Disclosure of the mechanisms of the medical preventional effect of fucoidan in the composition of food systems
Автор: Anjum V., Kadi A.M.Y., Bagale U., Potoroko I.Yu.
Рубрика: Пищевые ингредиенты, сырье и материалы
Статья в выпуске: 1 т.12, 2024 года.
Бесплатный доступ
It is very promising to study the molecular mechanism and basis of the action of fucoidan in food systems for the prevention of inflammatory bowel diseases (IBD) using network pharmacology as a new paradigm for drug design and molecular docking technology. Our results offer a theoretical framework for future clinical investigations. The GeneCards and OMIM network databases were used to seek and screen the targets for IBD activity in the treatment of gastric cancer lesions. The string data platform was used to create the protein interoperability network of fucoidan for the treatment of IBD, whereas Cytoscape 3.9.1 was used to create the compound-target network. Topological analysis was used to identify the main target. Finally, the drug-disease intersection target was analysed using enrichment and biological pathways. A total of 81 component targets were gathered, and using network topology and protein interoperability network analysis, 6758 targets for IBD were found, along with another 301 essential core targets. The key targets were evaluated to include STAT3, ESR1, BCL2, and AKT1, among others. 173 signaling pathways, including pathway in cancer, metabolic pathway, Neuroactive ligand-receptor interaction, and PI3K-Akt signaling pathway were screened from KEGG and GO functional enrichment analysis. Best binding of -6.5 kcal/mol with AKT1 was obtained from additional docking studies with fucoidan. Fucoidan acts on targets such as AKT1, ESR1, STAT3, and BCL2, as well as important inflammatory, immunological, and gastric cancer pathways, and can be utilised to prevent and manage IBD.
Fucoidan, network pharmacology, inflammatory bowel disease, mechanism studies, gastric cancer
Короткий адрес: https://sciup.org/147243170
IDR: 147243170 | УДК: 664.8.038+663 | DOI: 10.14529/food240102
Текст научной статьи Disclosure of the mechanisms of the medical preventional effect of fucoidan in the composition of food systems
All living things, including humans, must eat and digest their food to receive the necessary nutrients for continued physical vigour. Energy from nutrient absorption is used in many metabolic processes, primarily growth, development, and reproduction [1]. Colorectal cancer (CRC) is a clinical malignant carcinoma in the gastrointestinal tract, and its incidence and mortality are ranked as following the digestive malignancies of gastric cancer, esophageal cancer, and primary liver cancer [2]. Long-term intestinal inflammation is the primary cause of colorectal cancer (CRC), although other factors such as genetics, poor dietary choices, and way of life all contribute to the disease's progression [3]. According to global cancer statistics from 2018, there were 9.2 % new cases of colorectal cancer out of a to- tal of 17.0 million cases, and 6.1 % of those cases resulted in a death from cancer [4]. Thus, maintaining both host protection against invasive infections and host health depends heavily on a well-balanced diet. In this situation, immune-boosting dietary additives can offer the host additional advantages. As a result, scientists have been searching for naturally occurring compounds that may improve human health without having any negative effects. Among these, seaweed-derived bioactive substances, such as micro- and macroalgae, are intriguing candidates with a variety of advantageous properties, such as antioxidant, anti-inflammatory, and anti-cancer properties [5]. Fucoidans are known to be present in seaweed cell walls in order to maintain the stability of the cell membrane and shield the structure from dehydration [6]. The structure of fucoidans has been correlated with their biological and immunological activities, such as antioxidant, anticoagulant, antithrombotic, antiinflammatory, antiviral, antilipidemic, antimetastatic, antidiabetic and anticancer effects [7–9] suggest that dietary fucoidans can directly stimulate intestinal cells. Systematic investigation of the interactions of bioactive substances instead of drugs with targets, transport pathways in the digestive tract and disorders is possible using network pharmacology. It is especially appropriate for researching the chemicals that give marine medicine its therapeutic properties as well as its multi-component and multi-target qualities. The use of network pharmacology technologies is especially relevant for the study of bioactive chemicals that have therapeutic properties in marine medicine, as well as its multicomponent and multipurpose properties. In this regard, the main goal of our work is to study the biological effects and molecular mechanisms of brown seaweed polysaccharides in the treatment of inflammatory diseases of the gastrointestinal tract based on network pharmacology methods. Our results expand the use of brown seaweed in the prevention and treatment of inflammatory bowel disease and provide a starting point for future research, as well as help in the development of new preventive bioactive substances to replace drugs.
