Brain Ischemic Stroke Detection through Deep Learning: A Systematic Review on CT vs MRI vs CTA Images
Автор: Rathin Halder, Nusrat Sharmin
Журнал: International Journal of Education and Management Engineering @ijeme
Статья в выпуске: 5 vol.14, 2024 года.
Бесплатный доступ
Purpose: Ischemic brain strokes have a high morbidity and death rate, thus it’s vital to obtain a quick diagnosis and imaging. Computer-aided diagnosis (CAD) has become popular in medical imaging and diagnostic radiology research. In the field of medical image analysis, deep learning (DL) approaches have recently shown greater performance over earlier, more advanced machine learning techniques. Acute Ischemic stroke (AIS) is one of the medical sectors where DL has conducted substantial research. The systematic review examines the performance of deep learning models across different imaging modalities, highlighting their strengths and limitations in identifying ischemic strokes. Key aspects such as sensitivity, specificity, and overall accuracy are assessed, providing insights into the comparative effectiveness of CT, MRI, and CTA in stroke detection. In contrast with other reviews in this domain, this paper offers a concise summary of the most notable DL methods applied in the classification, detection, and segmentation of acute ischemic brain stroke, focusing on popular imaging techniques like Computed Tomography (CT), Magnetic Resonance Imaging (MRI), and CT Angiography (CTA). This survey also highlights datasets and data acquisition challenges and attempts to provide a comprehensive overview of data preprocessing, as well as insight into publicly available datasets. Methods and Results: This study aims to give an idea of how training and testing datasets should be handled by evaluat- ing recent studies. This review discusses the challenges associated with each imaging modality, including image noise, artifacts, and variability in acquisition protocols. Strategies to mitigate these challenges through preprocessing techniques and model optimization are explored, aiming to improve the robustness and reliability of deep learning-based stroke de- tection systems. Moreover, this research contains a brief discussion of recent deep learning architectures and an analysis of performances. Conclusions: Overall, this systematic review contributes to the understanding of current advancements in brain ischemic stroke detection through deep learning, offering valuable insights for researchers and clinicians seeking to leverage these technologies for improved patient outcomes. Future directions and potential research avenues are also discussed to guide further advancements in this critical area of medical imaging and diagnosis.
Brain Ischemic Stroke, Deep-learning, and Machine Learning
Короткий адрес: https://sciup.org/15019483
IDR: 15019483 | DOI: 10.5815/ijeme.2024.05.03
Текст научной статьи Brain Ischemic Stroke Detection through Deep Learning: A Systematic Review on CT vs MRI vs CTA Images
In recent years, stroke has emerged as a significant health concern, being the most common life-threatening cere brovascular disorder. After heart disease [1], it is the second most common factor in mortality globally, and it is second in terms of death. A stroke occurs due to the disruption of blood circulation to a specific portion of the brain. The affected brain cells eventually die from a lack of oxygen and nutrients from the blood. There are predominantly two categories of stroke: Hemorrhagic and Ischemic [2]. An ischemic stroke is a condition when blood vessels carrying oxygen-rich blood to the brain become clogged whereas hemorrhagic stroke is caused by blood vessels suddenly leaking or cracking open resulting in bleeding inside or around the brain. Ischemic strokes make for around 70% of all strokes, because of this, we are mainly focusing on ischemic strokes in this article.
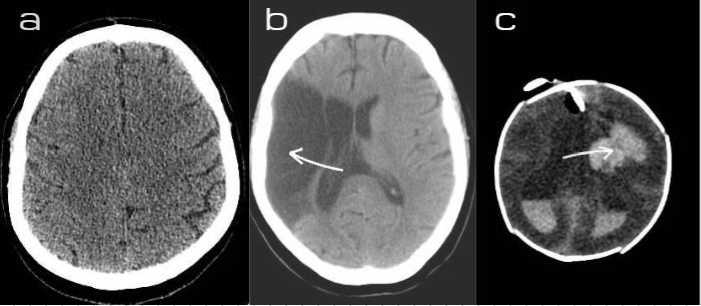
Fig. 1. a) Healthy brain b) Ischemic stroke c) Hemorrhagic stroke.
Ischemic strokes occur when blood vessels are blocked by a thrombus or an embolus that travels through the blood- stream and eventually creates a blockage. Based on the type of blockade, ischemic strokes are divided into two groups named thrombotic stroke and embolic stroke. When a clot develops inside a blood artery, it reduces blood flow to the brain and causes a thrombotic stroke. An embolic ischemic stroke, on the other hand, takes place when a blood clot moves from another region of the human anatomy to the brain.
For both cases, urgent response is required as the treatment for an ischemic stroke is determined by how promptly a patient can be taken to the hospital. They are also influenced by the patients´ medical history. Figure1 showed the state of the different types of stroke. This review will discuss different Computer Aided Diagnosis (CAD) techniques for Acute Ischemic Stroke (AIS).
Techniques for brain imaging are essential in the early assessment of individuals who may be experiencing a stroke. Imaging studies are crucial for making a stroke diagnosis because they give an accurate representation of the brain and assist determine how well each area functions. CT, CTA, and MRI techniques are the best protocols in evaluating AIS[3]. In this way, Computer-aided diagnosis (CAD) has recently become a reliable clinical practice for detecting many disorders including but not limited to brain stroke detection. These imaging techniques when paired with specific machine learning and deep learning approaches result in stroke diagnoses with reliable accuracy.
