Berberine in emulsions: possibilities to stimulate GABA production in yogurt
Автор: Anjum V., Kadi A.M.Y., Potoroko I.Yu.
Рубрика: Пищевые ингредиенты, сырье и материалы
Статья в выпуске: 3 т.12, 2024 года.
Бесплатный доступ
A wide range of bioactive components such as vitamins, bacteriocins, bioactive peptides and bioactive compounds can be obtained in the process of fermentation with lactic acid bacteria (LAB), which are commonly used in various fermented food products, including fermented milk products. The ingredients produced in the process of fermentation of raw milk form the attractiveness of finished products for consumers in the functional products segment due to the provided usefulness due to their bioactivity and health benefits. To increase the level of usefulness, a significant part of fermented milk products are enriched with bioactive ingredients that can act as activators of starter microflora. The fortificates used are relevant both as in situ components and functional additives. Berberine (BB) requires special attention as a fortificate; it has been used in various formulas for a long time due to its pharmacological action. Berberine has very limited oral bioavailability, despite a wide range of pharmacological action. Gut microbiota has become a hot topic in recent years for investigating the modes of action of herbal medicines. BB has minimal toxicity and provides therapeutic benefits at recommended dosages. Numerous studies have shown that BB can interact with gut microbiota and exhibit altered pharmacological effects as a result of its poor bioavailability. The proposed material evaluates the possibility of using BB in double emulsions for the fortification of fermented milk products using yogurt as an example, as the most attractive to consumers, as well as an answer to the question regarding its technological suitability as an activator for the synthesis of gamma-aminobutyric acid in the final product.
Lactic acid bacteria, berberine, emulsions, functional additives, toxicity, bioavailability
Короткий адрес: https://sciup.org/147244568
IDR: 147244568 | УДК: 664.8.038+663 | DOI: 10.14529/food240302
Текст научной статьи Berberine in emulsions: possibilities to stimulate GABA production in yogurt
A quaternary ammonium salt belonging to the protoberberine group of isoquinoline alkaloids is called berberine (BB) [1]. The latter are often found in plant roots, bark, and other materials that are used in traditional East Asian treatments. According to Cicero and Baggioni [2], some examples of these plants are Coptis chinensis, Berberis aristata, B. petiolaris, B. vulgaris, B. aquifolium, and B. thunbergii. Plant groups such as Annonaceae, Berberidaceae, Menispermaceae, and Papaveraceae are among those from which BB can be isolated. Phellodendron amurense and C. chinensis have been shown to have an abnormally high BB concentration [3].
For more than a millennium, humans have been employing BB in traditional herbal formulations to treat a wide range of illnesses, including typhoid, gastroenteritis, diabetes mellitus (DM), and secretory diarrhoea. These mixtures work incredibly well for eliminating heat, drying out moisture, extinguishing fire, and detoxifying [4, 5]. According to Jin et al. [6], BB and its derivatives exhibit a number of pharmacologic actions via different pathways. Because of its multipletarget actions, BB may be therapeutic against a variety of chronic diseases, including obesity, diabetes mellitus, inflammatory bowel disease (IBD), atherosclerosis, Alzheimer's disease, rheumatoid arthritis, and cardiovascular disorders [6].
Studies on animals and humans have shown that BB has the ability to modulate the immune system, reduce oxidative stress and inflammatory responses, decrease lipid accumulation, inhibit steatosis and fibrosis, enhance insulin sensitivity, and promote insulin secretion [7]. The field of intestinal microecology has garnered significant attention due to recent developments in microbial sequencing technology, metabolomics technology, and sterile animal models [8]. The host's immune system, metabolism, digestion and absorption of nutrients, and the emergence of disease are all significantly influenced by the gut microbiota. Regulating the host's health requires preserving the stability of the intestinal microecological environment. As a result, maintaining the stability of the intestinal micro-ecological environment is crucial for managing the host's health. There is mounting evidence in recent years that BB can change the gut microbiota's value and composition in an unhealthy state. The gut microbiota is a complex system that is revealing new factors that influence the onset and course of a number of disorders.
