций, авторы исследования присвоили каждому случаю подтип,согласно существующим классификациям.Далее определяли пациентов, схожих по молекулярно-генетическим характеристикам, и объединяли их в один молекулярный под-тип.Всего было выделено шесть подтипов: люминальный папиллярный, люминальный неспецифический люминальный нестабильный, стромальный, базаль-ный/плоскоклеточный и нейроэндокринно-подобный (табл. 1) [22].
В настоящее время молекулярно-генетическая классификации далека от реализации в рутинной практике из-за сложности и дороговизны методики оценки экспрессии м-РНК, кроме того, профиль экспрессии отражает свойства стромы опухоли и нормальной ткани.Поэтому для верификации подтипа используются суррогатные классификации на основании иммуногистохимического (ИГХ) исследования.
В работе V. D adhania и соавт. выполнен поиск ИГХ маркеров молекулярных подтипов РМП с целью внедрения методики в рутинную клиническую практику. Работа включала пациентов из бaзы Онкологического центра Андерсона. В рамках исследования использовалась панель из 15 антител (GATA3, KRT18 KRT20, uroplakin 2, cyclin D1, ERBB2/HER2, KRT5 KRT14, p63, p16, BCL2, aSMA, Миозин, Кальпонин и Десмин) для идентификации трех подтипов МИРМП – люминальный, базальный и p53-подобный. Выявить маркер для p53-подобного подтипа не удалось, а для двух основных подтипов МИРМП предлагается использование следующих маркеров: GATA3, CK 18 CK20, Уроплакин 2, Циклин D1, HER2 для люминального РМП и CK5/6, CK14, p63 для базального РМП. При этом для идентификации двух основных подтипов МИРМП с точностью в 91% оптимально использование всего двух антител C K5/6 (базальный) и GATA3 (люминальный) [24].
В 2013 г. участник научной группы по разработке классификации Лунд G. Sjödahl с соавт., используя имеющиеся данные об экспрессии генов,выполнили ИГХ исследование гистологического материала 237 пациентов. Работа включала UroA, UroB, SCCL, GU подтипы на всех стадиях РМП. Использовалась панель из 20 антител: CCNB1, CCND1, CCNE1, CDH1, P-Cad CDH2, CDKN2A, EGFR, ERBB2 (Her-2), FGFR3, KRT5 KRT6, KRT14, KRT20, DSC2/3 (desmocollin 2/3), E2F3, RB1 (retinoblastoma 1), MKI67, TP63 и uroplakin 3 (UPK3). Опухоли UroA подтипа характеризовались
UROMOL
Класс 1
Class 1
Класс 2
Class 2
Класс 3
Class 3
* экспрессия KRT5, KRT14, KRT15
ФКРТ5 KRT14, KRT15 and IncRNA
Гены раннего клеточного цикла * экспрессия уроплакинов Earlycell cycle lUroplakin expression
Гены позднего клеточного цикла f экспрессия KRT14, KRT20, уроплакинов Late cell cycle ФКРТ14, KRT20 uroplakin expression Class
НМИРМП NMIBC
UNC
MDA
TCGA
Lund
Люминальный
SCC-подобныи
UroA
Генетически нестабильный
Genomically unstable
Инфильтративный Infiltrate
Неизвестны None identified
Мишень Targets
Факторы транскрипции Transcription factors
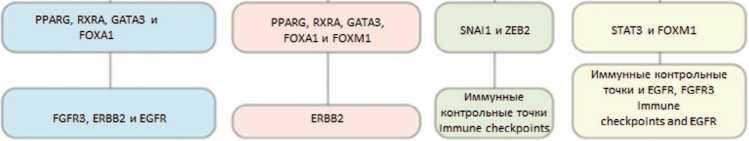
Рис. 1 Молекулярные подтипы рака мочевого пузыря а. Подтипы НМИРП на основании транскрипционных характеристик.
b. Номенклатура подтипов рака мочевого пузыря, определенн Университетом Северной Каролины (UNC), Центром онкологии М. Д. Андерсона (MDA), проектом Атласа генома рака (TCGA) и Университетом Лунда (Lund) [23]
Fig. 1 Molecular Subtypes of Bladder Cancer a. Subtypes of non-muscle-invasive bladder cancer (NMIBC) based on transcriptional characteristics.