Machine Learning (ML) has become a valuable tool for diagnosing neurological diseases. A few works worth mentioning using ML include ischemic stroke lesions identification utilizing the T1-weighted MRI scans to predict the stroke region by using the Gaussian Naive Bayes classification method[4], Gaussian Support Vector Machine (Gaussian SVM) and Cubic Support Vector Machine (Cubic SVM) [5]. But as brain image classification is a complex problem and the datasize is growing larger day by day deep learning approaches are becoming popular with better performance than ML techniques. The introduction of deep learning in the medical field has become an epoch-making element with its faster processing speed and capability of producing accurate results for stroke detection. There has been a good number of contributions in terms of using different architectures like VGG16[6, 7], ResNet architecture [8], UNET [9], CNN-based models[10], R-CNN models, etc.
Among many reviews [11] discussed the lesion segmentation using state-of-the-art deep learning approaches of modern times, [12] reviewed the same methods but kept it confined only within MR images, [13] went through the lesion characteristics, segmentation difficulty, algorithm maturity of ischemic stroke detection in comparison with other brain tumors. These reviews provide a good understanding of the challenges of ischemic stroke detection in deep learning approaches and identify the future scopes of work.
In this article, we try to provide a detailed review of various brain scan images to diagnose ischemic brain stroke depending on deep learning from recent publications.The following goals are what we hope to accomplish with this study in order to provide a quick summary of brain ischemic stroke: to discuss the performance of the existing deep learning techniques and the medical imaging tools used for ischemic stroke detection. The main contributions of the papers are listed below:
• To investigate datasets that include CT, MRI, and CTA imaging that are used to detect ischemic strokes, as well as produce a comparative study. One can gain an insight into the sort of dataset used in this domain from this article, as well as what extent of training and testing might achieve the best-suited results. With this, we tried to provide a detailed contributions classification or detection of ischemic brain stroke based on deep learning from recent publications, based on three different and main imaging techniques ( CT, MRI, and CTA) in the domain of brain ischemic strokes.
• To investigate datasets such as CT, MRI, and CTA those are used to classify ischemic strokes, as well as try to show comparisons among them. One can gain an idea of the dataset used in this domain from our article, such as how many subjects they should use to achieve the greatest results. To the best of our knowledge, this is the first work where comparison between three different imaging techniques.
• This work will give insight into the pre-processing techniques used for CT or MRI images.
• This study organizes some of those studies based on their degree of similarity thoroughly examines each organiza- tion and offers useful details on the use of deep-based techniques in brain ischemia.
2. Literature Search Strategy
2.1 Study Selection Criteria2.2 Statistical Analysis
The arrangement of this document is as follows: Section 2 presents Literature Search Strategy, Section 3 represents Brain ischemic stroke, Section 4 describes Deep Learning, Section 5 presents dataset for brain ischemic stroke, Section 6 describes image prepossessing, Section 7 represents Deep Learning applied in ischemic stroke, Section 8 Challenges in acute ischemic stroke, section 9 Discussion, and Conclusion.
The research for this review encompassed a thorough study of numerous published journal articles, conference papers, and book chapters. To synthesize the main ideas and contributions, Figure 3 illustrates a concept map. Research papers were identified using targeted keywords such as “Ischemic stroke,” “acute stroke,” “deep learning,” and “neuroimaging,” with a focus on analyzing different sources of datasets and their classification approaches. This information is presented in the concept map to enhance metacognitive skills.
Initially, 169 unique papers were shortlisted from a total of 550 records. Each paper was independently analyzed to gather information based on criteria such as type of stroke, publication journal, publication year, system platform, algorithms used, data type, number of images, and performance metrics. Performance measures were either directly reported or calculated from the analysis provided. Studies were identified through databases including Google Scholar, ScienceDirect, IEEE Xplore, Springer, and PLOS One. The PRISMA methodology was employed to improve the accessibility and coherence of the review process, ensuring accurate reporting and assessment of research from 2015 to 2022. The Literature Search Strategy for PRISMA is depicted in Figure 2. A total of approximately 500 primary studies on the relevant topic were initially identified from selected databases. After filtering, 210 studies were excluded based on inclusion and exclusion criteria, resulting in 169 primary studies after removing duplicate papers. Each study was comprehensively analyzed, with 120 studies reviewed in detail. Articles deemed irrelevant to the issue were eliminated, resulting in approximately 60 papers for further consideration.
Figure 3 presents a concept map, and a visual diagram summarizing the main ideas and contributions of the paper, focusing on the key points highlighted in the review.
A concept map in figure 3 is a visual diagram that represents the focus points of the paper. Here mainly the main ideas and contributions are summarized through the concept map.
120 publications were chosen after a thorough study, and following the paper’s brief introduction and information, 60 papers were included for SLR evaluation. Out of the 169 primary studies, a total of 60 are conference papers, accounting for 40% of the studies; 49% of the studies were published in journals; and the final 11% of the pertinent research was discovered in other sources. Considering the number of SLRs found in the literature we draw the conclusion that there is substantial evidence that SLR studies are deficient in the field of ischemic stroke detection using deep learning, despite the fact that 169 primary researches were taken into account in our SLR study and only 11% of these articles used SLR guidelines. Out of the 60 papers shown in Figure 4, we conducted a thorough analysis and gathered information to identify the challenges and current DL techniques used in ischemic stroke detection.
3. Brain Ischemic Stroke
3.1 Anatomical background of brain ischemic stroke3.2 Imaging Techniques of brain ischemic stroke
Ischemic stroke is a heterogeneous disease that occurs due to a variety of underlying pathological processes. The blockage of an artery is the main cause of Ischemic brain stroke. It happens when the circulatory system to the brain is significantly reduced as a result of clogged or restricted blood vessels.
