Восстановление функции кондуита радиальной артерии для шунтирования коронарной артерии
Автор: Рипп Т.М., Кондратьева Д.С., Афанасьев С.А., Муслимова Э.Ф., Козлов Б.Н., Мордовин В.Ф.
Журнал: Сибирский журнал клинической и экспериментальной медицины @cardiotomsk
Рубрика: Клинические исследования
Статья в выпуске: 3 т.33, 2018 года.
Бесплатный доступ
Авторы изучили влияние лерканидипина на функциональное состояние лучевой артерии, используемой в качестве шунта для аортокоронарного шунтирования у пациентов со стенозом коронарной артерии. Эндотелийзависимую вазодилатацию (ΔD) оценивали до операции с использованием ультразвука. Пациенты были разделены на группы I и II с ΔD≥8% и ΔD
Лучевая артерия, шунт, эндотелийзависимая вазодилатация, лерканидипин, дуплексное ультразвуковое исследование
Короткий адрес: https://sciup.org/149125232
IDR: 149125232 | DOI: 10.29001/2073-8552-2018-33-3-30-35
Текст научной статьи Восстановление функции кондуита радиальной артерии для шунтирования коронарной артерии
The use of arterial conduits becomes more common in the coronary artery bypass grafting (CABG). Radial artery is the second most commonly used conduit after the internal thoracic artery [1, 2]. Internal diameter of the radial artery grafts is comparable with diameter of coronary arteries; the artery is easy to access; sufficient length of radial artery conduits can be easily dissected out; radial artery grafts can adjust to high values of arterial blood pressure. A disadvantage in the use of radial artery is a susceptibility of this blood vessel to spasm due to its well-developed smooth muscle layer of the vascular wall [3, 4]. Dissection technique per se ( skeletonizing dissection and mechanical dilatation ) is regarded as a factor provoking spasm [5, 6]. Considering the risk of spasm, the radial artery conduits are used only when coronary stenosis is less than 75% [7]. Different approaches have been proposed and are currently used for auto-arterial conduit preparation including various dissection techniques and different options of the pharmacological antispasmodic protocol. For example, extrafascial harvesting technique, instead of skeletonization, significantly decreases the frequency of arterial spasm [8, 9]. Treatment of the arterial conduits with calcium antagonists, nitrates, α -adrenoblockers, their combinations, and calmodulin also prevents arterial spasm [10–12]. All these measures result in beneficial effects for most of patients. However, standard prophylaxis of spasm remains ineffective in 10 to 15% of patients where the use of radial artery leads to serious complications. In case of proper dissection technique, vasospasm can be caused by the abnormal endothelial function [13]. It has been known that atherosclerosis, arterial hypertension, and other risk factors of cardiovascular diseases affect functional activity of endothelium in both coronary and peripheral blood vessels including radial artery [14]. Endothelial dysfunction caused by deleterious factors (mechanical, metabolic, immune etc.) leads to the changes in the endocrine activity of the epithelium. Up to now, large pools of data have been accumulated regarding antihypertensive drugs with vasoprotective properties such as calcium antagonists. Data show that vasodilation in the presence of calcium antagonists is caused by both decrease in the transmembrane influx of calcium ions into the cells and NO/cGMP-mediated mechanisms [15]. Treatment with these drugs improves functional characteristics of the blood vessels. However, whether the preoperative use of calcium antagonists prevents the radial artery spasm during and after CABG remains unknown. In this context, the aim of our study was to elucidate the effects of therapy with calcium antagonist, lercanidipine, on functional properties of radial artery during preoperative preparation of a bypass graft.
