In vitro release studies of rilpivirine from in situ forming polymeric implants in buffer solution and in a gel phantom of muscle tissue
Автор: Ulianova Y.V., Ermolenko Y.V., Vanchugova L.V., Mityukov A.V., Gelperina S.E.
Журнал: Вестник Воронежского государственного университета инженерных технологий @vestnik-vsuet
Рубрика: Пищевая биотехнология
Статья в выпуске: 4 (98) т.85, 2023 года.
Бесплатный доступ
Development of in situ forming implants (ISFI) based on PLGA polymers is one of the most promising approaches to long-acting injectables. Evaluation of the drug release rate from such depot formulations requires methods that most closely simulate in vivo conditions. Gel phantoms mimic the elastic properties of muscle tissue and appear to be a promising replacement for conventional methods using physiologically relevant buffer solutions. Accordingly, the aim of the study was to select the optimal composition for the gel phantom formation and evaluate the effect of the phantom matrix on the release rate of rilpivirin used as a model drug from the PLGA ISFI. According to the results of the study, a 1% agarose gel was the best suited for a tissue phantom preparation and implant formation. It was also shown that the release profile of rilpivirin from the ISFI matrix depended on how the implant was formed (in a gel or freely in buffer). In the case of a phantom, the structure of the implant was less porous and retained its shape for 28 days of incubation at 37 °C. During this period, the ISFI formed in an agarose gel released considerably less rilpivirin compared to the ISFI formed without gel (11% vs 80%).
Isfi, plga, drug release, agarose gel, polyacrylamide gels
Короткий адрес: https://sciup.org/140304450
IDR: 140304450 | УДК: 640 | DOI: 10.20914/2310-1202-2023-4-70-75
Текст научной статьи In vitro release studies of rilpivirine from in situ forming polymeric implants in buffer solution and in a gel phantom of muscle tissue
DOI:
In situ forming implants (ISFI) occupy a special place among the various injectable depot formulation, due to their important advantages: simple manufacturing technology (a few steps, simple and inexpensive equipment) and less invasive administration procedure based on introducing a liquid biodegradable and biocompatible composition through a small needle thus avoiding microsurgery. Phase-sensitive ISFI is a low viscous solution comprising both the drug and the polymer in a biocompatible water-miscible organic solvent such as N-methylpyrrolidone (NMP), which forms a solid implant following intramuscular or subcutaneous injection as a result of the phase inversion process [1-5]. To date such ISFIs have been used successfully in commercial applications for the treatment of various diseases: prostate cancer (Eli-gard® ), central precocious puberty (Fensolvi® ), opioid dependence (Sublocade™), schizophrenia (Perseris™). One of the main parameters of depot formulations is the drug release rate from the carrier matrix. Nevertheless, current methods in vitro employed for evaluation of the release kinetics do not always substitute for the animal experiments [6]. For example, it was shown that not only the chemical composition of the injection site plays
an important role in the drug release from ISFI but also the physical structure and tissue stiffness are important [7]. Thus, the use of hydrogel phantoms that mimic muscle tissue to simulate in vivo conditions and to improve the in vitro-in vivo correlations (IVIVC) was proposed in some studies [6, 8, 9].
Therefore, one of the objectives of the present study was to choose a composition for the formation of a gel phantom that satisfy the following necessary requirements: (1) elastic modulus of the gel must correspond to the elastic modulus of muscle tissue, which is in the range from 15 to 34 kРа [10], (2) rapid gelation (or easy sample preparation), (3) possibility of introducing a viscous polymer solution into the phantom and (4) formation of an implant inside the gel. Another objective of the study was to evaluate the influence of the gel phantom on the drug release rate from the ISFI using rilpivirine as a model drug.
Materials and Methods
Chemicals. Rilpivirine (RPV) was obtained from Lomonosov Moscow State University, Russia; poly(lactic-co-glycolic acid) (PLGA, Pura-sorb® PDLG 5004, LA/GA ratio of 50:50, ester endcapped, η = 0.41 dL/g, Corbion, The Netherlands); N-methyl-2-pyrrolidone (NMP, Sigma, Germany); buffer solutions were prepared using PBS tablets
This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International License
Ульянова Ю.В.и др. Вестник ВГУИТ, 2023, Т. 85, №. 4, С. (MP Biomedicals, LLC, USA); polysorbate 20 (Tween 20® ) and trifluoroacetic acid (TFA) suitable for HPLC (Sigma, USA);, acrylamide (ААm) and N, N'-methylenebis(acrylamide) (MBA) (Acros Organics, Belgium), ammonium persulfate (APS) (Applichem, Germany); tetramethylethylenediamine (TEMED) and agarose (gel point, 34.5–37.5 °C) (Sigma, USA); acetonitrile (HPLC grade, Chem-lab, Belgium).