A fermented food high in nutrients, yogurt is created from milk and contains lactic acid bacteria, peptones, peptides, and other trace activators. According to Park et al. [9], yogurt has an intestine-cleaning effect by encouraging the growth of intestinal lactic acid bacteria. Korean yogurts occasionally contain ingredients including nonfat dry milk, soy protein, vegetables, sweet potatoes, pumpkins, plums, etc [9]. One excellent source of plant protein is soybean. One of the most prevalent amino acids in legumes, including soybean, red bean, and mung bean, is glutamic acid (Glu).
Microorganisms known as lactic acid bacteria (LAB) are commonly found in the intestine and play a role in the host's physiological metabolism (Table 1). Numerous metabolites are produced by LAB, including as vitamins, amino acids, bacteriocin, organic acids, and exopoly- saccharides. Numerous metabolites, such as organic acids, bacteriocin, amino acids, exopolysaccharides, and vitamins, can be produced by LAB. These metabolites are fundamental to LAB activity and have a significant effect on host health.
Numerous gut bacteria with a wide variety of species inhabit the intestine. By supporting the preservation of the intestinal epithelial barrier, fending off infections, and controlling immunological responses - all of which have an additional impact on the host's nutrition, metabolism, and behavior - metabolites of LAB can maintain the stability and balance of the gut microbiota. Recent LAB research has mostly concentrated on the influence of probiotics on gut microbiota and host health. By generating metabolites, LAB, as members of the gut microbiota, have positive effects on the intestinal lining.
LAB in yogurt preparation
The primary agent in yogurt fermentation is Lactobacillus delbrueckii , and most frequently a particular subspecies ( Lactobacillus delbrueckii subsp. bulgaricus ). This strain is the only one used to ferment some yogurts. At the normal temperature of 42-46 °C for yogurt fermentation, L. delbrueckii flourishes and can quickly eat a significant portion of the lactose in the yogurt, turning it into lactic acid. Numerous chemicals responsible for the distinct flavors and fragrances of yogurt are also produced by L. delbrueckii .
The other bacterium is Streptococcus thermophilus has a small role in the big scheme of yogurt fermentation. However, it is also essential because most yogurt fermentations cannot be finish without this microorganism. While it does create some of the acidity and flavorings seen in yogurt, S. thermophilus mostly functions as a support system for L. delbrueckii. It's interesting to note that S. thermophilus was probably absent from early yogurt because genetic data suggests that it developed to its current state over the last 3,000 years, possibly from a pathogenic species. Together, these two bacteria can break down milk proteins far more quickly than S. thermophilus can, resulting in the production of free amino acids and short peptides. By transforming them into new proteins and a variety of other substances required for its growth, S. thermophilus needs them to sustain its own growth. In exchange, S. thermophilus generates significant amounts of folic acid and ornithine, essential for L. delbrueckii's capacity to synthesis
Table 1
Beneficial characteristics and harmful infections of LAB species in the gut
|
LAB species |
Beneficial characteristics |
Harmful infection types |
Reference |
|
Lactobacillus acidophilus |
Probiotic characteristics; used for dairy products and biomedical treatment |
Dental abscess/caries; Endocarditis; Bacteremia |
[10, 11] |
|
Lacticaseibacillus casei |
Probiotic characteristics; used to ferment dairy products; applied in food, biotechnology and medical fields |
Bacteremia; Endocarditis |
[11, 12] |
|
Lactiplantibacillus plantarum |
Probiotic characteristics; ecological and metabolic adaptability; high acid tolerance response; bile salt hydrolysis ability |
Bacteremia; Cholecystitis |
[11, 13] |
|
Lacticaseibacillus rhamnosus |
Probiotic characteristics; acid- and bile-stability; great avidity for human intestinal mucosal cells |
Endocarditis; Bacteremia |
[14] |
|
Lacticaseibacillus paracasei |
Probiotic characteristics; modulate gut microbiota composition and improve gastrointestinal function |
Endocarditis; pancreatic necrosis |
[15, 16] |
|
Lactobacillus johnsonii |
Probiotic characteristics; interact with a great diversity of immune cells; increase the levels of long chain fatty acids |
N/A |
[17] |
|
Lactobacillus del-brueckii |
Probiotic characteristics; bile salt hydrolysis ability |
Urinary tract infection |
[18, 19] |
|
Limosilactobacillus reuteri |
Probiotic characteristics; widely used to prevent and treat numerous gastrointestinal disorders |
Bacteremia |
[20] |
|
Ligilactobacillus salivarius |
Probiotic characteristics; antimicrobial activity; innate immune responses in epithelial cells |
Bacteremia |
[21, 22] |
|
Lactobacillus gasseri |
Probiotic characteristics; used in food industry as supplements; inhibit increase in body weight and adipose tissue weight |
Liver abscesses |
[23, 24] |
|
Ligilactobacillus ruminis |
Probiotic characteristics; modulate inflammatory cytokines and gut microbiota modulation |
N/A |
[25] |
DNA, but which the latter unable to create in sufficient quantities (Figure 1). As a result, these two species are able to thrive in milk significantly more effectively than either could by fermenting together.