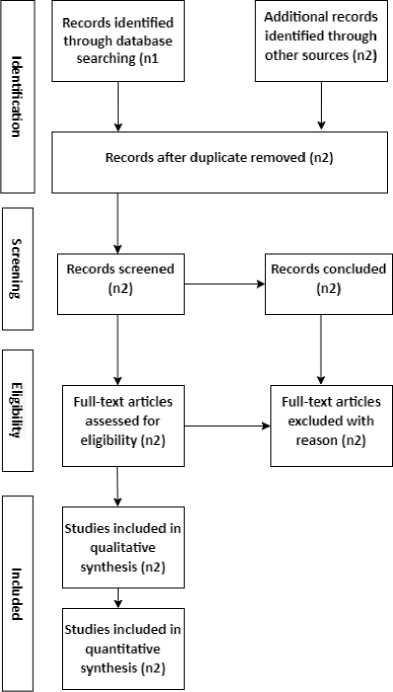
Fig. 2. PRISMA diagram for the representation of the searching method
Endothelial Cell Dysfunction: One mechanism is endothelial cell dysfunction, which happens when something in- flames or irritates the slick tunica intima lining of the artery. Toxins from cigarettes that circulate in the blood and harm the endothelium are one common irritation. That damage turns into a location for atherosclerosis, where a plaque develops. A buildup of fat, cholesterol, proteins, calcium, and immune cells occurs at this point, obstructing the flow of blood via the arteries. However, this delayed obstruction only partially closes the arteries because it often takes years for plaque to build up, so some blood still reaches the brain region even though less flow travels there. Therefore, strokes occur when an artery suddenly becomes completely or nearly completely blocked[14].
Embolism: An embolic stroke typically occurs when a blood clot that has broken free from its original location travels through the bloodstream and lodges itself in a narrower artery, or vein further down the circulatory system[15]. These blood clots can develop in the heart but most often they result from atherosclerosis.
Lacunar Stroke: The basal ganglia are nourished by the deep regions of the central cerebral artery, which are frequently affected by a specific type of ischemic stroke known as a lacunar stroke. The main causative agent is hyaline arteriolosclerosis, which develops when protein [16] fills the arteriole wall. The arterial wall can become quite thick as a result of hypertension or diabetes, which reduces the lumen size as a result the oxygen supply for the tissue, is reduced.
CT scan: A CT scan is a computerized x-ray imaging procedure where a patient is exposed to a rapidly spinning narrow x-ray beam around their body. The computer analyzes the resulting “slice” images of the body, and by collecting multiple successive slices, a 3D image of the patient can be generated. This facilitates the identification and localization of underlying structures, potential cancers, or anomalies. The method offers several advantages, including the ability to rotate the 3D image and view successive slices, making it easier to precisely pinpoint the issue’s location. In comparison to a standard X-ray, a CT scan provides more accurate and detailed information
In CT images, an ischemic stroke is characterized by a dark region with contrast, making it distinguishable from its surrounding tissues. On the other hand, a hemorrhage appears as a bright region, clearly contrasting against its surrounding areas. The key indicator of an ischemic stroke, a hypodense lesion, is not visible in the first few hours following the commencement of the stroke, although improvements in MRI technology have increased the possibility of seeing minor lesions in brain pictures[17]. As a result, it is now possible to identify cerebral microbleeds, which are minor brain hemorrhages linked to an increased risk of ischemic stroke and intracerebral bleeding. MRI is more precise but is only available in large hospitals, while CT is more common but less precise. Figure 5 illustrates the brain stroke detection system of a CAD (computer-aided diagnosis).
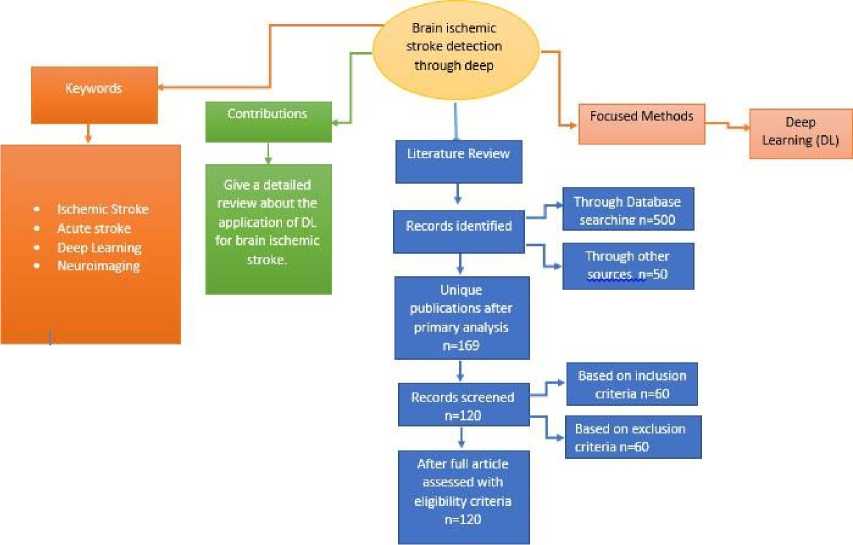
Fig. 3. Concept map for the representation of the paper
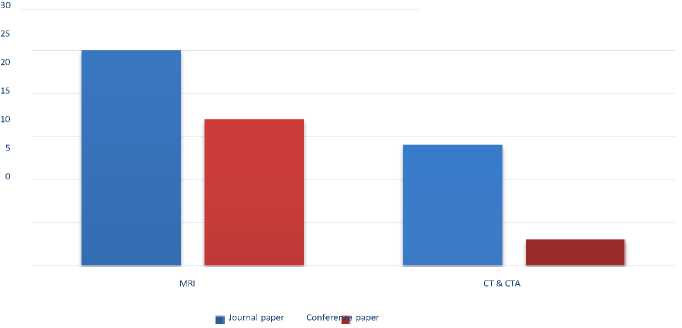
Fig. 4. Research Article Types
CT angiography: CT angiography is a medical procedure that integrated by a CT scan with the injection of a specific dye to produce images of blood vessels and tissues in a targeted body area. The dye is administered through an intravenous (IV) line in the arm or hand, and it acts as a contrast medium to highlight the tissues and blood vessels under examination. Because it can locate aberrant blood vessel forms inside the brain, it is currently utilized to identify cerebral ischemia.
