Methodological issues in pollen analysis of Pleistocene deposits in Denisova Cave
Автор: Bolikhovskaya N.S., Shunkov M.V.
Журнал: Archaeology, Ethnology & Anthropology of Eurasia @journal-aeae-en
Рубрика: Paleoenvironment, the stone age
Статья в выпуске: 3 т.52, 2024 года.
Бесплатный доступ
Denisova Cave, in the northwestern Altai, is a key Paleolithic complex in North Asia. Pleistocene deposits in the cave contain lithic industries and human fossils documenting the evolution of the cultural traditions of Denisovans in the second half of the Middle and in the Upper Pleistocene. This study addresses methodological issues in paleogeographic interpretation of pollen records relating to Quaternary deposits of cave sites. We present the results of the analysis of recent and subrecent spectra of cave sediments and soil samples taken at sites of characteristic plant communities in natural zones of the Anui River valley near Denisova Cave. Findin gs from taphonomic study of pollen microremains from loose sediments in the East Chamber of the cave make it possible to obtain a correct climato-stratigraphic and climato-phytocenotic interpretation of pollen spectra from Pleistocene deposits in Denisova Cave.
Короткий адрес: https://sciup.org/145147212
IDR: 145147212 | DOI: 10.17746/1563-0110.2024.52.3.017-029
Текст научной статьи Methodological issues in pollen analysis of Pleistocene deposits in Denisova Cave
Pollen analysis of Pleistocene sediments provides a sufficient amount of information concerning the climatostratigraphy and detailed reconstructions of landscape-climatic changes that occurred in the course of the environment’s development, since pollen and spores of higher plants as study objects are the only paleobotanical and paleontological fossil group present in deposits of all lithological facies and all stratigraphic subdivisions of the Quaternary. Microscopic sizes and features of the morphological structure of pollen and spores contribute to their distribution over the surface of land and water areas, as well as deposition in loose sediments. Thus, fossil pollen spectra from the Pleistocene and Holocene deposits should be considered a representation of the paleovegetation of the surrounding territory; and the noted changes in the pollen composition up the profile considered the most complete record of climatic-phytocenotic and floristic changes throughout the studied stage of the Quaternary.
Pollen analysis of deposits of Paleolithic sites is widely used to establish the geological age of Pleistocene strata and their particular climato-stratigraphic subdivision, as well as to determine the rank and relative age of warm and cold climatic stages, which alternated successively during the accumulation of loose sediments. Pollen data are the only source of paleobotanical information to reconstruct a detailed history of changes in the components of prehistoric man’s habitat. These changes occurred under the influence of global climatic fluctuations and were reflected in changes of zonal
vegetation types, floristic complexes, and regional and local climatic conditions during the alternation of interglacial and periglacial environments. Pollen records show repeated successions of plant communities and landscape-climatic transformations, which occurred during interglacial and cold stages. Throughout the regions of Northern Eurasia, climatic events of cold periods of glacial rank affected the environment to varying degrees. In some cold epochs, severe climatic conditions led to the spread of vast ice sheets on plains and glaciers in the mountains; and during other cold periods, contributed to the formation of underground glaciation—significant or insular development of permafrost rocks. Derived pollen data, showing the dominance or a significant amount of arctic-alpine and arctic-boreal taxa of tundra and forest-tundra vegetation in the periglacial pollen spectra, are often the only basis for identification of epochs of permafrost development in the paleoclimatic record.
The number of papers describing the findings of pollen studies of deposits in Paleolithic cave sites in the mountainous regions of Northern Eurasia is considerably smaller than that of publications addressing pollen records of Pleistocene deposits of continental plains in this territory.
E.M. Malaeva studied the Pleistocene strata in the Main Chamber and the Entrance Zone of Denisova Cave located in the valley of the Anui River, and proposed the first profound paleogeographic reconstructions of the northwestern Altai, based on the results of pollen analysis of the cave deposits (Derevianko, Malaeva, Shunkov, 2000; Derevianko et al., 2003). Further pollen study of Pleistocene deposits in the Anui basin—the Early Paleolithic site of Karama, the East Chamber of Denisova Cave, and the terminal Paleolithic layers of Kaminnaya Cave—made it possible to establish a comprehensive climatic and stratigraphic classification of the sites and to reconstruct the landscape and climatic conditions of interglacial and glacial epochs, as well as interstadial and stadial stages during the deposition period (Bolikhovskaya et al., 2011, 2017; Bolikhovskaya, Shunkov, 2014, 2020; Derevianko et al., 2000). The results of the study of pollen spectra from the deposits of the Middle Paleolithic site in Chagyrskaya Cave provided the basis for an assessment of the natural and climatic conditions of the Late Pleistocene in the Charysh River valley (Derevianko et al., 2018; Kolobova et al., 2020; Rudaya et al., 2017).