Important amino acid metabolites of LAB
The branched chain amino acid (BCAA) metabolism, the glutamate decarboxylase (GAD) system, the arginine deiminase (ADI) route, and the aromatic amino acid metabolism are the four amino acid metabolic processes found in LAB
-
[26]. Arginine deiminase (ArcA) in the ADI route converts arginine to citrulline and ammonia, and ornithine transcarbamylase then converts citrulline to ornithine and carbamoyl phosphate (Fig. 1). According to Majsnerowska et al. [27], the arginine/ornithine antiporter is in charge of bringing external arginine into the cells and removing ornithine from them. One characteristic of bacteria that is particularly prevalent in Lactobacillales is the ADI pathway. It has been observed that amino acids implicated in these
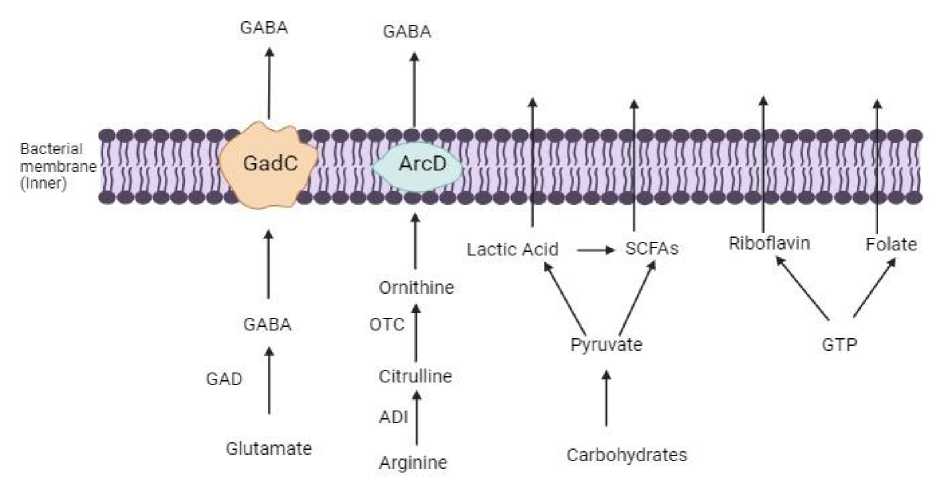
Fig. 1. Synthesis of amino acids, organic acids and vitamins in LAB
pathways are beneficial to human health. One non-essential amino acid that has been utilized to alleviate intestinal irritation is citrulline. An necessary amino acid, ornithine has a number of physiological roles, including promoting muscle growth, stimulating hormone growth, and acting as an anti-obesity agent. A neuroinhibitory molecule known as γ-Aminobutyric Acid (GABA), which is a non-protein amino acid often present in plants, animals, and microbiota, is one of the amino acid metabolites of LAB.