Preparation of ISFI formulations. RPV (12 mg) was dissolved in 12 mL of NMP. Then, PLGA (440 mg) was added to this solution. The components were dissolved by continuous mixing at room temperature to form homogenous mixtures. The final drug loading of RPV in RPV-polymer solution was 2.7% (w/w PLGA).
High-pressure liquid chromatography (HPLC). The assay of RPV was performed using a reverse phase HPLC method. The HPLC analysis was carried out with a LC-2030С 3D Plus HPLC system (SHIMADZU, Japan) equipped with a Photodiode Array (PDA) Plus Detector, a С18 pre-column and a Purospher® STAR RP-18 endcapped column (120 • 4 mm, 3 µm). The mobile phase consisted of 0.1% TFA in water (solvent A) and 0.1% TFA in acetonitrile (solvent B) delivered at a flow rate of 1 mL/min. A gradient elution was applied from 10% to 60% solvent B at 25 min. Column oven was set at 35 °C, injection volume was 20μl, and the analysis was carried out at 280 nm.
Phantom preparation and characterization. Polyacrylamide gels PAA_1, PAA_2, and PAA_3а were obtained by radical copolymerization of ААm (31%, 32%, and 11%) and MBA as a crosslinking agent (2.7%, 1.4%, 20% relative to ААm concentration) with redox initiation using TEMED (0.02%, 0.02, 0.1%) and APS (0.02%; 0.02%, 0.1%) in PBS solution followed by incubation of the mixture at room temperature for 24 h. The resulting hydrogels were washed with water for 3 days (water was changed three times per day). Cryogel PAA_3b was synthesized according to the procedure from [11]. A solution of ААm (11%), MBA (20% to ААm concentration) and TEMED (0.1%) in phosphate buffer (рН 7.4) was cooled to 7 °C, then an APS solution (0.1%) cooled to 7 °C was added to initiate polymerization. The solution was then quickly poured into a mold and placed in a – 25 °C freezer for 24 h. Young's modulus of phantoms was determined by mechanical testing using a RheoStress RS600 rheometer (Thermo HAAKE, USA). The microstructure of gels and ISFI was evaluated by SEM (JSM-6510LV microscope, JEOL Ltd, Japan).
Incorporation of the ISFI formulations into agarose gel. 1% (w/v) agarose gel was produced as described in [12]. Briefly, 2 ml of a 1% (w/v) gel solution was transferred to the mold and cooled on ice. Before the phase transition of agarose was complete, 0.2 ml of the RPV-polymer solution was injected into a cavity made in the upper layer. A warm 1% agarose solution was carefully added on the surface of the solution after 30 sec. The resulting phantom was cooled at 4 °C for 10 min.
Evaluation of RPV release . The release medium consisted of 2% w/v Tween 20 in 0.15 M PBS at рН 7.4. The release of RPV from ISFI formulations was evaluated by injecting 0.2 ml of the RPV-polymer solution or by placing agarose phantom with ISFI into 150 ml of release medium and further incubation under the sink conditions at 37 °C with continuous shaking (200 rpm, orbital shaker-incubator ES-20, Biosan, Latvia). Then 500 μL samples were taken at predetermined time intervals (30 min, 1, 2, 3, 5, 7, and 24 h, and then on daily basis). The concentration of the released RPV was measured by HPLC. All experiments were performed in triplicate.
Hydrolytic degradation of ISFI. The kinetics of ISFI degradation was studied using the capillary electrophoresis (CE) system CAPEL-105 M (Lu-mex, Russia) equipped with a spectrophotometric detector and a quartz capillary tube (i.d. 75 mm, e.l. 50 cm, t.l. 60 cm) and Elforun software (Lumex, Russia). After ISFI formation, the aliquots were taken at predetermined time intervals (1, 2, 3 day, etc.). All products of the hydrolytic degradation in the supernatants (100 µl) were further hydrolyzed by addition of 10 ml of 1 N NаОН, and the resulting concentration of lactic acid was measured by CE at 254 nm as described in [13]. All experiments were performed in triplicate.