b. Nomenclature of bladder cancer subtypes defined by the University of North Carolina (UNC), MD Anderson Cancer Center (MDA), The Cancer Genome
Atlas project (TCGA), and Lund University. The UNC, MDA, and TCGA studies included only NMIBC [23]
FGFR3 и EGFR
Таблица 1. Основные характеристики подтипов МИРМП согласно Консенсусной международной классификации [21] Table 1. Main characteristics of subtypes of MIBC according to the Consensus Molecular Classification of Muscle-invasive Bladder Cancer [21]
|
Частота подтипа МИРМП, % Frequency of MIBC subtype, % |
24% 8% 15% 15% 35% 3°о |
|||||
|
Графическое представление Graphic representation |
||||||
|
Молекулярный подтип Molecular subtype |
Люминальный папиллярный Luminal Papillary |
Люминальный неспецифический Luminal Non-Specified |
Люминальный нестабильный Luminal Unstable |
Стромальный Stromal-rich |
Базальный/ плоскоклеточный Basal/Squamous |
Нейроэндокринный Neuroendocrine |
|
Особенности канцерогенеза Oncogenic mechanisms features |
FGFR3+ PPARG+ CDKN2A- |
PPARG+ |
PPARG+, ERBB2+, геномная нестабильность, повышенная активность клеточного цикла |
EGFR+ |
TP53-, RB1-, Cell cycle |
|
|
Частые мутации Frequent mutations |
FGFR3 (40%), KDM6A (38%) |
ELF3 (35%) |
TP53 (76%), ERCC2 (22%), TMB+ |
TP53 (61%), RB1 (25%) |
TP53 (94%), RB1 (39%) |
|
|
Инфильтрация стромальными клетками Stromal infiltrate |
н/у |
Фибробласты Fibroblasts |
Фибробласты, миофибробласты fibroblasts, myofibroblasts |
Фибробласты, миофибробласты Fibroblasts, myofibroblasts |
Нейроэндокринная дифференцировка Neuroendocrine differentiation (72%) |
|
|
Клинические особенности Clinical features |
Превалирует среди стадии T2 T2 stage + |
Пожилые пациенты (80+) Older patients (80+) |
н/у |
Женщины+Т3/4 стадии Women+ T3/T4 stage+ |
Чаще у женщин, превалируют стадии T3/T4 More common in women, stages T3/T4 prevail |
н/у |
|
Медиана общей выживаемости (годы) Median overall survival (years) |
4 |
1,8 |
2,9 |
3,8 |
1,2 |
1 |
экспрессией структурных (KRT5) маркеров, маркеров клеточного взаимодействия (P-Cad) и тирозинкиназных рецепторов (EGFR)в области базальных клеток а CCNB1 – в области базальной мембраны, что говорит о пролиферативной активности в данной области и подтверждает сохранность структурной организации ткани. В отличие от UroA, опухоли подтипа SCCL экспрессировали KRT5, P-Cad, EGFR, KRT14, CCNB1 и в уротелиальном, и в базальном слое, что говорит о потере стуктурной организации. Подтип GU преимущественно экспрессировал маркеры межклеточного взаимодействия (E-Cad) и клеточного цикла (CCNB1 и MKI67) по всей поверхности опухоли. UroB подтип характеризовался схожим с UroA профилем марке- ров, но включал мышечноинвазивные формы, что может говорить о прогрессии подтипа UroA в UroB. При составлении кривой Каплан-Мейера пациенты с UroA подтипом обладали наиболее благоприятным прогнозом, с GU подтипом – промежуточным прогнозом и с SCCL подтипом – неблагоприятным прогнозом, что подтверждает клиническую роль маркеров [19].
В дальнейшем велась работа по оптимизации количества используемых маркеров. C.Hardy и соавт. использовалась панель из 24 антител (FGFR3, FOXA1 GATA3, PPARG, CDH1, CDH3, TP63, RXRA, CCNB1 CCND1, CDKN2A (p16), E2F3, RB1, KRT14, KRT20 KRT5, UPK3, CHGA, EPCAM, NCAM1, SYP, TUBB2B
-
VIM, ZEB2). В результате исследования удалось увеличить точность классификации LundTax, так использование всего трех маркеров: GATA3, KRT5 и p16 позволило идентифицировать базальный/люминаль-ный подтип с 93-96% точностью, а базальный/GU/Uro подтипы с 85-86% точностью. Эти результаты так же подтвердили результаты V. Dadhania и соавт. [25].
Использование ИГХ маркеров для анализа подтипов РМП,позволило не только упростить выявление подтипов,но и улучшить систематизацию классификации.
Опухолевая строма, CAFs
Молекулярно-генетические характеристики эпителиальных клеток опухоли не могут полностью объяснить чувствительность к терапии и прогноз заболевания [26]. Поэтому в настоящее время все большее внимание уделяется не только эпителиальным опухолевым клеткам,но и опухолевой строме как самостоятельному фактору прогрессии.
CAF оказывают канцерогенный эффект, индуцируя эпителиально-мезенхимальный переход (ЭМП Epithelial-mesenchymal transition – EMT). Так, в работе L. Zhuang и соавт. оценивалось влияние трансформирующего фактора роста бета (Transforming growth factor beta, TGFb1) на ЭМП. Результаты показали, что TGFβ1, секретируемые CAFs, способствуют ЭМП клеток рака мочевого пузыря. Это происходит через активацию не кодирующей РНК-ZEB2NAT, которая, в свою очередь, ингибирует экспрессию гена E-cadherin ответственного за клеточную адгезию. В результате потери контактов с соседними клетками эпителиоциты освобождаются из системы межклеточных связей [29]. Эти результаты подтвердились в работе J. Schulte и соавт., где сравнивался эффект TGFb, фактора роста фибробластов (acidic fibroblast growth fac- tor, aFGF) и фактора роста тромбоцитов (Platelet-derived growth factor, PDGF). Использование TGFb и совместное TGFb с aFGF индуцировало инвазию клеточной линии рака мочевого пузыря RT112, что подтверждалось экспрессией маркеров EMT (Snail, Slug, Zeb1 E-cadherin). Кроме того, факторы роста были связаны с высокой активностью маркеров CAFs [30].
CAFs формирует внеклеточный матрикс (ВКМ) за счет депонирования и ремоделирования компонентов матрикса. Депонирование происходит благодаря продукции коллагена, ламинина, гликопротеинов таких как Тенасцин С (TNC) или Фибронектин, протеогликанов и полисахаридов [31]. Кроме того, CAFs синтезируют ферменты, ремоделирующие внеклеточный матрикс. Некоторые из них, например трансглутаминаза и лизилоксидаза, отвечают за формирование связей между белковыми молекулами, другие, наоборот, разрушают ВКМ, например, матриксная метало-протеаза (Matrix metalloproteinases, MMPs) и ADAM-12 (дизентегрин и металлопротеаза-12) [32]. Ремоделирование ВКМ высвобождает провоспалительные факторы роста и цитокины,открывает участки для адгезии иммунных клеток и формирует путь для дальнейшего распространения опухоли [33].