In the mammalian central nervous system, GABA is a significant inhibitory neurotransmitter [28]. In order to prevent nerve cells from overheating, lower blood pressure, and reduce neuronal activity, GABA is widely utilized in the treatment of psychiatric illnesses such epilepsy, convulsions, and Parkinson's disease. In LAB, pyridoxal 5'-phosphate (PLP)-dependent GAD decarboxylates L-glutamate under anaerobic circumstances, irreversibly activating GABA in the process and consuming an H+ (Figure 1). Following intracellular synthesis, extracellular glutamate is transported inside by the Glu/GABA anti-porter GadC, while intracellularly generated GABA is sent to the exterior [29, 30].
Metabolites participate in the modulation of the gut microbiota
Numerous host regulators, including the immune system, neurological system, and food, work together to maintain the gut microbiota's homoeostasis. It is generally acknowledged that the most significant host-dependent regulation that determines individual variances in gut flora is diet. Additionally, the immune system is a major player in controlling the gut microbiota. Defects in Paneth cell secretion of antimicrobial peptides, changes in innate immune system sensors, and the endoplasmic reticulum stress response are all critical regulators of the gut microbial ecology [31]. Another important mechanism controlling the gut microbiota is the neurological system. It has been demonstrated that the gutbrain axis (GBA) serves as a conduit for the gut microbiota and the central nervous system (CNS) (Fig. 2).
Neuronal circuits of the enteric nervous system (ENS) receive signals directly from the central nervous system (CNS), and these circuits are important in the regulation of the gut microbiota [32]. The brain can communicate with the gut microbiota in both directions through a variety of pathways, including bacterial metabolites (such as SCFAs, folate, and EPS), neurotransmitter production (such as GABA and 5-hydroxy-tryptamine (5-HT)), enteroendocrine cell activation (such as glucagon-like peptide 1), immunomodulation (such as IL-6 and tumor necrosis factor-alpha (TNF-α)), and stimulation of the vagal nerve and the ENS [33].
The intricate regulatory network of the intestinal flora is influenced by a variety of factors, including the stability of the intestinal environment, the invasion of pathogenic bacteria, phar- macological therapy, emotional shifts, and other factors. Because of its substantial impact on gut microbiota, LAB is a popular probiotic for preserving both human and animal health. These benefits stem from the chemical and physical characteristics of the surface elements and metabolites. The effects of LAB metabolites on the gut microbiota have only been the subject of a few number of investigations to yet. Nevertheless, among the numerous metabolites of LAB, some function as dietary substrates for intestinal bacteria or cells, while others are antibacterial agents that prevent the growth of dangerous bacteria or neurotransmitters involved in brain regulation. These characteristics suggest that the metabolites of LAB play a significant role in the gut microbiota's control.
Effect of berberine on gut microbiota
It is recognized that the gut microbiota influences drug metabolism in a direct and indirect manner, especially when it comes to oral medications. By modulating the makeup of the gut microbiota among other multi-target processes, BB lowers blood levels of glucose and fats [34]. Additionally, BB has been shown to decrease gut microbiota diversity and alter the relative abundance of Bacteroides, Eubacterium, and Desulfovibrio [35]. The colon and terminal ileum of mice (C57BL/6) given BB treatment also revealed an enrichment of Bacteroides; however, the populations of Ruminococcus gnavu (Genus of Mediterraneibacter), Ruminococcus schinkii (Genus of Blautia), Lactobacillus acidophilus (Genus of Lactobacillus), Lactobacillus murinus (Genus of Ligilactobacillus), and Lactococcus lactis (Genus of Lactococcus) were decreased as a result of BB treatment [36].
According to recent research, BB influences the expression of multiple intestinal immunity components and has positive effects on the immune cells that make up the intestinal immune system. It has also been demonstrated that BB reduces low-grade inflammation and inhibits the mRNA expression of MIF, TNF-α, IL-10, IL-1b, and macrophage migration inhibitory factor (MIF) [37]. By decreasing the activity of Clostridium cluster XIVa and IV and their bile salt hydrolase (BSH), short-term exposure to BB modifies the populations of gut bacteria and causes taurocholic acid (TCA) to accumulate. The intestine farnesoid X receptor (FXR), when activated by TCA, can mediate the metabolism of glucose, lipids, and bile acids. An anaerobic bac- terium found in the gastrointestinal tract ferments fibers and other substrates to create butyrate, a short-chain fatty acid (SCFA) [38]. Additionally, it has been demonstrate that BB increases the number of butyrate-producing bacteria in the gut microbiota, which facilitates the acetyl CoA-butyryl CoA-butyrate pathway and the synthesis of butyrate. The butyrate then enters the bloodstream and lowers the glucose and fat levels [39]. Metabolic diseases known to be significantly influence by the gut flora. The application of BB treatment supported, among other things, by BB's capacity to speed up cellular glucose uptake and metabolism [40].