ResultsPolymer phantoms formation
Polyacrylamide gels were prepared by varying the concentration of ААm and MBA in phosphate buffer (PAA_1, PAA_2, and PAA_3а) and by a cryogelation technique (PAA_3b). The appearance and shape of polyacrylamide gels are shown in Figure 1А. The phantoms differed in the Young's modulus (6.13 kРа, 8.6 kРа, 8.9 kРа, and 9.59 kРа for for PAA_1, PAA_2, PAA_3а, and PAA_3b, respectively) and the degree of porosity (Fig.1В). 1% agarose gel with an elastic modulus of 11.38 kРа was also obtained.

Figure 1. A ) Appearance of the phantoms ( from left to right ): PAA_1, PAA_2, PAA_3а, PAA_3b and agarose gel; B ) SEM image of standard polyacrylamide gels ( left ) and cryogel phantoms (right) ; C ) Introduction of the PLGA solution into the gel. Compiled by the authors
ISFI formation in polymer phantoms
Rhodamine 6G was added to the PLGA solution for visual assessment of the implant formation in the phantom. It was found that the polymer solution was introduced only into the agarose gel during its gelation. In all other cases, when the polymer composition was injected through the 18G needle, the viscous solution came out through the hole from the needle (Fig.1С)
In vitro RPV release from the ISFI
The ISFIs formed without a phantom showed a triphasic RPV release profile: burst release during the first 24 h, then from 1 to 18 days a period of a slower RPV release (lag-phase), and then rapid RPV release period or the second burst (after 20 days) (Fig.2А). The initial 24-h release of RPV was 24.43 ± 1.73%, whereas after 28 days of incubation the ISFI released 81.76 ± 6.16% of RPV. Slower RPV-release from the ISFI formed in agarose gel, compared to ISFI formed without a phantom was observed in the gel conditions (Fig.2А). The overall release at the end of the 28-day study was 11.02 ± 0.34%. Implants formed without a phantom visually had a larger surface area and a large volume, and had a distinct macroporous structure (Fig.2В).
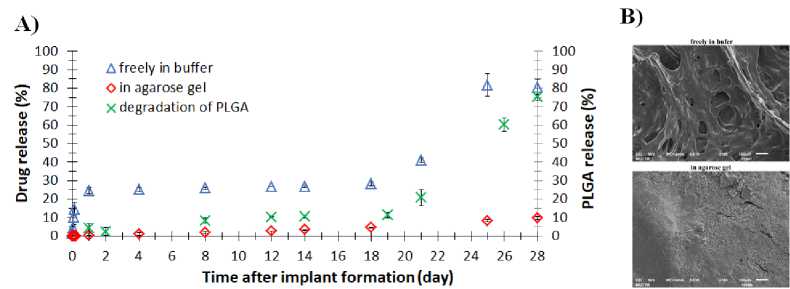
Figure 2. А ) Release profiles of RPV from ISFI (n = 3) and hydrolytic degradation profiles of PLGA from ISFI formed freely in buffer (n = 3); B ) SEM images highlighting the difference in microstructure for ISFI formed freely in buffer and in an agarose phantom. Compiled by the authors
Discussion
Polyacrylamide and agarose gels were used to phantoms phormation. All gels participating in the experiment were characterized by elasticity in the range from 6 to 11 kРа. The cryogels were characterized by macropores [11]. However, according to the SEM images (Fig. 1В) the microstructure of standard gels and cryogels was similar: all gels had highly developed porosity with a pore size of ~ 3 μm. The cryogel contained also macropores, but in small amounts. We assume that the absence of obvious differences in the structure is due to the high content of monomers in the reaction solution. Despite the porosity of the gels, it was impossible to inject the polymer solution into the phantom. However, rapid gelation of the agarose gel (15 min), in comparison with polyacrylamide gels (24 h), allowed for a cavity formation in the phantom for the introduction of a polymer solution. Another disadvantage of polyacrylamide gels is the need for thorough continuous washing to remove toxic acrylamide. Therefore, a 1% agarose gel was chosen as a model phantom to evaluate its effect on the RPV release rate from ISFI.