Methodology
Active components and corresponding target selection
The literature on the brown algae polysaccharide fucoidan was gathered from PubMed database. The latter is a platform that includes pharmacokinetic features, associated protein tar- gets. The metrics of OB (oral bioavailability; 30 %), and DL (drug-likeness; 0.18) were filtered out as they are critical components of the drug's Absorption, distribution, metabolism, elimination (ADME) profile [10]. Molsoft and SwissADME compute OB and DL, which are used to weed out substances that are unlikely to be pharmaceuticals. Protein targets that interacted with those putative active chemicals were then predicted using STITCH and Swiss Target Prediction databases. The target's probability was fixed at > 0.5.
Procurement of disease-associated targets genes
The known targets linked to the aetiology of inflammatory bowel disease were searched from two significant databases namely, OMIM and GeneCards [11]. Only the carefully chosen targets that were clearly linked to the illness were kept, and the repeating targets were eliminated, to lower the number of false positives. Both acquired genes then entered in the Venn diagram drawing tool, to find the intersected gene as potential genes.
Construction of Protein-protein interaction (PPI) network
The PPI network was built using the candidate genes importing to the STRING database. The PPI network was pictured with Cytoscape (v3.9.1) [12].
Biological pathway and enrichment analysis
The bioinformatics tool was used to analyze the enrichment of biological processes and pathways for the intersection target network between drugs and diseases. According to the enrichment results, the material basis, and molecular mechanisms of fucoidan in the prevention treatment of gastro-intestinal disease were predicted and analyzed.
Drug-target pathways network construction
The PPI constructed network from STRING database, then studied with Cytoscape to analyse the constructed compound-protein interaction (CPI) network to characterise the therapeutic action of Fucoidan for gastrointestinal disorders. The drug components, target or disease-related gene, and pathways are represented by the nodes in constructed network with different shaped and colours. An association between the nodes is called an “edge”.
Component target molecular docking
The respective target genes namely AKT1 (3O96), ESR1 (1X7E), BCL2 (6QGG), and STAT3 (6NJS) were acquired from the RCSB protein data library. The fucoidan was download- ed as SDF file as 3D image and further optimised in the AutoDock (AD) 4.2 tool. The ligand and target gene were imported into autodock Vina software in “pdbqt” format for molecular docking, and molecular graphics visualisation tool PyMOL and ligplot+ were used to analyze the interaction mode of the docking results. For docking, the entire receptor was enclosed inside a grid box, with a grid spacing of 1.0 Å, keeping the receptor rigid and the ligand as a flexible molecule. The ligand’s backbone and side-chain were flexible and allowed to dock with the receptor to form all possible conformations. After defining the binding site and receptor–ligand preparation, docking runs were launched from the command prompt. The interaction energy between the ligand and the receptor was calculated for the entire binding site and expressed as affinity (kcal/mol).
Results and discussion
Ligand and target gene preparation
The ligand structures were downloaded from PubChem and converted to pdb format using PyMol. The structures were energy minimized and saved in PDBQT format by MGL tools. The target gene was downloaded from Protein Data Bank and (PDB) converted to PDBQT format using AutoDock tools (Fig. 1).
The difficult phase in the development of therapeutic and prophylactic drugs is ADMET analysis which is carried out using the SwissADME tool to assess various pharmacoki- netic parameters that forecast ADME, as well as the toxicity (T) of the most highly significant novel therapeutic phytocomponents. Properties of ADMET of powerful therapeutic and prophylactic drugs for different paradigms, including GI absorption was found high, P-glycoprotein substrates, BBB penetration, and various inhibitors of cytochrome namely CYP1A2, CYP2C19, CYP2C9, CYP2D6, and CYP3A4 found none, created encouraging outcomes that significantly substantiate the potency of fractions. The size and chemo-physical characteristics of the skin are determined by the log Kp values (–8.56).