Berbeine as supplement with probiotics
Metabolic homoeostasis is merited by actions that modify the gut microbiota, such as taking probiotics or BB, a bacteriostatic drug, orally. In dairy goats, supplementing with BB during the transition phase can decrease plasma biochemical indications of negative energy balance and plasma biomarkers of inflammation (positive APPs), while also increasing intakes of dry matter, energy balance, net energy of lactation, and metabolized protein. Supplementing with BB improved antioxidant markers in blood, colostrum, and milk and enhanced colostrum and milk production; these findings suggest that BB may have a beneficial impact on transition dairy goat productivity, immunity, and antioxidative ability [41].
Another hot topic in gut microbiome research is the possibility of employing probiotics to treat metabolic or other illnesses. The variety of the target population, the uneven application of strains and formulas, and the disparate attributes and validity across the studies could be the causes of the debatable outcomes of probiotic intervention [42]. It's interesting to note that research has shown that native probiotics with genera like Lactobacillus and Bifidobacterium are more abundant in the feces of diabetes patients following antidiabetic treatment with a single dose of metformin or acarbose [43]. These probiotics are linked to an antidiabetic effect, but have also been shown to be inhibited by BB administration. So, it raises the question of whether administering probiotics in addition to a medication like BB could have greater antidiabetic effects than probiotics or BB alone.
Therefore, a number of research were planned and carried out to determine the impact of probiotics and the BB trial in treating various diesease in an effort to change gut microbiota dysbiosis. The
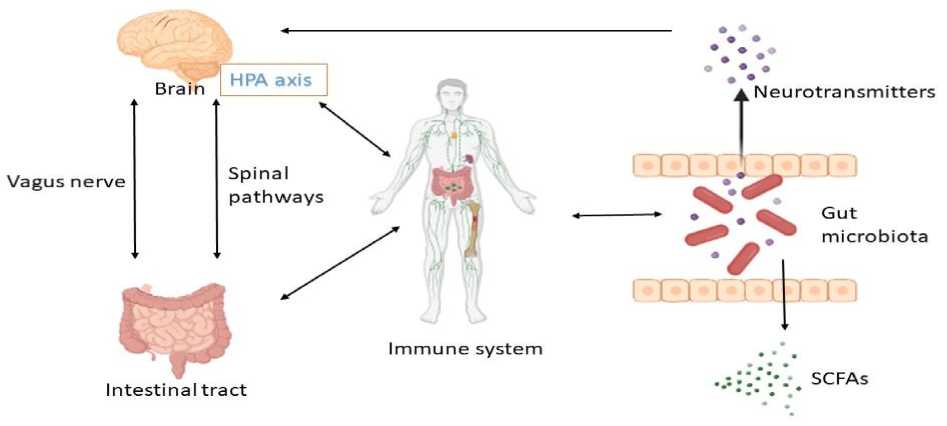
Fig. 2. Routes of communication in the gut-brain-axis (GBA). The Gut microbiota is one of the key regulators of gut-brain function. Communication within the occurs through these three ways: the immune pathway, the neuroendocrine pathway (neurotransmitters), and the vagus pathway, involving microbial metabolites such as SCFAs. LAB maintain homeostasis and affect host behaviors via the microbiota – gut – brain axis. HPA, hypothalamic-pituitary-adrenal axis, is central to homeostasis, stress responses, energy metabolism, and neuropsychiatric function
principal aim of these investigations was to ascertain and juxtapose the effectiveness of probiotics plus BB (Prob + BB), BB + placebo (BB), or probiotics + placebo (Prob) against placebo (Plac) in diminishing glycaemic haemoglobin (HbA1c) in individuals with diabetes. Additionally, the groups' secondary outcomes – such as clinical metabolic measurements – are assessed and contrasted. The potential for controlling the gut microbiota of BB and/or probiotics therapies is investigated, as well as how these microbial alterations in the gut linked with the antidiabetic impact in time-dependent antibiotic pretreatment, using comprehensive metagenomics and metabolomics analysis.