The use of hydrogels as a tissue model for evaluation of the drug release from ISFI has been described previously [8, 14-17]. In these studies, the hydrogel completely replaced the biorelevant medium. In our study, the main release medium was a buffer solution, into which a cylindrical phantom with an implant was placed. Due to this approach it was possible to avoid disruption of the gel structure during sampling, to replace the release medium if necessary, and to avoid an additional step of sample preparation for the analysis of the released RPV. Importantly, the ISFI formed without phantom (freely in buffer) and in agarose phantom demonstrated the distinct drug release profiles (Fig.2А). Thus, after 28 days of incubation the ISFI formed in a 1% agarose gel released ~11% of RPV as compared with 80% of RPV released without phantom. As previously observed [18], in bulk release models the mass transfer into the release medium is carried out mainly due to convective transport, while in the gel phantom mass transfer is controlled by diffusion as in vivo. Therefore, the RPV release rate in the gel phantom is significantly lower. It is important that when ISFI formed without a phantom, the RPV release profile from the implant is characterized by two burst-effects (Fig. 2А). A first burst release occurs as a result of the drug desorption and diffusion from the surface [19, 20]. This burst-effect is absent in the release profiles of RPV from ISFI formed in an agarose gel. Feasibly, it disappears due to the slow diffusion of RPV in the gel phase. The second burst-release of RPV is significant (from 30 to 80% of released RPV) and related to the onset of “erosion” of the polymer matrix and faster degradation of PLGA (Fig.2А). It should be noted that morphological differences in the structure were observed for the two studied implants. The ISFI formed in an agarose gel initially had a less porous and denser microstructure
(Fig. 2В), which could inhibit the penetration of water into the matrix, thereby slowing down the hydrolysis rate of polymer chains and the RPV release from the implant. It may be an additional explanation for the absence of the second bursteffect in a release profile of RPV from the ISFI formed in the agarose gel. The ISFI, formed without phantom, being more porous, quickly swelled, lost their shape, and began to disintegrate, which complicates a long-term experiment in the study of the kinetics release from depot formulations.
Conclusion
The phantom based on 1% agarose gel is the best suited for simulating muscle tissue to study the RPV release profile from the ISFI. The release profiles of RPV from ISFI formed in a phantom was characterized by the absence of burst-effects observed under the typical release conditions using liquid media. In addition, the ISFI formed in an agarose gel exhibited significantly slower release of RPV (11% vs 80% during 28 days), which is explained by the fact that in this case the RPV release was controlled by diffusion transport, as in vivo . Another advantage of gel tissue phantoms is their ability to maintain the ISFI structure during continuous experiments.
Acknowledgments
The research was carried out within the state assignment of Ministry of Science and Higher Education of the Russian Federation (project FSSM-2022-0003).
The authors gratefully acknowledge the gift of Purasorb® PDLG 5004 by Corbion N.V.
The authors are grateful to Arina Zaikina for SEM images (LLC "Teskan", St. Petersburg).
Список литературы In vitro release studies of rilpivirine from in situ forming polymeric implants in buffer solution and in a gel phantom of muscle tissue
- Ibrahim T.M., El-Megrab N.A., El-Nahas H.M. Optimization of injectable PLGA in-situ forming implants of anti-psychotic risperidone via Box-Behnken Design. Journal of Drug Delivery Science and Technology. 2020. vol. 58. pp. 101803.
- Ibrahim T.M., El-Megrab N.A., El-Nahas H.M. An overview of PLGA in-situ forming implants based on solvent exchange technique: effect of formulation components and characterization. Pharmaceutical Development and Technology. 2021. vol. 26. no. 7. pp. 709-728.
- Muddineti O. S., Omri A. Current trends in PLGA based long-acting injectable products: The industry perspective. Expert Opinion on Drug Delivery. 2022. vol. 19. no. 5. pp. 559-576.