Magnatic Resonance Imaging (MRI): MRI is a non-invasive medical imaging procedure that utilizes a combination of magnetic fields and radio waves generated by a computer to produce detailed three-dimensional anatomical images of the body’s organs and tissues. During an MRI, radiofrequency and magnetic waves are directed into the patient’s body to obtain the images. The atoms in a magnetic field emit energy, which is transmitted as a signal to the computer. The computer then processes this signal using mathematical algorithms to create the final image.
Fig. 5. CAD for Brain stroke detection system
4. Deep Learning
Machine learning is a broad field encompassing several subfields, including deep learning. Unlike traditional machine learning, which relies on fundamental concepts, deep learning utilizes artificial neural networks designed to emulate the way humans think and learn. It is a machine learning method that extracts features and tasks directly from data, encompassing various types of data such as images, texts, or sounds. Deep learning is often referred to as end-to-end learning, where tasks are learned entirely from data[18].
Artificial neural networks (ANNs) are a key component of deep learning and are inspired by biology, and modeled after the human brain. These networks consist of interconnected neurons[19], resembling the architecture of the human brain’s neural networks. Neurons are organized into layers within artificial neural networks, with the input layer receiving data and the output layer producing the desired output. Layers between the input and output layers are known as hidden layers, each performing different transformations on their inputs.
A specific type of artificial neural network called a convolutional neural network (CNN), is designed to handle pixel input, making it particularly suitable for tasks involving image detection and processing. CNNs, also known as comp nets, are commonly used for image analysis but can also be applied to other data analysis or classification tasks. CNNs excel at recognizing and interpreting patterns in data, making them valuable for image analysis tasks. Convolutional layers within CNNs apply specified modifications to input data before passing it to subsequent layers, a process known as convolution operation.
5. Dataset for Brain Ischemic Stroke
The use of computer-generated output as an aid for physicians in making diagnoses is known as computer-aided diagnosis (CAD). The main raw material for building and evaluating such systems for the classification of brain ischemic stroke data is datasets, which can consist of CT, CTA, or MRI scans. In this section, this study aims to elaborate on datasets suitable for establishing CAD systems.
For deep learning-based models, datasets are typically classified into two portions: Training and Testing. Training data comprises the initial data used to train deep learning models, where algorithms are taught how to make predictions or identify patterns by being fed training datasets. During the training process, the model’s performance is evaluated using a separate dataset known as the validation set, which differs from the training set. Once training is complete, the model is tested on another specific dataset called the test set to assess its final performance.
Computed tomography (CT) imaging plays a crucial role in the diagnostic process for patients with stroke symptoms in numerous hospitals. However, automated approaches have encountered difficulty in extracting potentially measurable information due to the subtle manifestations of ischemia in acute CT scans. The two basic MRI subtypes are diffusion- weighted imaging (DWI) and apparent diffusion coefficient (ADC). Diffusion-weighted imaging provides details about tissue integrity based on water molecule motion and is commonly considered a significant tool in studying the central nervous system. Tables 1 and 2 discuss the datasets used for deep learning-based automatic tools, which are believed to be helpful in the early diagnosis of intracranial infections and acute ischemic stroke. For these types of deep learning models, splitting datasets into training and testing portions is preferable as it facilitates model assessment. Table 2 showcases different types of public and private MRI datasets utilized to train various types of deep learningbased models.