Conclusion
Till the time of this compiling data, no research have been published with addition of berberine fortified in yogurt investigating increased GABA production by LAB. Various research studies have been conducted where berberine showed to play very significant role in gut microbiota through various pathways and help in releasing GABA for its important function to be performed in brain. Hence, considering its pharmacological effect, berberine can be used as potent ingrediant in diary product like yogurt to perform its medicinal and nutritional fuction in food.
Список литературы Berberine in emulsions: possibilities to stimulate GABA production in yogurt
- Caliceti C., Franco P., Spinozzi S., Roda A., Cicero A.F. Berberine: New Insights from Phar-macological Aspects to Clinical Evidences in the Management of Metabolic Disorders. Curr. Med. Chem. 2016; 23:, 1460–1476.
- Cicero A.F., Baggioni A. Berberine and Its Role in Chronic Disease. Adv. Exp. Med. Biol. 2016; 928: 27–45.
- Habtemariam S. Berberine and inflammatory bowel disease: A concise review. Pharmacol. Res. 2016; 113(Pt A): 592–599
- Wang K., Feng X., Chai L., Cao S., Qiu F. The metabolism of berberine and its contribution to the pharmacological effects. Drug Metab. Rev. 2017a; 49: 139–157.
- Yang Y., Kang N., Xia H., Li J., Chen L., Qiu F. Metabolites of protoberberine alkaloids in human urine following oral administration of Coptidis Rhizoma decoction. Planta. Med. 2010; 76: 1859–1863.
- Jin Y., Khadka D.B., Cho W.J. Pharmacological effects of berberine and its derivatives: a patent update. Expert Opin. Ther. Pat. 2016; 26: 229–243.
- Tang M., Yuan D., Liao P. Berberine improves intestinal barrier function and reduces inflam-mation, immunosuppression, and oxidative stress by regulating the NF-kB/MAPK signaling pathway in deoxynivalenol-challenged piglets. Environ Pollut. 2021; 289: 117865.
- Li J., Wei H. Establishment of an efficient germ-free animal system to support functional microbiome research. Sci China Life Sci. 2019; 62: 1400–3.
- Park Y.S., Kim Y.S., Shin D.W. Changes in physiochemical characteristics and microbial popu-lations during storage of lactic acid bacterial fermented vegetable yogurt. Food Sci. Biotechnol. 2003; 12: 654–658.
- Anjum N., Maqsood S., Masud T., Ahmad A., Sohail A., Momin A. Lactobacillus acidophilus: characterization of the species and application in food production. Crit Rev Food Sci Nutr. 2014; 54: 1241–1251.
- Rossi F., Amadoro C., Gasperi M., Colavita G. Lactobacilli infection case reports in the last three years and safety implications. Nutrients. 2022; 14: 1178.
- Hill D., Sugrue I., Tobin C., Hill C., Stanton C., Ross R.P. The Lactobacillus casei Group: History and Health Related Applications. Front Microbiol. 2018; 9: 2107.
- Fidanza M., Panigrahi P., Kollmann T.R. Lactiplantibacillus plantarum-Nomad and Ideal Pro-biotic. Front Microbiol. 2021; 12: 712236.