Material and Methods
Patients
A total of 34 patients (mean age of 59.4±8.6 years) were included in the study. All patients received full information and signed the informed consent form for participation in the study for surgical treatment of chronic coronary insuf- ficiency. Inclusion criteria were ischemic heart disease and cardiac angina of NYHA functional class III to IV with hemo-dynamically significant three-vessel coronary stenosis including right coronary artery with medium to high risk for open and percutaneous coronary interventions and SYNTAX Score ≥23; patients had history of arterial hypertension. Patients underwent CABG with the use radial artery conduit. Exclusion criteria were absence of signed informed consent form, acute myocardial infarction, previous cardiac surgeries, and high and very high risk for complications after the CABG/ cardiopulmonary bypass due to severe renal, hepatic, respiratory, and cerebral insufficiency.
Study protocol
The preoperative preparation procedure included ultrasonic scanning of radial artery. Anatomical assessment of the artery for the use as a conduit was performed according to the Allen’s test [16, 17]. Functional properties of radial artery were preoperatively assessed by duplex ultrasound with Philips-ATL HDI 5000 SonoCT ultrasound machine with linear probe. The artery was examined by using the reactive hyperemia test [18, 19]. Patients were asked to stop drinking tonic beverages 24 h before the study. After resting in supine position for 10 min , patients underwent the initial duplex ultrasound study of the radial artery. Then, the standard sphygmomanometer pneumatic cuff was placed around the forearm 2–3 cm lower than the position for the ultrasound probe and inflated to 300 mm Hg for 5 min . Duplex ultrasound study was repeated 30 s before the cuff deflation, right after the cuff deflation, and, then, every 15 s . The last measurement was taken at 120 s after the beginning of hyperemia. Radial artery diameter was measured on the intima-media border in the area of anatomical projection for the radial artery at distance of 3–6 cm from the bend of elbow. Diameters were measured for five cardiac cycles and the values were averaged. The change in arterial diameter was calculated by using the equation: Δ D = (Dmax–D0)/D0 • 100%, where Δ D was the change in diameter; D0 was an initial arterial diameter; and Dmax was the maximum arterial diameter 60 s after the beginning of increase in intravascular pressure [20].
Initial ultrasound duplex ultrasound studies and reactive hyperthermia tests allowed calculating the changes in arterial diameter. Patients were divided into two groups: group I ( n =11) included patients whose Δ D was >8%; group II ( n =23) included individuals whose Δ D was <8%. Patients of group II were randomized into subgroups IIA ( n =12) and IIB ( n =11). Patients of subgroup IIA and group I received preoperative preparation for CABG according to the common plan. Patients of subgroup IIB received additional course of treatment with calcium channel blocker, lercanidipine (Ler-carmen, Berlin-Chemie Menarini), in a daily dose of 2.5–5 mg during 5 to 7 days before surgery. After the course, the duplex ultrasound study and reactive hyperemia test were repeated in these patients to evaluate endothelium-dependent vasodilation of radial artery.
Study of tonic activity of isolated radial artery
Direct studies of tonic activity of the radial artery were performed by using 1- mm -long isolated rings dissected during a preparation of the conduit for CABG. The rings were
Table
Clinical characteristics of patients by groups
|
Parameters |
Group I |
Group IIA |
Group IIB |
|
Age |
59.3±6.1 |
58.9±10.2 |
57.8±11.2 |
|
Waist circumference, cm |
98.6±12.5 |
92.4±8.9 |
91.5±9.9 |
|
Glucose, mmol/l |
5.6±0.6 |
5.8±0.8 |
5.6±0.6 |
|
Cholesterol, mmol/l |
6.2±1.1 |
6.6±1.5 |
6.8±1.3 |
|
SBP office, mm Hg |
136.0±14.2 |
140.5±12.4 |
142.5±13.5 |
|
DBP office, mm Hg |
94.1±12.4 |
92.9±8.5 |
91.9±7.4 |
|
IVS, mm |
11.2±1.6 |
11.7±1.5 |
11.9±1.6 |
|
Diameter of radial artery, mm |
2.6±1.1 |
2.8±0.9 |
2.8±0.9 |
|
TPW LV, mm |
11.6±1.3 |
11.1±2.0 |
11.6±1.9 |
|
MMI LV, g/m2 |
101.7±18.5 |
97.6±23.1 |
100.4±21.3 |
Comments: SBP — systolic blood pressure, DBP — diastolic blood pressure, IVS — interventricular septum, LV — left ventricle, TPW LV — thickness of posterior wall of left ventricle, MMI LV — left ventricular myocardial mass index.