CT and CTA images
Table 1. Datasets for CT images
|
Reference |
Dataset |
How many Subject |
Train Image |
Test Images |
Image Dimension |
|
(Gautam and Raman 2021) |
Collected from Himalayan Institute of Medical Sciences (HIMS), Dehradun, India. |
Total 900 images (Ischemic 300, Hemorrhagic 300,and Normal 300) |
720 |
180 |
512 X 512 |
|
(Dourado Jr et al. 2019) |
To acquire the images from the database, the General Medical System HiSpeed TC system (GEMSH) was used. |
Total, 420 images ( 140 healthy brains, and,140 hemorrhagic and 140 ischemic) |
336 |
84 |
140 X 140 |
|
(Chin et al. 2017) |
256 |
128 |
128 |
512 X 512 |
|
|
(Lo, Hung, and Lin 2021) |
Training and Testing datasets are collected from two different institutions. |
1479 (Ischemic 681,Normal 798) |
1254 (Ischemic 573, Normal 681) |
225 (Ischemic 108, Normal 117) |
512 X 512 |
|
(Nishio et al. 2020) |
Data collected From Osaka Red Cross Hospital and Ichinomiya Nishi Hospital |
238 |
189 |
49 |
512 X 512 |
|
(Pan et al. 2021) |
116 ischemic and 26 normal |
58 ischemic, 13 normal |
58 ischemic, 13 normal |
||
|
(Shinohara et al. 2020) |
35 ischemic, 39 Normal |
1380 X 1100 |
|||
|
(Wu et al. 2021) |
Central Hospital Of Minhang District, Shanghai(DWI), China and Huashan Hospital (CT) |
251 ischemic stroke and 26 normal |
5.1 Challenges with the dataset
6. Image Preprocessing
MRI images
Table 2. Datasets for MRI images
|
Reference |
Dataset |
How many Subject |
Train Image |
Test Images |
Image Dimension |
|
(L. Liu, Chen, et al. 2020) |
SPES dataset and tested on SPES and LHC datasets, |
(435 2D slices) |
1344 2D image slices |
90 2D image slices |
|
|
(Rajendra Acharya et al. 2019) |
Obtained from stroke patients at the IMS and SUM Hospital, Bhubaneswar, Odisha, India |
267 images |
267 images |
||
|
(Zhao et al. 2021) |
577 |
398 |
179 |
||
|
(R. Zhang et al. 2018) |
Sonata, Siemens Medical, Erlangen, Germany |
242 |
90 |
62 |
|
|
(Do et al. 2020) |
390 |
319 |
71 |
||
|
(Gaidhani, R.Rajamenakshi, And Sonavane 2019) |
Data were obtained From ATLAS (Anatomical Tracings of Lesions after Stroke |
600 |
400 |
200 |
197 X 233 |
|
(Karthik et al. 2021 |
ISLES 2015 |
ISLES 2015 |
ISLES 2015 |
224 X 224 |
|
|
(Kumar et al. 2020) |
ISLES 2017 |
75 |
43 |
32 |
256 X 256 |
In general, obtaining data for research on this topic is a challenging task. According to this review, only two authors utilized publicly shared datasets, while the majority of researchers relied on private databases. For instance, Kumar et al.[20] developed a DL framework for ischemic lesion segmentation in 2020, utilizing the ISLES 2017 DWI data to train and evaluate their CSNet model. Another example is the SPES online MRI dataset used by L. Liu, Chen, et al.[21] to train their model, with LHC datasets employed for testing purposes. Although these three MRI datasets are accessible, most researchers tend to rely on their private datasets collected from various hospitals. After reviewing the majority of the current work in this field, it is evident that anyone wishing to conduct research in this area should prioritize obtaining suitable datasets.
In medical image analysis, image pre-processing plays a significant role. To preserve image quality, different kinds of image processing are used such as image denoising, enhancement, contrast adjustment, and so many other techniques. Filters are so important in this area of pre-processing which is performed to achieve many requirements. In this section, various image preprocessing techniques used in the literature will be discussed.
CT and CTA images
Table 3. Table for CT images and CTA images
|
Reference |
Image preprocessing Techniques |
|
(Gautam and Raman 2021) |
Contrast adjustment, Image Fusion, SWML, SMDG |
|
(Nishio et al. 2020) |
Normalization, Horizontal flipping, Cropping, and Random rotation. |
|
(Pan et al. 2021) |
Shift and rotation, Ellipse fitting |
|
(Shinohara et al. 2020) |
Median filters, Gaussian filters, Flipping, Rotation |
|
(Wu et al. 2021) |
Spm12, Rotation, Ellipse fitting |
|
(Chin et al. 2017) |
Binarized by Otsu algorithm, Data Augmentation |
|
(Clerigues et al. 2019) |
Image Fusion, FSL FLIRT |
|
(Takahashi et al. 2019) |
ROI, Shifting, Rotation, Flipping |
CT scans are capable of identifying complex bone fractures, as well as other bone and joint problems. Moreover, they can detect various medical conditions, including certain cancers, benign tumors, heart disease, blood clots, bowel disorders such as blockages and Crohn’s disease, as well as diseases affecting the brain and spinal cord, and injuries. A median filter was used to remove noise generated[22]. A linear filter called the Gaussian is frequently used to blur pictures or lower noise levels. Edge detection is aided by the technique of “unsharp masking,” which is the subtraction of two Gaussian filters. Edge blurring and decreased contrast can, however, result from employing the Gaussian filter alone.The skull of a CT, CTA, MRI is the non-stroke area, so removing the skull is so useful for stroke detection [23].
L. Liu, Chen, et al.[21] and Wu et al.[9] use SPM12 software to convert the DICOM format to the NIFTI format of the images. Multiple visuals are placed into a common statistical distribution in terms of size and pixel values during the image normalization process, which is frequently employed in the production of data sets to train the AI model. However, a single image can also be normalized within itself. Regularly, both spatial and intensity normalization are involved in the process. In our statistical review in Table 4 almost of above, the authors use image normalization to increase the quality of images. Images were exposed to online image augmentation techniques such as horizontal flipping, cropping, and random rotation while the models were being trained.[6]. Table 3 referred to the details of preprocessing techniques of CT and CTA images.
7. Deep Learning Applied in Brain Ischemic Stroke
MRI images
Table 4. Table for MRI images
|
Reference |
Image preprocessing Techniques |
|
(L. Liu, Chen, et al. 2020) |
SPM12, Registration method MRIcron software (BET2), Gaussian method |
|
(Do et al. 2020) |
Cropping, contrast stretching, Histogram analysis, flipping, Gaussian filter |
|
(Gaidhani, R.Rajamenakshi, and Sonavane 2019) |
3D image to 2Darray, Median filter, them Normalization |
|
(Kumar et al. 2020) |
Normalization |
|
(C.-F. Liu et al. 2021) |
UNet BrainMask, in-plane (IP), Normalization |
|
(Stier et al. 2015) |
FSL Brain Extraction Tool (BET) , Registration methods |
|
(Yu et al. 2020) |
Coregistered, Normalization, Reperfusion, Thresholding |
|
(H. Zhang et al. 2021) |
Median filter, N4 bias field correction, FSL BET, FSL FLIRT, Interpolation |
|
(Oman et al. 2019) |
Cropping, flipping |
|
(Barman et al. 2019) |
Flipping |
As an image preprocessing approach, various types of filters are applied for feature extraction. Normalization, flipping, registration, shifting, rotation, and many more image processing techniques are used to prepare an image for building up trainable or testable datasets of images. Table 4 illustrates the details of preprocessing of MRI images. The decomposition of data files such as CT, MRI, or CTA is an important element of image preprocessing, and most authors utilize the spm12 software to solve this issue.