- Gupta T., Kaur H., Kapila S., Kapila R. Potential probiotic Lacticaseibacillus rhamnosus MTCC-5897 attenuates Escherichia coli induced inflammatory response in intestinal cells. Arch Microbiol. 2021; 203: 5703–5713
- Miao Z., Zheng H., Liu W.H., Cheng R., Lan H., Sun T., Zhao W., Li J., Shen X., Li H., Feng H., Hung W.L., He F. Lacticaseibacillus paracasei K56 Attenuates High-Fat Diet-Induced Obesity by Modulating the Gut Microbiota in Mice. Probiotics Antimicrob Proteins. 2022. DOI: 10.1007/s12602-022-09911-x
- Tang Q., Hao Y., Wang L., Lu C., Li M., Si Z., Wu X., Lu Z. Characterization of a bacterial strain Lactobacillus paracasei LP10266 recovered from an endocarditis patient in Shandong, China. BMC Microbiol. 2021; 21: 183
- Zheng D., Wang Z., Sui L., Xu Y., Wang L., Qiao X., Cui W., Jiang Y., Zhou H., Tang L., Li Y. Lactobacillus johnsonii activates porcine monocyte derived dendritic cells maturation to modulate Th cellular immune response. Cytokine. 2021; 144: 155581
- Wang X.L., Liu Z.Y., Li Y.H., Yang L.Y., Yin J., He J.H., Hou D.X., Liu Y.L., Huang X.G. Effects of Dietary Supplementation of Lactobacillus delbrueckii on Gut Microbiome and Intestinal Morphology in Weaned Piglets. Front Vet Sci. 2021; 8: 692389.
- Maillet F., Passeron A., Podglajen I., Ranque B., Pouchot J. Lactobacillus delbrueckii urinary tract infection in a male patient. Med Mal Infect. 2019; 49: 226–228
- Dargenio C., Dargenio V.N., Bizzoco F., Indrio F., Francavilla R., Cristofori F. Limosilactobacillus reuteri Strains as Adjuvants in the Management of Helicobacter pylori Infection. Medicina (Kaunas). 2021; 57: 733.
- Guerrero Sanchez M., Passot S., Campoy S., Olivares M., Fonseca F. Ligilactobacillus salivarius functionalities, applications, and manufacturing challenges. Appl Microbiol Biotechnol. 2022; 106: 57–80.
- Salminen M.K., Rautelin H., Tynkkynen S., Poussa T., Saxelin M., Valtonen V., Jarvinen A. Lactobacillus bacteremia, species identification, and antimicrobial susceptibility of 85 blood isolates. Clin Infect Dis. 2006; 42: e35-44
- Kim J., Yun J.M., Kim M.K., Kwon O., Cho B. Lactobacillus gasseri BNR17 Supplementation Reduces the Visceral Fat Accumulation and Waist Circumference in Obese Adults: A Randomized, Double-Blind, Placebo-Controlled Trial. J Med Food. 2018; 21: 454–461.
- Mann S., Park M.S., Johnston T.V., Ji G.E., Hwang K.T., Ku S. Oral probiotic activities and biosafety of Lactobacillus gasseri HHuMIN D. Microb Cell Fact. 2021; 20: 75
- Yang B., Li M., Wang S., Ross R.P., Stanton C., Zhao J., Zhang H., Chen W. Lactobacillus ruminis Alleviates DSS-Induced Colitis by Inflammatory Cytokines and Gut Microbiota Modulation. Foods. 2021; 10: 1349.
- Moore J.F., DuVivier R., Johanningsmeier S.D. Changes in the free amino acid profile of pick-ling cucumber during lactic acid fermentation. J Food Sci. 2022; 87: 599–611.
- Majsnerowska M., Noens E.E.E., Lolkema J.S. Arginine and Citrulline Catabolic Pathways Encoded by the arc Gene Cluster of Lactobacillus brevis ATCC 367. J Bacteriol. 2018; 200: e00182-18.
- Kim J., Lee M.H., Kim M.S., Kim G.H., Yoon S.S. Probiotic Properties and Optimization of Gamma-aminobutyric Acid Production by Lactiplantibacillus plantarum FBT215. J Microbiol Biotechnol. 2022a; 32: 1–10.
- Kim J., Yoon Y.W., Kim M.S., Lee M.H., Kim G.A., Bae K., Yoon S.S. Gamma-aminobutyric acid fermentation in MRS-based medium by the fructophilic Lactiplantibacillus plantarum Y7. Food Sci Biotechnol, 2022b; 31: 333–341.