Иммунологический фенотип микроокружения опухоли является важным маркером прогноза заболевания и эффективности лечения. В зависимости от количественного и качественного состава опухолевой ткани выделяют «горячие» опухоли, инфильтрирова-ные иммунными клетками (CD4+, CD8+, миелоидные клетки), и «холодные» опухоли с преобладанием регуляторных Т-клеток, миелоидных клеток-супрессоров и макрофагов 2го типа [34]. В работе H. Gil-Julio проводилось исследование механизмов «уклонения» РМП от иммунного ответа. Tак, наличие капсулы было статистически значимо связано (р = 0,027) с экспрессией (FAP+) CAFs и низкой инфильтрацией (CD8+) Т-кле-ток,что характерно для опухолей «холодного» типа [35]. Таким образом, CAF обладают иммуносупрессивным действием,обусловленным физическим свойством к секвестрации иммунных клеток во внеклеточном матриксе, и секреторными свойствами к продукции иммуномодулирующих хемокинов.
Опухолевые клетки и CAFs активно взаимодействуют друг с другом. В работе S. Pavlides и соавт. предложена модель возникновения эффекта Варбурга с участием опухолевой стромы. Так, эпителиальные опухолевые клетки индуцируют анаэробный гликолиз в соседних стромальных фибробластах за счет трансформации фибробластов в CAFs. Опухоль-ассоцииро-ванные фибробласты продуцируют лактат и пируват а эпителиальные опухолевые клетки используют их в митохондриальном цикле трикарбоновых кислот, способствуя высокой пролиферативной способности. Эти данные подтверждались низкой экспрессией
Кавеолина-1 (Caveolin-1, Cav-1) в строме рака молочной железы (РМЖ) и высокой экспрессией метаболитов анаэробного гликолиза, а модель взаимодействия эпителиальных опухолевых клеток и CAFs получила название «Обратный эффект Варбурга» [36].
Вышеперечисленные функции CAFs в значительной степени зависят от органа и изменяются даже внутри одной ткани. Концепция гетерогенности CAFs может косвенно подтверждаться наличием подтипов опухолей при раке молочной железы, толстой кишки мочевого пузыря. В настоящее время ведется активная работа по поиску подтипов CAFs.
Гетерогенность CAFs
В работе D. Öhlund и соавт. изучалась гетерогенность стромы рака поджелудочной железы. Удалось выявить популяцию (aSMA+) CAFs в непосредственной близости от эпителия опухоли, получившую название мышечных CAFs (myofibroblastic CAFs, myCAFs), и популяцию (IL-6+) CAFs на некотором удалении от паренхимы опухоли, получившую название воспалительных CAFs (inflammatory CAFs, iCAFs). Результаты исследования говорят о гетерогенности стромы и наличии разных механизмов активации CAFs [37].
В дальнейшем на основании анализа профилей экспрессии генов из баз данных Gene Expression Omnibus (GEO) и TCGA по секвенированию плоскокле-точноого рака головы и шеи, меланомы, рака легких удалось выделить 5 подтипов CAFs: десмопластические (desmoplastic CAF), миофибробласты (myofibroblast CAF), воспалительные (inflammatory like CAF-1 и 2) и пролиферативные (proliferating CAF) [38]. Профили экспрессии генов приведены в таблице 2.
Таблица 2. Профиль экспрессии генов в зависимости от молекулярно-генетического подтипа CAFs [39] Table 2. Gene expression profile according to the molecular-genetic subtype of CAFs [39]
|
Подтипы CAFs CAFs subtype |
Профиль экспрессии генов Gene expression profile |
|
Десмопластические Desmoplastic CAF |
MYH11, MCAM, TAGLN и MYLK |
|
Миофибробласты Myofibroblast CAF |
COL1A1, COL3A1 |
|
Воспалительные 1 Inflammatory like CAF-1 |
CFD, C3, CXCL14 и CXCL12 |
|
Воспалительные 2 Inflammatory Like CAF-2 |
CXCL2, ICAM1, TNFAIP3 |
|
Пролиферативные Proliferating CAF |
CDC45, CDK1, BIRC5, TOP2A |
За последнее десятилетие появилось большое количество публикаций, указывающих на важную роль микроокружения опухоли и CAFs в развитии и прогрессировании РМП [40].
Z. Chen и соавт. на основании анализа экспрессии генов идентифицировали iCAFs и myCAFs. iCAFs экспрессировали цитокины и хемокины,включая CXCL12, IL6, CXCL14, CXCL1 и CXCL2, что аналогично подтипу iCAFs в работе D. Öhlund. Благодаря высокой секреторной активности, iCAFs стимулировали пролиферацию эпителиальных клеток опухоли ангиогенез сосудов и инфильтрацию опухоли иммунными клетками. (RGS5+) фибробласты обладали признаками, характерными для myCAFs, и в большей степени отвечали за адгезивные свойства и формирование экстрацеллюлярного матрикса [41]. Эти данные нашли подтверждение в работе Н. Wang и соавт., в которой авторам удалось выявить Wnt/β-catenin и Notch сигнальные пути, участвующие в развитии опухоли и подтвердить наличие (RGS5+) myCAFs и (PDGFRα+) iCAFs [21]. В дальнейшем Z. Ma с соавт. выявили подтип CAFs, индуцируемый интерфероном (interferon IFN) 1-го типа, что принципиально отличает его от myCAFs и iCAFs, активируемых трансформирующим фактором роста (T ransforming growth factor, TGF). irCAFs обладают канцерогенным эффектом за счет активации WNT5A сигнального пути, при ИГХ исследовании идентифицируются антителами к SLC14A1 характеризуются плохим ответом на химотерапию и иммунотерапию [42].