mounted in the temperature stabilized (36 °C) chamber perfused with oxygenated Krebs- Henseleit solution; isometric transducer ( MX -1S, Russia) was used to measure tonic activity. By using the micromanipulators, the arterial rings were stretched to produce a preload of 1 mN of force [21]. Record-
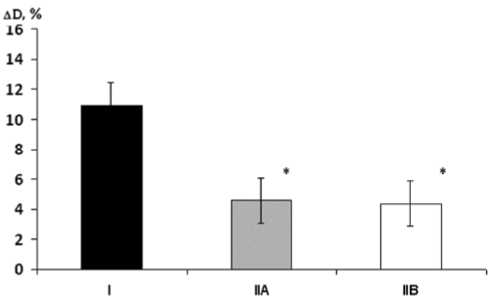
Fig. 1. Initial endothelium-dependent vasodilation ( Δ D) in groups
I, IIA, and IIB
* p < 0.01 when compared with group I.
ing of changes in the arterial tone continued for 5 h . Time duration from dissection of the arterial ring to beginning of its perfusion did not exceed 20 min .
Statistics
The data were processed by using statistical methods (STATISTICA for Windows 10.0 (StatSoft, USA)). Data were described with the mean (M) ± standard deviation (SD). The quality of the data was checked using distribution histograms, in the case of pronounced deviations from the random distribution, the data were re-checked by primary documents for errors in values and violation of the criteria for selecting patients. All variables belonged to the 2 types of distribution: normal and binomial. Significant differences between the normally distributed quantitative values were determined by using Student’s t- test ( paired or independent). Non-parametric Mann — Whitney criterion was used to compare independent variables; Wilcoxon criterion was used to compare paired values. To evaluate relationships between the studied parameters, the Pearson’s correlation coefficient and Spearman’s rank correlation coefficient were calculated . Values were considered statistically significant when p was <0.05.
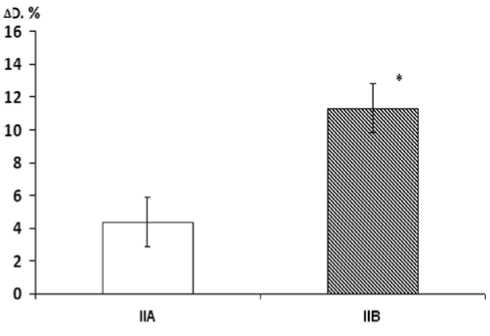
Fig. 2. Changes in parameters of endothelium-dependent vasodilation ( Δ D) in group IIA (without lercanidipine) and group IIB (lerca-nidipine treatment)
* p < 0.05 between groups.
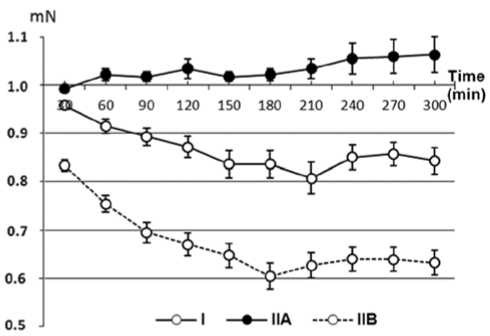
Fig. 3. Changes in the tone of isolated radial artery segments in the experiment. Vertical axis: changes in the tension of arterial segment ( mN ). Horizontal axis: duration of the experiment ( min )
* p < 0.001 when compared with group IIA; # p < 0.001 when compared with group I.
Results
Clinical studies
Table demonstrates that main clinical parameters of patients from the studied groups were comparable.