Table 5. Performance analysis table
|
Reference |
DL method |
Layer Information |
Type of Data |
Performance measure Accuracy(%) |
|||
|
Accuracy (%) |
Sensitivity |
Specificity |
DSC |
||||
|
(Gautam and Raman 2021) |
Image fusion, P CNN |
2 convo and 2 FC, ReLU, maxpooling, dropout, softmax and classification |
CT |
92.22 |
0.9222 |
0.92 |
|
|
(Chin et and al. 2017) |
Otsu algorithm, CNN |
2 convo 1 pooling and a single FC , ReLU |
CT |
92.969 |
0.9516 |
0.9090 |
|
|
(Nishio et al. 2020) |
Two-stage CNN, YOLOv3 and VGG16 |
CT |
0.373 |
0.34 |
|||
|
(Pan et al. 2021) |
ResNet, Maximal connected region (MCR), Maximum a posteriori probability (MAP) |
CT |
89.71 |
0.8817 |
0.9125 |
||
|
(Shinohara et al. 2020) |
DCNN |
CT |
86.5 |
0.829 |
0.897 |
||
|
(Wu et al. 2021) |
U-net, ResNet, Maximum a posteriori probability (MAP) |
CT and MRI |
85.71 |
0.8431 |
0.8462 |
||
|
(L. Liu, Chen, et al. 2020) |
Res-CNN |
10 convo 4 residual, 4 concate 4 deconvo BN LReLU. |
MRI |
0.74 |
|||
|
(Zhao et al. 2021) |
Multi feature map fusion network ,VGG16 |
Convo, BN, ReLU, MaxPooling, GAP, FC, Sigmoid |
MRI |
0.65 |
|||
|
(R. Zhang et al. 2018) |
(FC DenseNET) |
DWI |
0.7815 |
0.89 |
|||
|
(Do et al. 2020) |
RRCNN, ( VGG16 + ResNet) |
Convo, MaxPool, FC, Softmax |
DWI |
87.3 |
0.839 |
0.90 |
0.89 |
|
(Gaidhani, Rajamenakshi, and Sonavane 2019) |
(LegNet, SegNet) |
Convo, maxpool, FC |
DWI |
98.94 |
0.97 |
0.97 |
|
|
(Karthik et al.2021) |
FCN |
Conv, DeConv, Dropout, Softmax |
DWI |
0.8009 |
0.9457 |
0.78 |
|
|
(Kumar et al. 2020 |
CSNet |
Convo, ReLU, max-pool, |
DWI |
0.89 |
0.83 |
In this section, various architectures of deep learning models used for classifying ischemic brain strokes are reviewed, and their performances are analyzed in Table 5.
7.1 Deep learning methods used CT and CTA images
7.2 Deep learning methods used MRI images
8. Challenges in Acute Ischemic Stroke
9. Discussion and Conclusion
A new classification strategy based on image fusion and deep learning is presented in this research[24]. The fusion approach was used to enhance the sharpness of the stroke region. Later, a novel CNN architecture was presented for classifying brain stroke: hemorrhagic, ischemic, and normal using CT or CTA images. This [2] CNN module, which contains one fully connected layer, one max-pooling layer, and two convolutional layers, is used. In this study, they trained and evaluated a CNN module that could detect an ischemic stroke using 256 patch images.
In their study [6], they sought to create and evaluate a two-stage DL model for an autonomous AIS detection system. This study [10] tried to explore the recognition information of the acute infarct core on non-contrast CT ior CTA images using a deep learning residual network and two optimization approaches, which have a distinctive and crucial diagnostic value. This research [25] was to create a DL-assisted interactive framework for identifying the hyperdense middle cerebral artery (MCA) sign on non-contrast computed tomography in patients with acute ischemic stroke. To categorize input visuals as AIS-positive or AIS-negative, a deep convolutional neural network [25] was employed. In this study, [9], a novel two-stage CNN method for accurately identifying ischemic stroke on non-contrast CT or CTA images has been reported. Several trials have shown promising results.