Следующим этапом изучения гетерогенности CAFs стал поиск связи подтипа опухоли с подтипом CAFs. В работе A. Costa с соавт. изучали гетерогенность CAFs при различных подтипах рака молочной железы (РМЖ). С помощью проточной цитометрии c использованием 6 маркеров (FAP, integrin b1/CD29, aSMA S100-A4/FSP1, PDGFRb и CAV1) удалось выявить четыре подтипа CAFs. CAF-S1 (CD29 сред., FAP выс., FSP1 сред., aSMA выс., PDGFRb сред., CAV1 низ.) и CAF-S4 (CD29 выс. FAP отр., FSP1 сред., aSMA выс., PDGFRb сред., CAV1 сред.) были характерны для агрессивных HER2 и TN раков мочевого пузыря. CAFs-S1 были связаны с иммуносупрессивным фенотипом за счет привлечения регуляторных Т-клеток и ингибирования цитотоксических CD8+ T-клеток. CAF-S4 не участвовали в иммунном ответе и были связаны с сокращением мышц, регуляцией актинового цитоскелета [43].
J. Calvete и соавт. предприняли попытку оценить связь между FAP маркером CAFs и маркерами базального (CK5/6, CD44) и люминального (CK20, GATA3) подтипов для РМП. Выявлена сильная статистически значимая связь FAP с низкой активностью CK20 и GATA3, соответственно p =0,0001 и p =0,005. В исследовании не удалось выявить связь между базальным подтипом опухоли и (FAP+) CAFs [44].
Изучение гетерогенности CAFs улучшает наше понимание механизмов развития РМП, открывает новые возможности лечения. Полногеномное секвенирование (bulk RNA sequencing) позволяет провести сравнительный анализ опухолей, но не дает пространственного представления о профиле экспрессии генов. ИГХ мар- керы позволяют визуализировать распределение антител, обладают высокой специфичностью и чувствительностью. Главным недостатком является ограниченный выбор антител для проведения исследования, поиск которых продолжается в настоящее время (табл. 3).
ЗАКЛЮЧЕНИЕ
Внутриопухолевая гетерогенность при РМП является актуальным направлением современной молекулярной биологии и онкоурологии. Предложенные молекулярно-генетические классификации подтверждают неоднородность РМП. Однако они трудновоспроизводимы и имеют неясное клиническое применение. Со- гласно Консенсусной международной классификации стромальный, люминальный неспецифический и базаль-ный/плоскоклеточный подтипы экспрессируют сигнатуры стромы опухоли, в том числе опухоль-ассоциированных фибробластов. Это косвенно подтверждает роль CAFs в канцерогенезе и течении РМП за счет эпителиально-мезенхимального перехода, изменения метаболизма, инвазии и резистентности опухоли к иммунотерапии. Однако большинство исследований, посвященных гетерогенности РМП, основаны на секвенировании мРНК с последующим анализом профилей экспрессии генов. Таким образом, существует необходимость в поиске суррогатных ИГХ маркеров CAFs и молекулярно-генетических подтипов применимых в клинической практике.
Таблица 3. Используемые маркеры для идентификации CAFs по данным различных авторов
Table 3. Markers Used for CAF Identification according to different authors
|
Маркер Markers |
Экспрессия CAFs (по данным ИГХ) CAFs expression profile (according to IHC) |
Экспрессия CAFs по результатaм анализа профиля экспрессии генов B.Liu и др. [45] CAFs expression profile according to B. Liu et al.) [45] |
|
Гладкомышечный актин альфа α-smooth muscle actin, α-SMA |
+ [29, 46, 47] |
– |
|
Виментин Vimentin, VIM |
+ [29, 46, 48] |
+ |
|
Фибробласт-специфичный протеин 1 или S100A4 Fibroblast-specific protein-1, FSP-1 or S100A4 |
+/– [47] |
– |
|
Десмин Desmin (DES) |
– [48] |
– |
|
Подопланин Podoplanin (PDPN) |
– [50, 51] |
– |
|
Рецепторы тромбоцитарного фактора роста α или CD140a latelet-derived growth factor receptor-α, PDGFR-α) or CD140a |
– [46] |
+ |
|
Рецепторы тромбоцитарного фактора роста β Platelet-derived growth factor recepto–β (PDGFR-β) |
+ [46, 47] |
+ |
|
Кальдесмон 1 Caldesmon 1 (CALD1) |
+ [48, 52] |
+ |
|
Тенасцин-С Tenascin-C (TNC) |
+ [53] |
+ |
|
Периостин Periostin (POSTN) or osteoblast-specific factor (OSF-2) |
– [54] |
– |
|
E-кадгерин Cadherin-1 or Epithelial cadherin (E-cadherin) |
– [47] |
+ |
|
Периостин Periostin (POSTN) or osteoblast-specific factor (OSF-2) |
+ [46, 55] |
+ |
|
Белок, ассоциированный с микрофибриллам 5 Microfibrillar-associated protein 5 (MFAP5) |
+ [46] |
– |
|
Белок, активирующий фибробласты fibroblast activation protein, FAP |
+/– [44, 46, 47] |
+ |
|
Кислый секретируемый белок, богатый цистеином Secreted protein acidic and rich in cysteine (SPARC) |
– [47] |
– |
ИШАТШШШШШ
ПТШТУРАШШПШ non-muscle invasive bladdercancer recurrence using machine learning of quantitative nuclear features. Mod Pathol 2022;35(4):533-8.
ЛНТЕРАТУРА/REFERENOES
Oncol 2020;38(10):797.e7-797.e13.