Unknown processes contribute to gastric and intestinal disorder. Several theories have been put out to clarify the process at play. In the meantime, a total of 81 target genes were gathered that interacted with those suspected components from both Swiss Target Prediction and STITCH database after removal of duplication, whereas 6758 were obtained from GeneCard and OMIM after removing duplicate genes. In the end, 301 intersection genes or candidate genes were gathered for additional processes for foster research on the mechanism of fucoidan on treating inflammatory bowel.
PPI network construction and analysis
Using STITCH, the 6758 potential genes were chosen to create a first constructed PPI (Fig. 2a). 301 important targets (Fig. 2b) were ultimately gathered for pathway enrichment analysis, with AKT1 (164), STAT3 (133), BCL2 (112) and ESR1 (108) ranking highest.
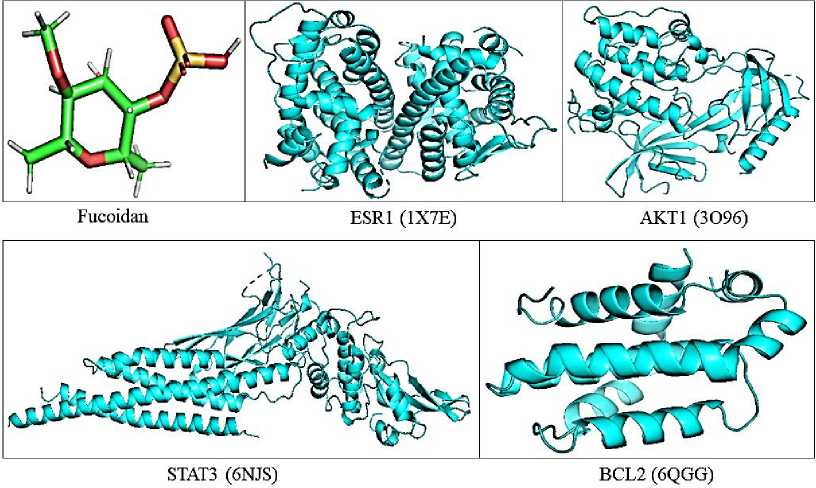
Fig. 1. 3D structure of Fucoidan and target gene
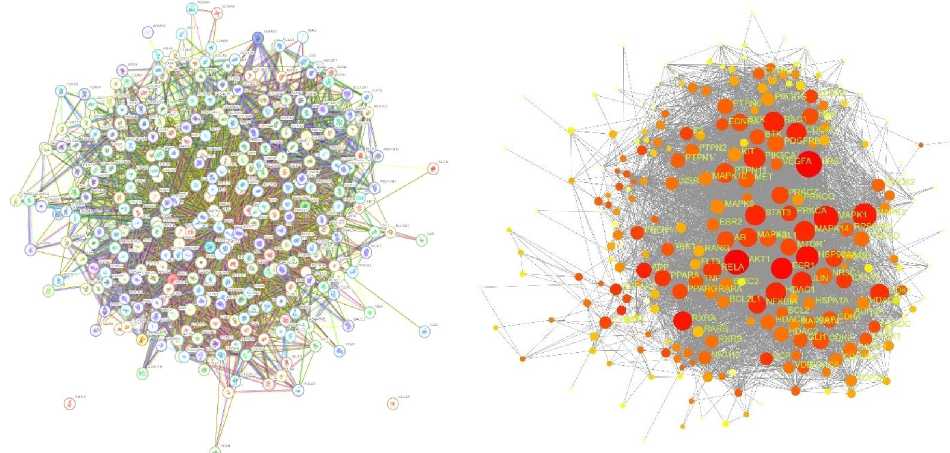
а)
б)
Fig. 2. a – PPI network; b – Intersection genes for pathway enrichment
Then, the Cytoscape 3.9.2 software was used to conduct a topology analysis. Screens with a value greater than two times (20) the average degree value were used to obtain the core targets (Fig. 3). The following 10 key targets were obtained: TNF (170), AKT1 (163), ALB (139), SRC (138), STAT3, CASP3, BCL2, ESR1, PPARG and JUN. The analysis results suggest that the fucoidan may play a therapeutic role mainly by acting on these 10 targets.