Using multi-modality MRI sequences, the Res-CNN [21] network is employed to segment the acute ischemic stroke lesions. When compared to the six traditional CNN approaches, it performs better. Res-architecture CNN’s is primarily U-shaped. Up-sampling operators were employed in U-shape architecture to replace unspooling operators, while skip connections were utilized to directly connect opposing contracts. The model’s loss rate can be significantly lowered, thereby increasing performance. In [7] developed a DL method titled multi-feature map fusion network (MFMF-Network) based on two types of subjects: weakly labeled subjects are trained for classification, and fully labeled subjects are tuned for segmentation. DenseNets significantly surpassed ResNets when they were originally introduced for the categorization of 2D natural photographs due to their novel dense connection model. These qualities are needed, especially in healthcare applications where training datasets are frequently smaller and data dimensionality might be larger. In [26], they make use of DenseNets’ capacity to segment crisp strokes in 3D space. Comparative experiment findings show that the approach is superior to other cutting-edge 2D or 3D CNN models. Diffusion-weighted imaging was used in[27] to construct a DL model for automatic binary categorization of the Alberta Stroke Program Early Computed Tomographic Score (ASPECTS) in acute stroke patients (DWI). This study[22] used categorization and segmentation of pictures of brain strokes. LeNet and auto encoder-decoder were the two DL models they used for classification and segmentation. They will try to improve the segmentation algorithm’s precision and identify the precise location of the brain stroke by utilizing a variety of powerful deep-learning pre-trained models. In this article[11], a method for segmenting ischemic lesions was proposed by using multi-scaled encoded feature maps, and the ROI align-based patch CNN constructs a multi- level deconvolutional ensemble. This technique efficiently develops dense, reliable, pixel-class discriminatory feature representations at the encoder blocks. This study’s [20] hybrid model of the Fractal Net and U-Net combines the best aspects of both models and adds denser feature retrieval layers at the encoder. The segmentation process is split into a Classifier and a Segmenter in the model. In [28] propose DRANet, a new deep learning architecture featuring an attention module. DRANet has developed to segment ischemic stroke and WMH lesions accurately.In their independent testing dataset, the suggested model DAGMNet[29] outperformed generic models like UNet and FCN, as well as DeepMedic. This strategy presented a low rate of false-positive detection while being noticeably better at segmenting tiny lesions, which was a key issue for earlier techniques.
The above discussion leads to the conclusion that deep learning offers significant benefits in detecting ischemic stroke. However, several limitations exist within the currently used architectures, and these need to be highlighted for the purpose of improving generalizability. Many deep learning algorithms require a dataset of considerable magnitude, which is currently lacking. Thus, the limited sample size stands out as the primary limitation.
Another significant limitation is the absence of labeled regions of interest in existing dataset images. This lack necessitates researchers to manually identify findings even after acquiring datasets, resulting in considerable time consumption. Therefore, resolving this issue promptly is crucial to save time and resources.
Moreover, researchers encounter difficulties in gaining access to resources due to confidentiality and privacy rules surrounding the data. Additionally, there is currently no definitive method available to verify the accuracy of the data. Hence, extensive research efforts will be necessary in the future to ensure data accuracy and reliability.
In conclusion, this systematic review provides a comprehensive evaluation of deep learning techniques for the detection of brain ischemic strokes across different imaging modalities, including CT, MRI, and CTA. Our analysis reveals the potential of deep learning models in enhancing stroke detection efficiency, offering promising avenues for improving patient care and outcomes in clinical settings. This review aims to showcase deep learning approaches employed to address various stroke-related challenges in the medical field. Furthermore, this review underscores the importance of addressing technical challenges and optimizing model performance through preprocessing techniques and algorithmic enhancements. By overcoming these obstacles, deep learning-based stroke detection systems can achieve greater robustness and reliability, facilitating more accurate and timely diagnoses for patients presenting with stroke symptoms. Additionally, it summarizes various image preprocessing techniques that have been applied thus far, intending to assist researchers in future investigations. Furthermore, it outlines the challenges encountered in ischemic stroke detection and presents a summarized database table along with references to aid researchers, thereby saving their time. Thus, this paper offers a comprehensive understanding of ischemic stroke-related work in the domain of medical image processing, leveraging significant advancements in deep learning techniques. While exploring various stroke-related matters using state-of-the-art techniques, this study acknowledges the challenges inherent in comparing studies due to differences in performance metrics, datasets, methodologies, and tuning parameters across different tasks. In summary, this systematic review contributes valuable insights to the ongoing discourse on stroke detection methodologies, highlighting the transformative potential of deep learning in revolutionizing diagnostic approaches and improving patient outcomes in stroke care.
Список литературы Brain Ischemic Stroke Detection through Deep Learning: A Systematic Review on CT vs MRI vs CTA Images
- WHO. The top 10 causes of death. https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death , December 2020. Accessed: 2022-03-06.
- Chiun-Li Chin, Bing-Jhang Lin, Guei-Ru Wu, Tzu-Chieh Weng, Cheng-Shiun Yang, Rui-Cih Su, and Yu-Jen Pan. An automated early ischemic stroke detection system using cnn deep learning algorithm. In 2017 IEEE 8th Interna- tional conference on awareness science and technology (iCAST), pages 368–372. IEEE, 2017.
- Karl-Olof Lo¨vblad, Stephen Altrichter, Vitor Mendes Pereira, Maria Vargas, Ana Marcos Gonzalez, Sven Haller, and Roman Sztajzel. Imaging of acute stroke: Ct and/or mri. Journal of Neuroradiology, 42(1):55–64, 2015.
- Ye Xin and Fu-Gang Han. Diagnostic accuracy of computed tomography perfusion in patients with acute stroke: a meta-analysis. Journal of the Neurological Sciences, 360:125–130, 2016.
- Shiliang Huang, Qiang Shen, and Timothy Q Duong. Quantitative prediction of acute ischemic tissue fate using support vector machine. Brain research, 1405:77–84, 2011.
- Mizuho Nishio, Sho Koyasu, Shunjiro Noguchi, Takao Kiguchi, Kanako Nakatsu, Thai Akasaka, Hiroki Yamada, and Kyo Itoh. Automatic detection of acute ischemic stroke using non-contrast computed tomography and two-stage deep learning model. Computer Methods and Programs in Biomedicine, 196:105711, 2020.
- Bin Zhao, Zhiyang Liu, Guohua Liu, Chen Cao, Song Jin, Hong Wu, and Shuxue Ding. Deep learning-based acute ischemic stroke lesion segmentation method on multimodal mr images using a few fully labeled subjects. Computational and Mathematical Methods in Medicine, 2021, 2021.