Сведения об авторах:
Жестков И.А. – врач-уролог общеклинического отделения, аспирант кафедры урологии и андрологии факультета фундаментальной медицины ФГБОУ ВО «МГУ им. М.В. Ломоносова»; Москва, Россия;
Олейникова Н.А. – к.м.н., научный сотрудник, отдел клинической патологии, Медицинский научно-образовательный центр ФГБОУ ВО «МГУ им. М.В. Ломоносова»; Москва, Россия; РИНЦ Author ID 824855,
Охоботов Д.А. – д.м.н.; доцент кафедры урологии и андрологии факультета фундаментальной медицины ФГБОУ ВО «МГУ им. М.В. Ломоносова»; Москва, Россия;
РИНЦ Author ID 759176,
Камалов Д.М. – к.м.н., врач уролог, Медицинский научно-образовательный центр МГУ имени М.В.Ломоносова, научный сотрудник научного отдела урологии и андрологии Медицинского научно-образовательного центра МГУ имени
М.В. Ломоносовa; Москва, Россия;
Дзитиев В.К. – к.м.н., заведующий урологическим отделением, научный сотрудник отдела урологии и андрологии МНОЦ ФГБОУ ВО «МГУ им. М.В. Ломоносова»;
Москва, Россия;
Варенцов М.Г. – аспирант кафедры урологии и андрологии факультета фундаментальной медицины ФГБОУ ВО «МГУ им. М.В. Ломоносова»; Москва, Россия;
Нестерова О.Ю. – к.м.н., врач-уролог МНОЦ МГУ им. М.В. Ломоносова, научный сотрудник отдела урологии и андрологии МНОЦ МГУ им. М.В. Ломоносова, старший преподаватель кафедры урологии и андрологии факультета фундаментальной медицины МГУ имени М.В. Ломоносова; Москва, Россия;
Тивтикян А.С. – врач-уролог, стажер-исследователь отдела урологии и андрологии Медицинского научно-образовательного центра ФГБОУ ВО «МГУ им. М.В. Ломоносова»; Москва,Россия;
Мальков П.Г. – д.м.н., заведующий отделом клинической патологии, Медицинский научнообразовательный центр, Московский государственный университет им. М.В. Ломоносова; Москва, Россия; РИНЦ Author ID 824314,
Камалов А.А. – профессор, д.м.н., академик РАН,директор, Медицинский научнообразовательный центр МГУ им. М.В. Ломоносова;заведующий кафедрой урологии и андрологии, факультет фундаментальной медицины,МГУ им. М.В.Ломоносова; Москва, Россия; РИНЦ Author ID 759356,
Вклад авторов:
Жестков И.А. – написание текста рукописи, анализ клинических и литературных данных, взаимодействие с редакцией журнала, 20%
Олейникова Н.А. – написание текста рукописи, анализ клинических и литературных данных, 20%
Охоботов Д.А. – научное руководство, критический обзор, 5%
Камалов Д.М. – научное руководство, критический обзор, 5%
Дзитиев В.К. – научное руководство, критический обзор, 5%
Варенцов М.Г. – анализ данных, подготовка иллюстраций, 15%
Нестерова О.Ю. – научное руководство, критический обзор, 5%
Тивтикян А.С. – анализ данных, написание текста рукописи, 15%
Мальков П.Г. – научное руководство, критический обзор, 5%
Камалов А.А. – научное руководство, критический обзор, научное редактирование, 10%
Конфликт интересов: Авторы декларируют отсутствие явных и потенциальных конфликтов интересов, связанных с публикацией настоящей статьи.
Финансирование: Статья подготовлена без спонсорской поддержки.
Статья поступила: 1
Результаты рецензирования: 1
Исправления получены: 1
Принята к публикации: 3
Information about authors:
Moscow,Russia;
Oleynikova N.A. – PhD, Research Fellow, Department of Clinical Pathology,Medicaland Educational Scientific Center, Lomonosov Moscow State University; Moscow, Russia;
RSCIAuthor ID 824855,
Okhobotov D.A. – Dr. Sci., Associate Professor, Department of Urology and Andrology, Faculty of Fundamental Medicine, Lomonosov Moscow State University; Moscow, Russia; RSCI Author ID 759176,
Kamalov D.M. – PhD, urologist of Medical and EducationalScientific Center of Lomonosov Moscow State University and researcher of Department of Urology and Andrology, Medical and Educational Scientific Center, Lomonosov Moscow State University; Moscow, Russia;
DzitievV.K. – PhD, Head of the Urological Department,Research Fellow,Departmentof Urology and Andrology, Medical and EducationalScientific Center, Lomonosov Moscow State University;Moscow, Russia;
Kamalov A.A. – Professor,Dr. Sci., Member of the Russian Academy of Sciences, Director, Medical and Educational Scientific Center, LomonosovMoscow State University; Head of the Department of Urology and Andrology,Faculty of Fundamental Medicine, Lomonosov Moscow State University; Moscow, Russia; RSCI Author ID 759356,
Authors’ contributions:
Zhestkov I.A. – writing the manuscript, analyzing clinical and literature data, interacting with the editorial board, 20%
Oleynikova N.A. – writing the manuscript, analyzing clinical and literature data, 20%
Okhobotov D.A. – scientific supervision, critical review, 5%
Kamalov D.M. – scientific supervision, critical review, 5%
Dzitiev V.K. – scientific supervision, critical review, 5%
Varentsov M.G. – data analysis, preparing illustrations, 15%
Nesterova O.Yu. – scientific supervision, critical review, 5%
Tivtikyan A.S. – data analysis, writing the manuscript, 15%
Malkov P.G. – scientific supervision, critical review, 5%
Kamalov A.A. – scientific supervision, critical review, scientific editing, 10%
Conflict of interest. The authors declare that there are no obvious or potential conflicts of interest related to the publication of this article.