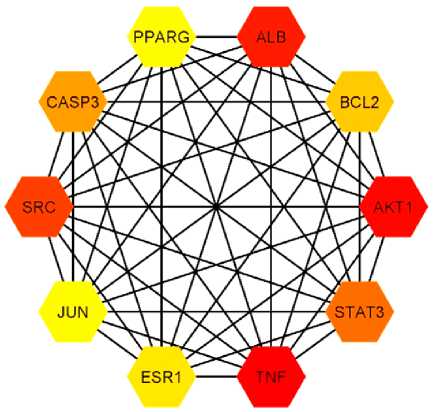
Fig. 3. Top ranked target genes based on degree
The KEGG study demonstrated a therapeutic and prophylactic effect, examination demonstrated the therapeutic effect that fucoidan plays in the treatment of inflammatory bowel disease by acting on the pathway. Here, ten significant signalling pathways (Fig. 4) with p < 0.01 were selected for additional investigation created on 301 key targets. The top three were neuroactive ligand-receptor interaction, AGE-RAGE signalling pathway in diabetic complication, chemical carcinogenesis-receptor activation.
Biological pathway and functional enrichment analysis
The SR plot bioinformatics tool was used to analyze the enrichment of biological processes and pathways for intersection targets of fucoidan and disease (Fig. 4).
Molecular Docking Studies
The docking process was carried out using AutoDock Vina. The binding affinities of ligands are represented as kcal/mol (see: Table). Docking studies were carried out to find out the enhancing activity of the ligands on AD 4.2 using PyMOL and LigPlot visualizer. The affinity value of less than 5 kcal/mol depicts negligible binding, whereas values closer to 10 kcal/mol indicate efficient binding. The four core targets (AKT1, STAT3, BCL2, and ESR1) screened related to fucoidan with a degree of > 20.
The protein-ligand docking studies are represented in Fig. 5–8. Fucoidan exhibited stronger binding affinity with AKT1 (3O96). The H-bonds and hydrophobic contacts between the docked complexes are shown in following figures. The ESR1, AKT1, STAT3, and BCL2 on the surface caused the first tethering and strong
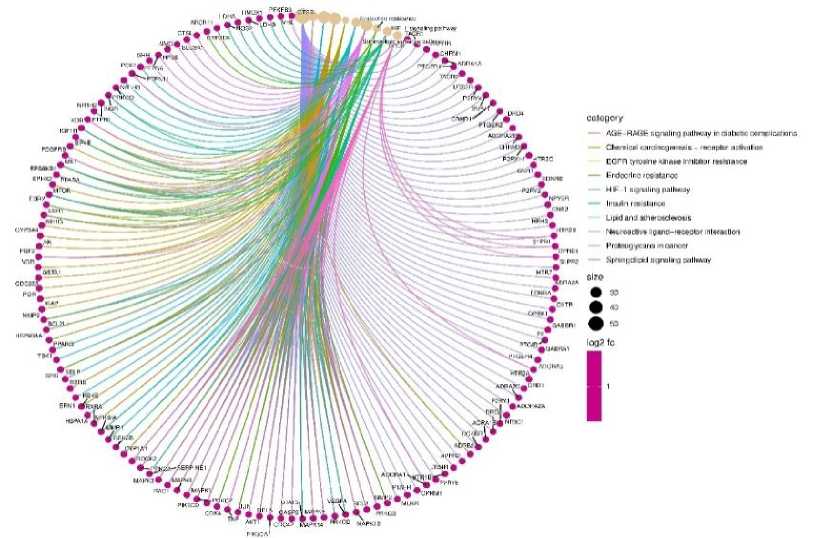
Fig. 4. Kegg pathway analysis
Table
Molecular interactions of Fucoidan with amino acids of proteins
Owing to its anatomical position, the gastrointestinal (GI) tract is a specialized barrier to maintain intestinal homeostasis by physically separating the intestine from the external environment [13]. In addition to nutrient uptake, the GI tract plays an important role in maintaining intestine immunity by identifying “Good” or “Bad” foreign antigens and enabling their interaction with diverse intestinal cells. Fucoidan promoted G2/M phase apoptosis in MGC-803
cell line and cell-cycle arrest in gastric cancer by downregulating, AKT1, STAT3, Bcl-2, and cy-clin B1[14] further upregulating CASP-3. It was evident from molecular docking showed good binding with AKT1 (–6.5 kcal/mol) interacting with Gln-79 (A; 3.01), Thr-82 (A; 2.89, 2.86,
3.06), Asp-292 (A; 2.73), Gly-294 (A; 3.15) as shown in Fig. 5.