- Franciosi E., Carafa I., Nardin T., Schiavon S., Poznanski E., Cavazza A., Larcher R., Tuohy K.M. Biodiversity and gamma-aminobutyric acid production by lactic acid bacteria isolated from tradi-tional alpine raw cow's milk cheeses. Biomed Res Int. 2015: 625740.
- Fabersani E., Marquez A., Russo M., Ross R., Torres S., Fontana C., Puglisi E., Medina R., Gauffin-Cano P. Lactic Acid Bacteria Strains Differently Modulate Gut Microbiota and Metabolic and Immunological Parameters in High-Fat Diet-Fed Mice. Front Nutr. 2021; 8: 718564.
- Taraskina A., Ignatyeva O., Lisovaya D., Ivanov M., Ivanova L., Golovicheva V., Baydakova G., Silachev D., Popkov V., Ivanets T., Kashtanova D., Yudin V., Makarov V., Abramov I., Lukashina M., Rakova V., Zagainova A., Zorov D., Plotnikov E., Sukhikh G., Yudin S. Effects of Traumatic Brain Injury on the Gut Microbiota Composition and Serum Amino Acid Profile in Rats. Cells. 2022; 11: 1409.
- Horvath T.D., Haidacher S.J., Engevik M.A., Luck B., Ruan W., Ihekweazu F., Bajaj M., Hoch K.M., Oezguen N., Spinler J.K., Versalovic J., Haag A.M. Interrogation of the mammalian gut-brain axis using LC-MS/MS-based targeted metabolomics with in vitro bacterial and organoid cultures and in vivo gnotobiotic mouse models. Nat Protoc. 2022. DOI: 10.1038/s41596-022-00767-7
- Zhang X., Zhao Y., Zhang M., Pang X., Xu J., Kang C. et al. Structural changes of gut microbiota during berberine-mediated prevention of obesity and insulin resistance in high-fat diet-fed rats. PloS One, 2012; 7: e42529.
- Cui H.X., Hu Y.N., Li J.W., Yuan K., and Guo Y. Preparation and Evaluation of Antidiabetic Agents of Berberine Organic Acid Salts for Enhancing the Bioavailability. Molecules, 2018; 24: 103.
- Guo Y., Zhang Y., Huang W., Selwyn F.P., Klaassen C.D. Doseresponse effect of berberine on bile acid profile and gut microbiota in mice. BMC Complement. Altern. Med. 2016; 16: 394.
- Gong J., Hu M., Huang Z., Fang K., Wang D., Chen Q., et al. Berberine Attenuates Intestinal Mucosal Barrier Dysfunction in Type 2 Diabetic Rats. Front. Pharmacol. 2017; 8: 42.
- Roediger W.E. Utilization of nutrients by isolated epithelial cells of the rat colon. Gastroenterology. 1983; 83: 424–429.
- Wang Y., Shou J.W., Li X.Y., Zhao Z.X., Fu J., He C.Y., et al. Berberine-induced bioactive metabolites of the gut microbiota improve energy metabolism. Metabolism, 2017b; 70: 72–84.
- Cok A., Plaisier C., Salie M.J., Oram D.S., Chenge J., Louters L.L. Berberine acutely activates the glucose transport activity of GLUT1. Biochimie, 2011; 93: 1187–1192.
- Ghavipanje N., Fathi Nasri M.H., Farhangfar S.H., Ghiasi S.E., Vargas-Bello-Pérez E. The Im-pact of Dietary Berberine Supplementation during the Transition Period on Blood Parameters, Antioxi-dant Indicators and Fatty Acids Profile in Colostrum and Milk of Dairy Goats. Vet. Sci. 2022; 9: 76.
- Suez J., Zmora N., Segal E., Elinav E. The pros, cons, and many unknowns of probiotics. Nat. Med. 2019; 25: 716–729.
- Wu H., Esteve E., Tremaroli V., Khan M.T., Caesar R., Mannerås-Holm L., Ståhlman M. et al. Metformin alters the gut microbiome of individuals with treatment-naive type 2 diabetes, contributing to the therapeutic effects of the drug. Nat. Med. 2017; 23: 850–858.