Financing. The article was made without financial support.
Список литературы Внутриопухолевая гетерогенность паренхимы и стромы рака мочевого пузыря
- Antoni S, Ferlay J, Soerjomataram I, Znaor A, Jemal A, Bray F. Bladder cancer incidence and mortality: a global overview and recent trends. Eur Urol 2017:71(1):96-108. https://doi.org/10.1016/J.EURURO.2016.06.010.
- Willis D, Kamat M. Nonurothelial bladder cancer and rare variant histologies. Hematol Oncol Clin North Am 2015;29(2):237-52. https://doi.org/10.1016/j.hoc.2014.10.011.
- Smith AB, Deal AM, Woods ME, Wallen EM, Pruthi RS, Chen RC, et al. Muscle-invasive bladder cancer: evaluating treatment and survival in the National Cancer Data Base. BJU Int 2014;114(5):719-26. https://doi.org/10.1111/bju.12601.
- Grossman B., Natale R, Tangen C, Speights V, Vogelzang,N., Trump D, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med 2003;349(9):859-66. https://doi.org/10.1056/NEJMoa022148.
- Tokuyama N, Saito A, Muraoka R, Matsubara S, Hashimoto T, Satake N, et al. Prediction of non-muscle invasive bladder cancer recurrence using machine learning of quantitative nuclear features. Mod Pathol 2022;35(4):533-8. https://doi.org/10.1038/s41379-021-00955-y.
- Fernandez-Gomez J, Solsona E, Unda M, Martinez-Piñeiro L, Gonzalez M, Hernandez R, et al. Prognostic factors in patients with non-muscle-invasive bladder cancer treated with bacillus Calmette-Guérin: multivariate analysis of data from four randomized CUETO trials. Eur Urol 2008;53(5):992-1001. https://doi.org/10.1016/j.eururo.2007.10.006.
- Xylinas E, Kent M, Kluth L, Pycha A, Comploj E, Svatek R, et al. Accuracy of the EORTC risk tables and of the CUETO scoring model to predict outcomes in non-muscle-invasive urothelial carcinoma of the bladder. Br J Cancer 2013;109(6):1460-6. https://doi.org/10.1038/bjc.2013.372.
- Zlotta A, Fleshner N, Jewett M. The management of BCG failure in non-muscle-invasive bladder cancer: an update. Can Urol Assoc J 2009;3(6 Suppl 4):S199-205. https://doi.org/10.5489/cuaj.1196.
- Sawazaki H, Ito K, Asano T, Kuroda K, Horiguchi A, Tsuda H, et al. Expressions of P-Glycoprotein, multidrug resistance Protein 1 and Annexin A2 as predictive factors for intravesical recurrence of bladder cancer after the initial transurethral resection and immediate single intravesical instillation of adriamycin. Asian Pac J Cancer Prev 2021;22(5):1459-66. https://doi.org/10.31557/APJCP.2021.22.5.1459.
- Ayari C, LaRue H, Hovington H, Decobert M, Harel F, Bergeron A, et al. Bladder tumor infiltrating mature dendritic cells and macrophages as predictors of response to bacillus Calmette-Guérin immunotherapy. Eur Urol 2009;55(6):1386-95. https://doi.org/10.1016/j.eururo.2009.01.040.
- Rebello R, Oing C, Knudsen K, Loeb S, Johnson D, Reiter R, et al. Prostate cancer. Nat Rev Dis Primers 2021;7(1):9. https://doi.org/10.1038/s41572-020-00243-0.
- Perou CM, Sørlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, et al. Molecular portraits of human breast tumours. Nature 406(6797):747-52. https://doi.org/10.1038/35021093.
- Damrauer J, Hoadley K, Chism D, Fan C, Tiganelli C, Wobker S, et al. Intrinsic subtypes of high-grade bladder cancer reflect the hallmarks of breast cancer biology. Proc Natl Acad Sci USA 2014;111(8):3110-5. https://doi.org/10.1073/pnas.1318376111.
- Choi W, Porten S, Kim S, Willis D, Plimack E, Hoffman-Censits J, et. al. Identification of distinct basal and luminal subtypes of muscle-invasive bladder cancer with different sensitivities to frontline chemotherapy. Cancer Cell 2014;25(2):152-65. https://doi.org/10.1016/j.ccr.2014.01.009.
- Millikan R, Dinney C, Swanson D, Sweeney P, Ro J, Smith T, et al. Integrated therapy for locally advanced bladder cancer: final report of a randomized trial of cystectomy plus adjuvant M-VAC versus cystectomy with both preoperative and postoperative M-VAC. J Clin Oncol 2001;19(20):4005-13. https://doi.org/10.1200/JCO.2001.19.20.4005.
- 1Cancer Genome Atlas Research Network. Comprehensive molecular characterization of urothelial bladder carcinoma. Nature 2014;507(7492):315-22. https://doi.org/10.1038/nature12965.
- Lindgren D, Frigyesi A, Gudjonsson S, Sjödahl G, Hallden C, Chebil G, et al. Combined gene expression and genomic profiling define two intrinsic molecular subtypes of urothelial carcinoma and gene signatures for molecular grading and outcome. Cancer Res 2010;70(9):3463-72. https://doi.org/10.1158/0008-5472.CAN-09-4213.