Fucoidan has also been demonstrated to have an impact on colon cancer, where it causes apoptosis, DNA fragmentation, G0/G1 arrest, and downregulation of Bcl-2, ESR1, and upregulation
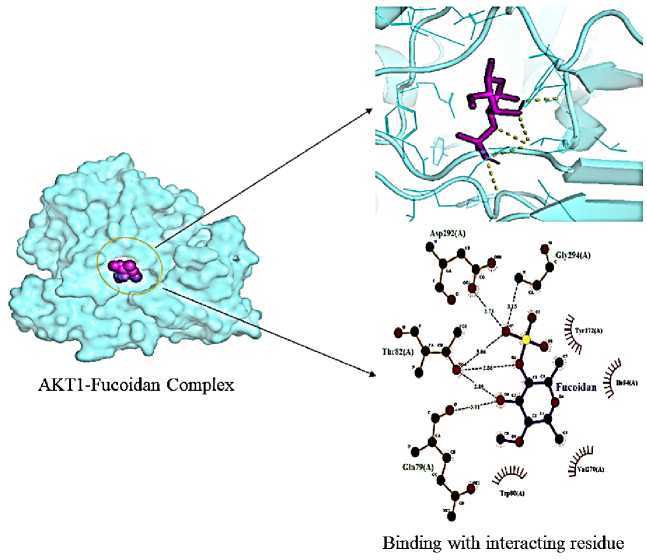
Fig. 5. Interaction of fucoidan with AKT1 (3096) receptor
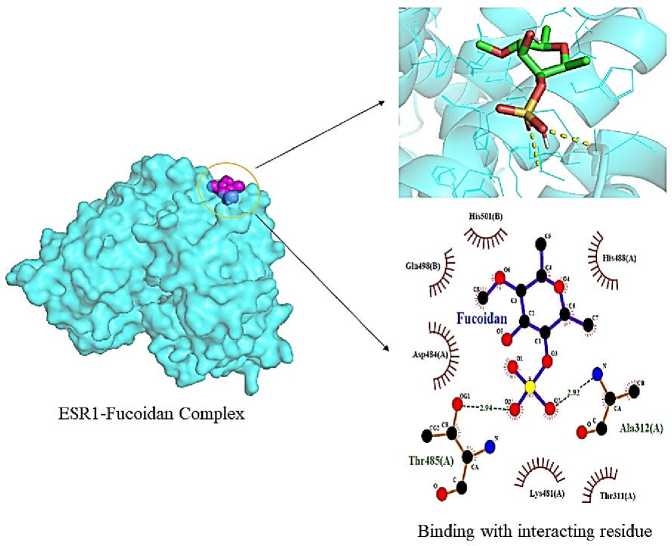
Fig. 6. Interaction of fucoidan with ESR1 (1X7E) receptor
of p21. Fucoidan showed interaction with ESR1 with binding energy of –5.0 kcal/mol interacting with Thr-485 (A; 2.94), Ala-312 (A; 2.92) as evident from Fig. 6. The increased permeability of paracellular by fluorescein isothiocyanatedextran indicated the significance of boosting the fucoidan bioavailability in maximizing the impact on immunity.
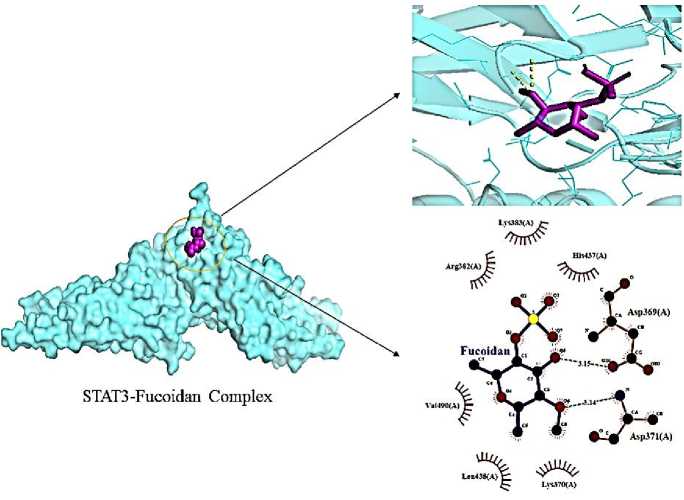
Dietary fucoidan has immunomodulatory and anti-inflammatory properties that can also improve the functioning of the gut immune system. Moreover, fucoidan helps treat conditions linked to inflammation, such as inflammatory bowel disorders. According to a recent study, fucoidan may have a protective effect on colon health since it was shown to reduce STAT3, TNF- a , IL-10, IL-6, and IL-1 levels in the colon cells of mice when given after antibiotic therapy [15]. It interacted with amino acid residue Asp-371 (A; 3.14), Asp-369 (A; 3.15) of STAT3 receptor showing bind energy of –4.9 kcal/mol (Fig. 7).