- Pandya A., Vora L., Umeyor C., Surve D. et al. Polymeric in situ forming depots for long-acting drug delivery systems. Advanced Drug Delivery Reviews. 2023. P. 115003. doi: 10.1016/j.addr.2023.115003
- Wang X., Burgess D.J. Drug release from in situ forming implants and advances in release testing. Advanced Drug Delivery Reviews. 2021. vol. 178. pp. 113912. doi: 10.1016/j.addr.2021.113912
- Kožák J., Rabišková M., Lamprecht A. In-vitro drug release testing of parenteral formulations via an agarose gel envelope to closer mimic tissue firmness. International Journal of Pharmaceutics. 2021. vol. 594. pp. 120142. doi: 10.1016/j.ijpharm.2020.120142
- Patel R.B., Solorio L., Wu H., Krupka T. et al. Effect of injection site on in situ implant formation and drug release in vivo. Journal of controlled release. 2010. vol. 147. №. 3. pp. 350-358. doi: 10.1016/j.jconrel.2010.08.020
- Sun Y., Jensen H., Petersen N.J., Larsen S.W. et al. Concomitant monitoring of implant formation and drug release of in situ forming poly (lactide-co-glycolide acid) implants in a hydrogel matrix mimicking the subcutis using UV–vis imaging. Journal of pharmaceutical and biomedical analysis. 2018. vol. 150. pp. 95-106. doi: 10.1016/j.jpba.2017.11.065
- Klose D., Azaroual N., Siepmann F., Vermeersch G. et al. Towards more realistic in vitro release measurement techniques for biodegradable microparticles. Pharmaceutical research. 2009. vol. 26. pp. 691-699.
- Mathur A.B., Collinsworth A.M., Reichert W.M., Kraus W.E. et al. Endothelial, cardiac muscle and skeletal muscle exhibit different viscous and elastic properties as determined by atomic force microscopy. Journal of biomechanics. 2001. vol. 34. no. 12. pp. 1545-1553. doi: 10.1016/S0021-9290(01)00149-X
- Lozinsky V.I. Cryogels on the basis of natural and synthetic polymers: preparation, properties and application. Russian Chemical Reviews. 2002. vol. 71. no. 6. pp. 489-511. doi: 10.1070/RC2002v071n06ABEH000720
- Solorio L., Babin B.M., Patel R.B., Mach J. et al. Noninvasive characterization of in situ forming implants using diagnostic ultrasound. Journal of Controlled Release. 2010. vol. 143. no. 2. pp. 183-190. doi: 10.1016/j.jconrel.2010.01.001
- Kumskova N., Ermolenko Y., Osipova N., Semyonkin A. et al. How subtle differences in polymer molecular weight affect doxorubicin-loaded PLGA nanoparticles degradation and drug release. Journal of microencapsulation. 2020. vol. 37. no. 3. pp. 283-295.
- Ye F., Larsen S.W., Yaghmur A., Jensen H. et al. Drug release into hydrogel-based subcutaneous surrogates studied by UV imaging. Journal of pharmaceutical and biomedical analysis. 2012. vol. 71. pp. 27-34. doi: 10.1016/j.jpba.2012.07.024
- Li Z., Mu H., Larsen S.W., Jensen H. et al. An in vitro gel-based system for characterizing and predicting the long-term performance of PLGA in situ forming implants. International Journal of Pharmaceutics. 2021. vol. 609. pp. 121183. doi: 10.1016/j.ijpharm.2021.121183
- Bassand C., Verin J., Lamatsch M., Siepmann F., et al. How agarose gels surrounding PLGA implants limit swelling and slow down drug release. Journal of Controlled Release. 2022. vol. 343. pp. 255-266. doi: 10.1016/j.jconrel.2022.01.028
- Lefol L.A., Bawuah P., Zeitler J.A., Verin J. et al. Drug release from PLGA microparticles can be slowed down by a surrounding hydrogel. International Journal of Pharmaceutics: X. 2023. vol. 6. pp. 100220. doi: 10.1016/j.ijpx.2023.100220
- Larsen C., Larsen S.W., Jensen H., Yaghmur A. et al. Role of in vitro release models in formulation development and quality control of parenteral depots. Expert opinion on drug delivery. 2009. vol. 6. №. 12. pp. 1283-1295. doi: 10.1517/17425240903307431
- Ulianova Y., Ermolenko Y., Tkachenko S., Trukhan V. et al. Tuning the release rate of rilpivirine from PLGA-based in situ forming implants. Polymer Bulletin. 2023. vol. 80. no. 10. pp. 11401-11420.
- Hopkins K.A., Vike N., Li X., Kennedy J.et al. Noninvasive characterization of in situ forming implant diffusivity using diffusion-weighted MRI. Journal of Controlled Release. 2019. vol. 309. pp. 289-301. doi: 10.1016/j.jconrel.2019.07.019