The results of initial reactive hyperemia test showed that Δ D was 10.96±1.87%, 4.36±1.89%, and 4.36±1.89% in patients from groups I, IIA, and IIB, respectively. Patients from groups IIA and IIB had similarly low ( р =0.01) increase in Δ D compared with group I (Fig. 1).
Reactive hyperemia tests, repeated after the course of ler-canidipine treatment, showed that Δ D in group IIB significantly increased to 11.32±2.22% which was significantly higher ( p =0.02) than both the initial value in this group and the value of this parameter in group IIA after treatment (Fig. 2). After lercanidipine treatment, values of Δ D in group IIB did not significantly differ from Δ D in group I.
One patient of group IIB did not respond to the reactive hyperemia test: no increase in his Δ D was detected according to results of the repeated reactive hyperemia test. One patient of group IIB complained about brief headache after taking lercanidipine; this patient refused to stop therapy or change medication; no other complications were observed.
Study of tonic activity of isolated radial artery
Direct measurements of tonic activity of isolated radial artery rings (Fig. 3) showed that relaxation of the arteries in group I began at first minutes of chamber perfusion; decrease in the tone of arterial rings was 0.04 mN 30 min after of perfusion; tonic force of arterial segments decreased by 0.8 mN after 210 min .
After that, tonic force increased by 0.05 mN and remained unchanged until the end of the experiment (at 0.15 mN below initial values). Dynamic changes in tonic activity of the rings from radial artery in group IIA were opposite: tonic tension of the rings remained steady for 4 h ; the last hour of the experiment showed increase in arterial tension by 0.5 mN . Tonic activity of the ring from radial artery correlated well with duplex ultrasound data from reactive hyperemia test ( r =-0.87, p =0,001). The study of the tonic activity of arterial rings from group IIB showed that, similarly to group I, relaxation of the arteries began in the very beginning of the perfusion, during the first minutes of the examination. Decrease in tonic force of the arterial rings in group IIB exceeded the corresponding values for group I. Indeed, amplitude of radial artery relaxation was 0.17 mN ; tonic force of segments decreased by 0.4 mN at 180 min compared with initial values. Starting from 210 min , we observed insignificant increase in arterial tension by 0.05 mN with corresponded tonic force of the segment of 0.65 mN which lasted until the end of the experiment.
Discussion
Ultrasonic scanning of radial artery demonstrated diversity in parameters of endothelium-dependent vasodilation in two groups of patients. The data showed that the radial artery in all patients was suitable as a conduit for CABG according to the Allen’s test. However, duplex ultrasound of the endothelium-dependent vasodilation provided evidence for dividing patients into two groups. Changes in diameter of the radial artery in group I were more than 8% (ΔD≥8%), whereas changes in diameter of the radial artery in group II were less than 8% (ΔD<8%). This parameter characterizes functional condition of patient’s radial artery [22]. Therefore, one may suggest preservation of the regulatory function of the epithelium and endothelium-dependent vasodilatation of the radial artery in group I and abnormality in the regulatory function of the endothelium in group II. Perhaps, disruption of endocrine activity of the endothelium is caused by atherosclerosis which was the primary disease in this category of patients. Severe atherosclerosis, presented with atherosclerotic arterial stenosis, significantly reduces increases in the radial artery diameter in noninvasive testing [23].
Different functional properties of the radial arteries in groups I and II were confirmed by the results of the direct examination of the tonic activity in the radial artery rings. In group I, the arterial rings with preserved endotheliumdependent vasodilatation continued to relax during the entire experiment; on the contrary, we observed constriction of the arterial rings in subgroup IIA. Development of the arterial spasm can be caused by mechanical stimulation of the radial artery during its dissection for CABG [24, 25]. Apart from this, the underlying reason for the arterial spasm can be abnormal function of endothelium in the radial artery of patients with ischemic heart disease. It has been well known that the vascular endothelium exerts endocrine activity, which depends on endothelial functional status. The data showed the existence of a certain pattern in the endothelial release of the vasoconstrictors and vasodilators [14]. Intact endothelium produces vasodilators. Endothelial dysfunction, caused by mechanical, infectious, metabolic, and immune influences, results in altered endocrine activity of the epithelium leading to predominant synthesis of vasoconstrictors [13]. Our data suggest that, in patients with positive reactive hyperemia test results, the isolated rings of the radial artery preserved both their tonic responsiveness and their ability to decrease the tonic force. The use of such arteries for conduits in CABG would obviously require minimum pharmacological tone control and perioperative myocardial infarction prevention.