The lead to drop in pro-inflammatory and an increase in anti-inflammatory cytokines. Though fucoidan's molecular size affects the absorption by intestine epithelial cells. Fucoidan inhibits the insulin-like growth factor (IRF)-I receptor via the Ras/Raf/ERK pathway, hence controlling cell viability and the biological cycle [16]. Numerous intracellular pathways involving signal transduc- tion molecules, including NF-κB, MAPKs, STAT, and PI3K pathways, up-regulate these factors and enzymes.
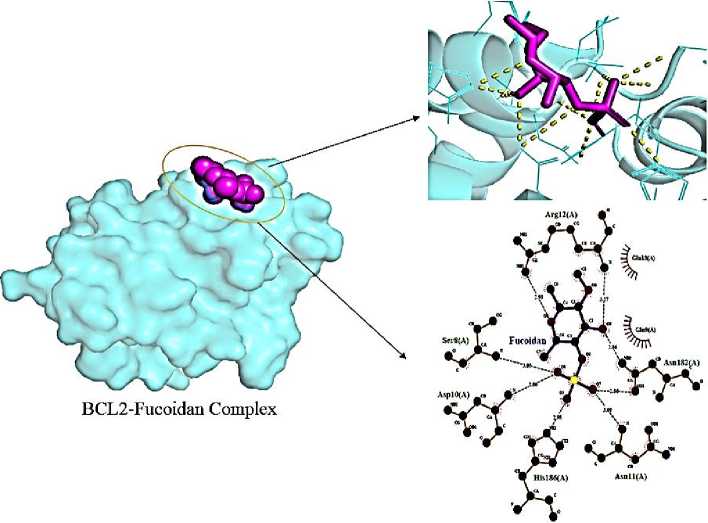
It also downregulated Bcl-2, raised Bax, and triggered apoptosis in prostate cancer through G1 cell cycle arrest [17]. It was shown that by inhibiting NF-ΟB activation and MAPK phosphorylation, fucoidan could lower the levels of proinflammatory mediators (PGE2, NO, TNF- a , IL-1 P , IL-6). Through docking binding with BCl2 (–5.5 kcal/ml) interacted with Asp-10 (A; 2.80), Ser-8 (A; 3.05), Arg-12 (A; 2.98, 3.27), Asn-182 (A; 2.86, 2.86), Asn-11 (A; 3.09) (Fig. 8).
Conclusion
Given the intimate relationship between the intestine and nutrition intake, natural bioactive substances have been investigated as possible preventive therapeutic agents to improve human intestinal immunity. Many concerns remain unsolved despite research on the impact of fucoidans in animal models and cell lines. For example, it is unclear if consumption of fucoidans directly alters cellular phenotypes or increases humoral immune responses. Therefore, developing diet-based therapies for bowel disorders, such as inflammatory bowel disease or colorectal malignancies (CRCs), will be made easier with a greater understanding of how fucoidan functions in the intestinal area as a food
Binding with interacting residue
Fig. 7. Interaction of fucoidan with STAT3 (6NJS) receptor
Binding with interacting residue
Fig. 8. Interaction of fucoidan with BCL2 (6QGG) receptor
supplement or dietary additive. Therefore, the ppreventive therapy of inflammatory receptors with fucoidan medicines has multi-component, multi-target, and multi-channel features, as determined by our study using network pharmacology and molecular docking. Our findings also indicate the primary active ingredients, primary targets, and associated pathways of fucoidan in the management of gastrointestinal disorders and inflammatory bowel disease, laying the groundwork for future research into the therapeutic efficacy of fucoidan.