- Sjödahl G, Lauss M, Lövgren K, Chebil G, Gudjonsson S, Veerla S, et al Molecular taxonomy for urothelial carcinoma. Clin Cancer Res 2012;18(12):3377-86. https://doi.org/10.1158/1078-0432.CCR-12-0077-T.
- Sjödahl G, Lövgren K, Lauss M, Patschan O, Gudjonsson S, Chebil G, et al. Toward a molecular pathologic classification of urothelial carcinoma. Am J Pathol 2013;183(3):681-91. https://doi.org/10.1016/j.ajpath.2013.05.013.
- Sjödahl G, Eriksson P, Liedberg F, Höglund M. Molecular classification of urothelial carcinoma: global mRNA classification versus tumour-cell phenotype classification. J Pathol 2017;242(1):113-25. https://doi.org/10.1002/path.4886.
- Wang H, Mei Y, Luo C, Huang Q, Wang Z, Lu G, et al. Single-Cell Analyses Reveal Mechanisms of Cancer Stem Cell Maintenance and Epithelial-Mesenchymal Transition in Recurrent Bladder Cancer. Clin Cancer Res 2021;27(22):6265-78. https://doi.org/10.1158/1078-0432.CCR-20-4796.
- Kamoun A, de Reyniès A, Allory Y, Sjödahl G, Robertson A, Seiler R, et al. Bladder Cancer Molecular Taxonomy Group. A Consensus Molecular Classification of Muscle-invasive Bladder Cancer. Eur Urol 2020;77(4):420-33. https://doi.org/10.1016/j.eururo.2019.09.006.
- Sanli O, Dobruch J, Knowles M, Burger M, Alemozaffar M, Nielsen M, et al Bladder cancer. Nat Rev Dis Primers 2017;3:17022. https://doi.org/10.1038/nrdp.2017.22.
- Dadhania V, Zhang M, Zhang L, Bondaruk J, Majewski T, Siefher-Radtke A, et al. MetaAnalysis of the Luminal and Basal Subtypes of Bladder Cancer and the Identification of Signature Immunohistochemical Markers for Clinical Use. EBioMedicine 2016;12:105-17. https://doi.org/10.1016/j.ebiom.2016.08.036.
- Hardy C, Ghaedi H, Slotman A, Sjödahl G, Gooding R, Berman D, et al. Immunohistochemical Assays for Bladder Cancer Molecular Subtyping: Optimizing Parsimony and Performance of Lund Taxonomy Classifiers. J Histochem Cytochem 2022;70(5):357-75. https://doi.org/10.1369/00221554221095530.
- Xiao Y, Yu D. Tumor microenvironment as a therapeutic target in cancer. Pharmacol Ther 2021;221:107753. https://doi.org/10.1016/j.pharmthera.2020.107753.
- Caramelo B, Zagorac S, Corral S, Marqués M, Real F. Cancer-associated Fibroblasts in Bladder Cancer: Origin, Biology, and Therapeutic Opportunities. Eur Urol Oncol 2023;6(4):366-75. https://doi.org/10.1016/j.euo.2023.02.011.
- Yang Y, Yang Y, Yang J, Zhao X, Wei X. Tumor microenvironment in ovarian cancer: function and therapeutic strategy. Front Cell Dev Biol 2020;8:758. https://doi.org/10.3389/fcell.2020.00758.
- Zhuang J, Lu Q, Shen B, Huang X, Shen L, Zheng X, et al. TGFβ1 secreted by cancer-associated fibroblasts induces epithelial-mesenchymal transition of bladder cancer cells through lncRNA-ZEB2NAT. Sci Rep 2015;5:11924. https://doi.org/10.1038/srep11924.
- Schulte J, Weidig M, Balzer P, Richter P, Franz M, Junker K, et al. Expression of the E-cadherin repressors Snail, Slug and Zeb1 in urothelial carcinoma of the urinary bladder: relation to stromal fibroblast activation and invasive behaviour of carcinoma cells. Histochem Cell Biol 2012;138(6):847-60. https://doi.org/10.1007/s00418-012-0998-0.
- Santi A, Kugeratski F, Zanivan S. Cancer associated fibroblasts: the architects of stroma remodeling. Proteomics 2018;18(5-6):e1700167. https://doi.org/10.1002/pmic.201700167.
- Edwards D, Handsley M, Pennington C. The ADAM metalloproteinases. Mol Aspects Med 2008;29(5):258-89. https://doi.org/10.1016/j.mam.2008.08.001.
- Kalluri R. Basement membranes: structure, assembly and role in tumour angiogenesis. Nat Rev Cancer 2003;3(6):422-33. https://doi.org/10.1038/nrc1094.
- Liu YT, Sun ZJ. Turning cold tumors into hot tumors by improving T-cell infiltration. Theranostics 2021;11(11):5365-86. https://doi.org/10.7150/thno.58390.
- Gil-Julio H, Perea F, Rodriguez-Nicolas A, Cozar J, González-Ramirez A, Concha A, et al. Tumor escape phenotype in bladder cancer is associated with loss of HLA Class I Expression, T-Cell exclusion and stromal changes. Int J Mol Sci 2021;22(14):7248. https://doi.org/10.3390/ijms22147248.
- Pavlides S, Whitaker-Menezes D, Castello-Cros R, Flomenberg N, Witkiewicz A, Frank P, et al. The reverse Warburg effect: aerobic glycolysis in cancer associated fibroblasts and the tumor stroma. Cell Cycle 2009;8(23):3984-4001. https://doi.org/10.4161/cc.8.23.10238.
- Öhlund D, Handly-Santana A, Biffi G, Elyada E, Almeida A, Ponz-Sarvise M, et al. Distinct populations of inflammatory fibroblasts and myofibroblasts in pancreatic cancer. J Exp Med 2017;214(3):579-96. https://doi.org/10.1084/jem.20162024.