The preoperative reactive hyperemia test results can help the doctors to discern whether the radial artery of a given patient can be used as the conduit for CABG; these test results can also help to make a decision about timely administration of necessary pharmacological treatment. Our study showed the positive effects of additional administration of calcium antagonist, lercanidine.
Our data showed that administration of lercanidipine during preoperative preparation in patients with duplex ultrasound changes in the radial artery diameter less than 8% in the reactive hyperemia test contributed to the functional recovery of the radial artery. Beneficial effects of lercanidipine were confirmed by the results of repeated reactive hyperemia test and by direct examination of the tonic activity of the vascular rings isolated from the radial artery. In case of lercan-idipine treatment, vascular relaxation significantly exceeded relaxation of the arteries with initially preserved endothelium-dependent vasodilation. Mechanism of this effect was likely related to increase in NO production [26]. It has been known that in the presence of calcium antagonists cGMP increase in the vascular smooth muscle cells leads to increase in the expression level and activity of NO-synthase (15). Apart from that, vasoprotective action of calcium antagonists may be related to their antioxidant properties [27]. Indeed, some of the dihydropyridine calcium antagonists, especially those with lipophilic properties, such as amlodipine, lacidipine, and nisoldipine, inhibit lipid peroxidation in cell membranes [28]. It has been shown that the endothelial dysfunction is caused with the NO-dependent system damage by oxygen free radicals (reactive oxygen species), the products of lipid peroxidation [29], without changes in receptors or signaling pathways. Due to an increase in the nitric oxide half-life time, calcium antagonists improve endothelial function, inhibit smooth muscle cell proliferation, and prevent cytokine-mediated apoptosis. Beneficial effects of calcium antagonists on the vascular endothelium are caused by their ability to inhibit thromboxane A2 synthesis and stimulate prostacyclin and NO release [30]. It has been also shown that calcium antagonists augment bradykinin release [31]. A significant factor determining the extent of vasoprotective effects may be suppression of the processes involved in atherogenesis [32]. It has been demonstrated that dihydropyridine calcium antagonists exert their antisclerotic (cholesterol ester hydrolysis and attenuation of intracellular lipid accumulation), antiproliferative (suppression of migration and proliferation of smooth muscle cells and microphages), and antiplatelet effects via the NO-dependent mechanisms.
Conclusion
The radial artery duplex ultrasound study with the reactive hyperemia test provides a way for adequate evaluation of the functional properties of radial artery. Values of radial artery Δ D>8% in the reactive hyperemia test represent a prog-nostically good sign and suggest about preserved functional condition of the artery. This is confirmed by decrease in tonic force of the isolated arterial segment. Changes in diameter of radial artery less than 8% are prognostically unfavourable and may be considered as a criterion for contraindication of the use of the radial artery as a graft unless prior medicinal correction is performed before surgery. The use of calcium channel blocker, lercanidipine, in patients, whose reactive hyperemia test showed Δ D of less than 8%, at the preoperative preparation stage allows recovery of endothelium-dependent vasodilation and prevention of intraoperative constriction in isolated arterial segments. Therefore, the use of lercanidipine provides a way for effective correction of the functional condition of the arterial conduit from radial artery.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Список литературы Восстановление функции кондуита радиальной артерии для шунтирования коронарной артерии
- Achouh P., Isselmou K. O., Boutekadjirt R., D'Alessandro C., Pagny G. Y., Fouquet R., Fabiani J. N., Acar C. Reappraisal of a 20-year experience with the radial artery as a conduit for coronary bypass grafting. Eur. J. Cardiothorac. Surg. 2012; 41(1): 87-92.