Список литературы Disclosure of the mechanisms of the medical preventional effect of fucoidan in the composition of food systems
- Martin G.B., Rodger J., Blache D. Nutritional and environmental effects on reproduction in small ruminants. Reprod. Fertil. Dev. 2004; 16: 491-501. DOI: 10.1071/rd04035
- Haraldsdottir S., Einarsdottir H.M., Smaradottir A., Gunnlaugsson A., Halfdanarson T.R. Colo-rectal cancer-review. Laeknabladid. 2014; 100: 75-82.
- Birt D.F., Phillips G.J. Diet, genes, and microbes: complexities of colon cancer prevention. Toxicol Pathol. 2014; 42: 182-188. DOI: 10.1177/0192623313506791
- Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018; 68: 394-424. DOI: 10.3322/caac.21492
- Subramaniam S., Selvaduray K.R., Radhakrishnan A.K. Bioactive compounds: Natural defense against cancer? Biomolecules. 2019; 9: 758. DOI: 10.3390/biom9120758
- Vo T.S., Kim S.K. Fucoidans as a natural bioactive ingredient for functional foods. J. Funct. Foods. 2013; 5: 16-27. DOI: 10.1016/j.jff.2012.08.007
- Li B., Lu F., Wei X.J., Zhao R.X. Fucoidan: Structure and bioactivity. Molecules 2008; 13: 1671-1695. DOI: 10.3390/molecules13081671
- Xue M., Ji X., Liang H., Liu Y., Wang B., Sun L., Li W. The effect of fucoidan on intestinal flora and intestinal barrier function in rats with breast cancer. Food Funct. 2018; 9: 1214-1223. DOI: 10.1039/c7fo01677h
- Zuo T., Li X., Chang Y., Duan G., Yu L., Zheng R., Xue C., Tang Q. Dietary fucoidan of Acaudina molpadioides and its enzymatically degraded fragments could prevent intestinal mucositis induced by chemotherapy in mice. FoodFunct. 2015; 6: 415-422. DOI: 10.1039/c4fo00567h
- Qian C., Zhang Y.L., Ma Y.H., et al. A network pharmacology approach to investigate the mechanism of Shuxuening injection in the treatment of ischemic stroke. JEthnopharmacol. 2020; 257: 112891. DOI: 10.1016/j.jep.2020.112891
- Gfeller D., Grosdidier A., Wirth M., et al. Swiss Target Prediction: a web server for target prediction of bioactive small molecules. Nucleic Acids Res. 2014; 42: W32-8. DOI: 10.1093/nar/gku293
- Szklarczyk D., Franceschini A., Kuhn M., et al. STRING v11: protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019; 47: D607- D613. DOI: 10.1093/nar/gky1131
- Ahluwalia B., Magnusson M.K., Ohman L. Mucosal immune system of the gastrointestinal tract: Maintaining balance between the good and the bad. Scand. J. Gastroenterol. 2017; 52: 11851193. DOI: 10.1080/00365521.2017.1349173
- Yu R.X., Hu X.M., Xu S.Q., Jiang Z.J., Yang W. Effects of Fucoxanthin on Proliferation and Apoptosis in Human Gastric Adenocarcinoma MGC-803 Cells via JAK/STAT Signal Pathway. Eur. J. Pharmacol. 2011; 657: 10-19. DOI: 10.1016/j.ejphar.2010.12.006
- Wang L., Ai C., Wen C., Qin Y., Liu Z., Wang L., Gong Y., Su C., Wang Z., Song S. Fucoidan isolated from Ascophyllum nodosum alleviates gut microbiota dysbiosis and colonic inflammation in antibiotic-treated mice. Food Funct. 2020; 11: 5595-5606. DOI: 10.1039/d0fo00668h
- Kim I.H., Nam T.J. Fucoidan downregulates insulin-like growth factor-I receptor levels in HT-29 human colon cancer cells. Oncol. Rep. 2018; 39: 1516-1522. DOI: 10.3892/or.2018.6193
- Kotake-Nara E., Asai A., Nagao A. Neoxanthin and Fucoxanthin Induce Apoptosis in PC-3 Human Prostate Cancer Cells. Cancer Lett. 2005; 220: 75-84. DOI: 10.1016/j.canlet.2004.07.048