- Kaiming He, Xiangyu Zhang, Shaoqing Ren, and Jian Sun. Deep residual learning for image recognition. In Proceedings of the IEEE conference on computer vision and pattern recognition, pages 770–778, 2016.
- Guoqing Wu, Xi Chen, Jixian Lin, Yuanyuan Wang, and Jinhua Yu. Identification of invisible ischemic stroke in noncontrast ct based on novel two-stage convolutional neural network model. Medical Physics, 48(3):1262–1275, 2021.
- Jiawei Pan, Guoqing Wu, Jinhua Yu, Daoying Geng, Jun Zhang, and Yuanyuan Wang. Detecting the early infarct core on non-contrast ct images with a deep learning residual network. Journal of Stroke and Cerebrovascular Diseases, 30(6):105752, 2021.
- R Karthik, R Menaka, Annie Johnson, and Sundar Anand. Neuroimaging and deep learning for brain stroke detection-a review of recent advancements and future prospects. Computer Methods and Programs in Biomedicine, 197:105728, 2020.
- Darwin Castillo, Vasudevan Lakshminarayanan, and Mar´ıa Jose´ Rodr´ıguez-A´ lvarez. Mr images, brain lesions, and deep learning. Applied Sciences, 11(4):1675, 2021.
- Yue Zhang, Shijie Liu, Chunlai Li, and Jianyu Wang. Application of deep learning method on ischemic stroke lesion segmentation. Journal of Shanghai Jiaotong University (Science), pages 1–13, 2021.
- David G Harrison et al. Cellular and molecular mechanisms of endothelial cell dysfunction. The Journal of clinical investigation, 100(9):2153–2157, 1997.
- Victor F Tapson. Acute pulmonary embolism. New England Journal of Medicine, 358(10):1037–1052, 2008.
- Adria` Arboix and Josep Llu´ıs Mart´ı-Vilalta. Lacunar stroke. Expert review of neurotherapeutics, 9(2):179–196, 2009.
- RS Jeena and Sukesh Kumar. A comparative analysis of mri and ct brain images for stroke diagnosis. In 2013 annual international conference on emerging research areas and 2013 international conference on microelectronics, communications and renewable energy, pages 1–5. IEEE, 2013.
- Seonwoo Min, Byunghan Lee, and Sungroh Yoon. Deep learning in bioinformatics. Briefings in bioinformatics, 18(5):851–869, 2017.
- John J Hopfield. Artificial neural networks. IEEE Circuits and Devices Magazine, 4(5):3–10, 1988.
- Amish Kumar, Neha Upadhyay, Palash Ghosal, Tamal Chowdhury, Dipayan Das, Amritendu Mukherjee, and De- bashis Nandi. Csnet: A new deepnet framework for ischemic stroke lesion segmentation. Computer Methods and Programs in Biomedicine, 193:105524, 2020.
- Liangliang Liu, Shaowu Chen, Fuhao Zhang, Fang-Xiang Wu, Yi Pan, and Jianxin Wang. Deep convolutional neural network for automatically segmenting acute ischemic stroke lesion in multi-modality mri. Neural Computing and Applications, 32(11):6545–6558, 2020.
- Bhagyashree Rajendra Gaidhani, RR Rajamenakshi, and Samadhan Sonavane. Brain stroke detection using convolu- tional neural network and deep learning models. In 2019 2nd International conference on intelligent communication and computational techniques (ICCT), pages 242–249. IEEE, 2019.
- Noah Stier, Nicholas Vincent, David Liebeskind, and Fabien Scalzo. Deep learning of tissue fate features in acute ischemic stroke. In 2015 IEEE international conference on bioinformatics and biomedicine (BIBM), pages 1316– 1321. IEEE, 2015.
- Anjali Gautam and Balasubramanian Raman. Local gradient of gradient pattern: a robust image descriptor for the classification of brain strokes from computed tomography images. Pattern Analysis and Applications, 23(2):797– 817, 2020.
- Yuki Shinohara, Noriyuki Takahashi, Yongbum Lee, Tomomi Ohmura, and Toshibumi Kinoshita. Development of a deep learning model to identify hyperdense mca sign in patients with acute ischemic stroke. Japanese Journal of Radiology, 38(2):112–117, 2020.
- Rongzhao Zhang, Lei Zhao, Wutao Lou, Jill M Abrigo, Vincent CT Mok, Winnie CW Chu, Defeng Wang, and Lin Shi. Automatic segmentation of acute ischemic stroke from dwi using 3-d fully convolutional densenets. IEEE transactions on medical imaging, 37(9):2149–2160, 2018.
- Luu-Ngoc Do, Byung Hyun Baek, Seul Kee Kim, Hyung-Jeong Yang, Ilwoo Park, and Woong Yoon. Automatic as- sessment of aspects using diffusion-weighted imaging in acute ischemic stroke using recurrent residual convolutional neural network. Diagnostics, 10(10):803, 2020.
- Liangliang Liu, Lukasz Kurgan, Fang-Xiang Wu, and Jianxin Wang. Attention convolutional neural network for accurate segmentation and quantification of lesions in ischemic stroke disease. Medical Image Analysis, 65:101791, 2020.
- Chin-Fu Liu, Johnny Hsu, Xin Xu, Sandhya Ramachandran, Victor Wang, Michael I Miller, Argye E Hillis, and Andreia V Faria. Deep learning-based detection and segmentation of diffusion abnormalities in acute ischemic stroke. Communications Medicine, 1(1):1–18, 2021.