- Ruttmann E., Fischler N., Sakic A., Alber H., Chevtchik O., Schistek R., Ulmer H., Grimm M. Second internal thoracic artery versus radial artery in coronary artery bypass grafting: a longterm, propensity score-matched follow-up study. Circulation. 2011; 124(12): 1321-1329.
- He G. W., Yang C. Q., Starr A. Overview of the nature of vasoconstriction in arterial grafts for coronary surgery. Ann. Thorac. Surg. 1995: 59: 676-683.
- Friedman D. T., Pettersson G., Smedira N. G., Li J., Ellis S. G. Radial artery bypass grafts have an increased occurrence of angiographically severe stenosis and occlusion compared with left internal mammary arteries and saphenous vein grafts. Circulation. 2004; 109: 2086-2091.
- Acar C., Jebara V. A., Portoghese M., Beyssen B., Pagny J. Y., Grare P., Chachques J. C., Fabiani J. N., Deloche A., Guermonprez J. L. Revival of the radial artery for coronary artery bypass grafting. Ann. Thorac. Surg. 1992; 54: 652-660.
- He G. W., Yang C. Q. Radial artery has higher receptor-mediated contractility but similar endothelial function compared with mammary artery. Ann. Thorac. Surg. 1997; 63: 1346-1352.
- Maniar H. S., Sundt T. M., Barner H. B., Prasad S. M., Peterson L., Absi T., Moustakidis P. Effect of target stenosis and location on radial artery graft patency. J. Thorac. Cardiovasc. Surg. 2002; 123: 45-52.
- Ali E., Saso S., Ahmed K., Athanasiou T. When harvested for coronary artery bypass graft surgery, does a skeletonized or pedicled radial artery improve conduit patency. Interact. Cardiovasc. Thorac. Surg. 2010; 10(2): 289-292.
- Miyagi N., Oshima N., Shirai T., Sunamori M. Skeletonised harvesting improves early-term and mid-term perfect patency of a radial artery graft. Jpn J. Thorac. Cardiovasc. Surg. 2006; 54: 472-476.
- Attaran S., John L., El-Gamel A. Clinical and potential use of pharmacological agents to reduce radial artery spasm in coronary artery surgery. Ann. Thorac. Surg. 2008; 85(4): 1483-1489.
- Shipulin V. M., Kozlov B. N., Korovin N. V., Afanasiev S. A. Intraoperative preparation of the radial artery for coronary artery bypass grafting. Angiology and Vascular Surgery. 2005; 2: 122-129.
- Vecherskiĭ Yu. Y.u, Andreev S. L., Murashev B. Yu. New aspects of using dihydropyridine calcium antagonists during coronary bypass surgery. Ter. Arkh. 2010; 82(12): 19-22.
- Moens A. L., Goovaerts I., Claeys M. J., Vrints C. J. Flow-Mediated Vasodilation A Diagnostic Instrument, or an Experimental Tool? Chest. 2005; 127: 2254-2263.
- Inoue T., Matsuoka H., Higashi Y., Ueda S., Sata M., Shimada K. E., Ishibashi Y., Node K. Flow-mediated vasodilation as a diagnostic modality for vascular failure. Hypertens. Res. 2008; 31(12): 2105-2113.
- Ding Y., Vaziri N. D. Nifedipine and diltiazem but not verapamil up-regulate endothelial nitric-oxide synthase expression. J. Pharmacol. Exp. Ther. 2000; 292: 606-609.
- Kohonen M., Teerenhovi O., Terho T., Laurikka J., Tarkka M. Is the Allen test reliable enough? Eur. J. Cardiothorac. Surg. 2007; 32(6): 902-905.
- Yokoyama N., Takeshita S., Ochiai M., Hoshino S., Koyama Y., Oshima A., Isshiki T., Sato T. Direct assessment of palmar circulation before transradial coronary intervention by color Doppler ultrasonography. Am. J. Cardiol. 2000; 86: 218-221.