- Galbo PM Jr, Zang X, Zheng D. Molecular Features of Cancer-associated Fibroblast Subtypes and their Implication on Cancer Pathogenesis, Prognosis, and Immunotherapy Resistance. Clin Cancer Res 2021;27(9):2636-47. https://doi.org/10.1158/1078-0432.CCR-20-4226.
- Hanahan D. Hallmarks of cancer: new dimensions. Cancer Discov 2022;12(1):31-46. https://doi.org/10.1158/2159-8290.CD-21-1059.
- Chen Z, Zhou L, Liu L, Hou Y, Xiong M, Yang Y, et al. Single-cell RNA sequencing highlights the role of inflammatory cancer-associated fibroblasts in bladder urothelial carcinoma. Nat Commun 2020;11(1):5077. https://doi.org/10.1038/s41467-020-18916-5.
- Ma Z, Li X, Mao Y, Wei C, Huang Z, Li G, Interferon-dependent SLC14A1+ cancer-associated fibroblasts promote cancer stemness via WNT5A in bladder cancer. Cancer Cell 2022;40(12):1550-1565.e7. https://doi.org/10.1016/j.ccell.2022.11.005.
- Costa A, Kieffer Y, Scholer-Dahirel A, Pelon F, Bourachot B, Cardon M, et al. Fibroblast heterogeneity and immunosuppressive environment in human breast cancer. Cancer Cell 2018;33(3):463-479.e10. https://doi.org/10.1016/j.ccell.2018.01.011.
- Calvete J, Larrinaga G, Errarte P, Martín A, Dotor A, et al. The coexpression of fibroblast activation protein (FAP) and basal-type markers (CK 5/6 and CD44) predicts prognosis in high-grade invasive urothelial carcinoma of the bladder. Hum Pathol 2019;91:61-8. https://doi.org/10.1016/j.humpath.2019.07.002.
- Liu B, Zhan Y, Chen X, Hu X, Wu B, Pan S. Weighted gene co-expression network analysis can sort cancer-associated fibroblast-specific markers promoting bladder cancer progression. J Cell Physiol 2021;236(2):1321-31. https://doi.org/10.1002/jcp.29939.
- Mezheyeuski A, Segersten U, Leiss L, Malmström P, Hatina J, Östman A, et al. Fibroblasts in urothelial bladder cancer define stroma phenotypes that are associated with clinical outcome. Sci Rep 2020;10(1):281. https://doi.org/10.1038/s41598-019-55013-0.
- Schulte J, Weidig M, Balzer P, Richter P, Franz M, Junker K, et al. Expression of the E-cadherin repressors Snail, Slug and Zeb1 in urothelial carcinoma of the urinary bladder: relation to stromal fibroblast activation and invasive behaviour of carcinoma cells. Histochem Cell Biol 2012;138(6):847-60. https://doi.org/10.1007/s00418-012-0998-0.
- Council L, Hameed O. Differential expression of immunohistochemical markers in bladder smooth muscle and myofibroblasts, and the potential utility of desmin, smoothelin, and vimentin in staging of bladder carcinoma. Mod Pathol 2009;22(5):639-50. https://doi.org/10.1038/modpathol.2009.9.
- Alexa A, Baderca F, Lighezan R, Izvernariu D. Myofibroblasts reaction in urothelial carcinomas. Rom J Morphol Embryol 2009;50(4):639-43.
- Zhou Q, Wang Z, Zeng H, Zhang H, Liu Z, Huang Q, et al. Identification and validation of poor prognosis immunoevasive subtype of muscle-invasive bladder cancer with tumor-infiltrating podoplanin+ cell abundance. Oncoimmunology 2020;9(1):1747333. https://doi.org/10.1080/2162402X.2020.1747333.
- Okajima E, Tomizawa M, Shimada K, Negishi T, Nishiyama N, Kitamura H. D2-40/podoplanin expression in cancer stroma by immunohistochemical staining is associated with poor prognosis in bladder cancer patients after radical cystectomy. Urol Oncol 2020;38(10):797.e7-797.e13. https://doi.org/10.1016/j.urolonc.2020.05.020.
- Du Y, Jiang X, Wang B, Cao J, Wang Y, Yu J, et al. The cancer-associated fibroblasts related gene CALD1 is a prognostic biomarker and correlated with immune infiltration in bladder cancer. Cancer Cell Int 2021;21(1):283. https://doi.org/10.1186/s12935-021-01896-x.
- Guan Z, Sun Y, Mu L, Jiang Y, Fan J. Tenascin-C promotes bladder cancer progression and its action depends on syndecan-4 and involves NF-κB signaling activation. BMC Cancer 2022;22(1):240. https://doi.org/10.1186/s12885-022-09285-x.
- Miyai K, Kawamura K, Ito K, Matsukuma S, Tsuda H. Prognostic impact of stromal periostin expression in upper urinary tract urothelial carcinoma. BMC Cancer 2022;22(1):787. https://doi.org/10.1186/s12885-022-09893-7.
- Takeuchi T, Tonooka A, Okuno Y, Hattori-Kato M, Mikami K. Oct4B, CD90, and CD73 are upregulated in bladder tissue following electro-resection of the bladder. J Stem Cells Regen Med 2016;12(1):10-5. https://doi.org/10.46582/jsrm.1201003.
- Said N, Frierson H, Sanchez-Carbayo M, Brekken R, Theodorescu D. Loss of SPARC in bladder cancer enhances carcinogenesis and progression. J Clin Invest 2013;123(2):751-66. https://doi.org/10.1172/JCI64782.