- Afanasiev S. A., Ripp T. M., Kozlov B. N., Kondratieva D. S., Mordovin V. F., Shipulin V. M. Method of the noninvasive assessment of functional competence of the radial artery for use as a vascular graft during performing coronary bypass operations. Patent for an invention RUS 2421139 24.06.2009.
- Leluk V. G., Leluk S. E. Ultrasonic Angiology. Moscow: Medicine Press; 2003: 121-124.
- Balakhonova T. V., Pogorelova O. A., Alidzhanova Kh. G., Soboleva G. N., At'kov O. Y. Noninvasive investigation of endothelial function in patients with hypertension and hypercholesterolemia (HCE). Ter. Arkh. 1998; 70(4): 15-19.
- Kondrat'eva D. S., Afanas'ev S. A., Evtushenko A. V., Evtushenko V. V., Shipulin V. M. Сhronotropic and inotropic dependence of the myocardium in patients with rheumatic heart disease before and after amiodarone treatment. Fiziol. Cheloveka. 2008; 34(1): 52-56.
- Zhen W., Tong H., Wang Y., Sun Y., Huang W., Ma Y., Tian J., Wu L. Coronary bypass revascularization with radial artery and internal mammary artery grafts. Chin. Med. J. 2002; 115(1): 55-57.
- Atkov O. Y., Balahonova T. V., Pogorelova O. A. Non-invasive ultrasound detection of endothelial dysfunction. Eur. J. Ultrasound. 1998; 7(1): 37-45.
- Bokeria L. A., Sigaev I. Iu., Katsiia G. V., Berishvili I. I., Piskun A. V., Buziashvili Iu. I., Alekian B. G., Chigogidze N. A. Results of hospital bypass angiography in patients with coronary artery disease undergoing arterial and vein myocardial revascularization. Angiol. Sosud Khir. 2003; 9(2): 32-38.
- Carpentier A., Guermonprez J. L., Deloche A., Frechette C., Du-Bost C. The aorta-to-coronary radial artery bypass graft. A technique avoiding pathological changes in grafts. Ann. Thorac. Surg. 1973; 16: 111-112.
- Nakayama N., Ikezono K., Ohura M., Yabuuchi Y. Effects of the new long-acting dihydropyridine calcium antagonist pranidipine on the endothelium-dependent relaxation in isolated rat aorta in vitro. Arzneimittelforschung. 1993; 43(12): 1266-1270.
- Luscher T. F. Endothelial dysfunction as a therapeutic target. The ENCORE trials. Eur. Heart J. 2000; 2 (Suppl D): 20-25.
- Taddei S., Virdis A., Ghiadoni L., Uleri S., Magagna A., Salvetti S. Lacidipine restores endothelium-dependent vasodilation in essential hypertensive patients. Hypertension. 1997; 30: 1606-1612.
- Vaziri N. D., Rodríguez-Iturbe B. Mechanisms of Disease: oxidative stress and inflammation in the pathogenesis of hypertension. Nature Clinical Practice Nephrology. 2006; 2(10): 582-593.
- Berkels R., Taubert D., Bartels H., Breitenbach T., Klaus W., Roesen R. Amlodipine increases endothelial nitric oxide by dual mechanisms. Pharmacology. 2004; 70: 39‑45.
- Cominacini L., Pasini A. F., Pastorino A. M., Garbin V., Davoli A., Rigoni A., Campagnola M., Tosetti M. L., Rosato P., Gaviraghi G. Comparative effects of different dihydropyridines on the expression of adhesion molecules induced by TNF-alpha on endothelial cells. J. Hypertens. 1999; 17: 1837-1841.
- Ishii N., Matsumura T., Shimoda S., Araki E. Anti-atherosclerotic potential of dihydropyridine calcium channel blockers. J. Atheroscler. Thromb. 2012; 19(8): 693-704.