In other mountainous regions of Northern Eurasia (except for Western Europe), thorough pollen studies have been carried out and profound reconstructions of Pleistocene environments have been made at the Paleolithic cave sites in the Caucasus—the Tsutskhvat multi-layered system (Mamatsashvili, 1978), Ortvala and Sakazhia (Nioradze et al., 1978; Nioradze, Mamatsashvili, 1989), Kudaro I and Kudaro III (Levkovskaya, 1980;
Lyubin, 1989), Apiancha (Klopotovskaya, 1985), Vorontsovskaya (Levkovskaya, 1992), Barakaevskaya (Levkovskaya, 1994), Ortvala-Klde, Dzudzuana, Khvedelidzeebis-Mgvime, Tsiltos-Ngvime, and Rganis-Klde (Lordkipanidze, 1989, 1992), and Treugolnaya (Levkovskaya, 2007). Abundant pollen materials were collected and environment reconstructions were made at Paleolithic sites in the caves of Molochny Kamen in Ukrainian Transcarpathia (Gladilin, Pashkevich, 1977) and Bukovynka in the foothills of the Carpathians (Gerasimenko, Ridush, Avdeyenko, 2019), cave sites in the foothills of the Crimean Mountains (Gubonina, 1985) and Mountainous Crimea (Gerasimenko, 2005; Gerasimenko, 2004, 2007; Gerasimenko et al., 2014; Gerasimenko, Ridush, Avdeenko, 2016).
The majority of the above-mentioned studies provide methodical substantiation of pollen indication of paleoclimatic and paleophytocenotic events in the time of accumulation of cave sediments, based on a comparative analysis of pollen spectra derived from recent and subrecent samples collected in caves and at zonal and local plant community sample sites in the adjacent areas. However, the publications provide little information on the taphonomic features of pollen, spores, and other palynomorphs in cave sediments, which would serve as a confirmation of the representativeness of the derived pollen materials. To determine all factors in the formation of pollen complexes and to differentiate between the autochthonous or allochthonous components therein, pollen analysis of cave deposits should be supplemented with studies of the composition and taphonomic features of all plant microremains present in the macerate of each sample.
In order to get a substantiated climatostratigraphic and paleophytocenotic interpretation of the derived pollen data, traditional methodological studies were carried out, identifying the degree of correspondence between the composition and percentage of pollen and spores in samples of modern sediments collected at geobotanical sites of the Anui River valley, and those of their producing plants. Furthermore, taking into account the features of cave sedimentation at sites of limited exposure and the low probability of the influx of plants from outside, a comprehensive study of the taphonomic peculiarities of all plant microremains presented in macerates of samples from Pleistocene deposits in the East Chamber of Denisova Cave was performed.
Denisova Cave is located in the upper Anui River valley, which stretches from the southeast to the northwest between the Bashchelak (2420 m asl) and Anui (1800 m) mountain ranges (Fig. 1). In the area of the cave, the transverse profile of the valley is asymmetrical and close to V-shaped. The left side of the valley rests on the slopes of Mount Karakol (1315 m asl), the right side on the slopes of Mount Sosnovaya

Fig. 1. Upper Anyi River valley. The arrow marks the location of Denisova Cave.
(1112 m asl). The width at the bottom is about 120 m. The water-edge elevation mark is 662 m asl. The slope of the left side of the valley is slightly concave; that of the right side is convex, turning at the bottom into subvertical walls up to 10–15 m high.
The cave faces southwest; it is located on the right side of the valley, in a large block of Silurian limestones, 30 m above the modern river edge. The cave consists of three subhorizontal gallery-like chambers interconnected through the Main Chamber. The East and South Chambers stretch deep into the cave speleosystem and demonstrate the same stages of filling with loose sediments as those in the Main Chamber. To provide a reliable paleogeographic interpretation of the entire amount of pollen data derived in the process of detailed pollen analysis of Pleistocene deposits in the East Chamber, the studies were carried out focused on resolution of the main methodological issues of the palynology of cave sites.
This article presents the results of the palynotaphonomic study of plant microremains collected from Pleistocene deposits of the East Chamber of Denisova Cave and the findings from the analysis of recent and subrecent spectra from the cave, as well as from sample sites of distinctive plant communities of various environmental zones in the Anui valley.
Palynotaphonomic studiesof Pleistocene deposits in the East Chamber
Scientific publications rarely contain full information on the composition of pollen complexes and on the taphomorphological features of pollen and spore grains in cave deposits. Therefore, during the study of the Pleistocene deposits in the Denisova East Chamber (Bolikhovskaya et al., 2017), special attention was paid to palynotaphonomy. The profile of Pleistocene deposits in this chamber consists mainly of loams with light, medium, or heavy granulometric composition, varying in thickness and color, and unevenly saturated with fragments of bedrock, detritus, bone remains, and coprolites of small and large mammals. According to the lithological and genetic analysis, the Pleistocene stratum of the East Chamber can be subdivided into three units, separated by clear signs of sedimentation hiatus (Fig. 2). The lower unit (layers 17.2 and 17.1) is composed of ocher-yellow loams
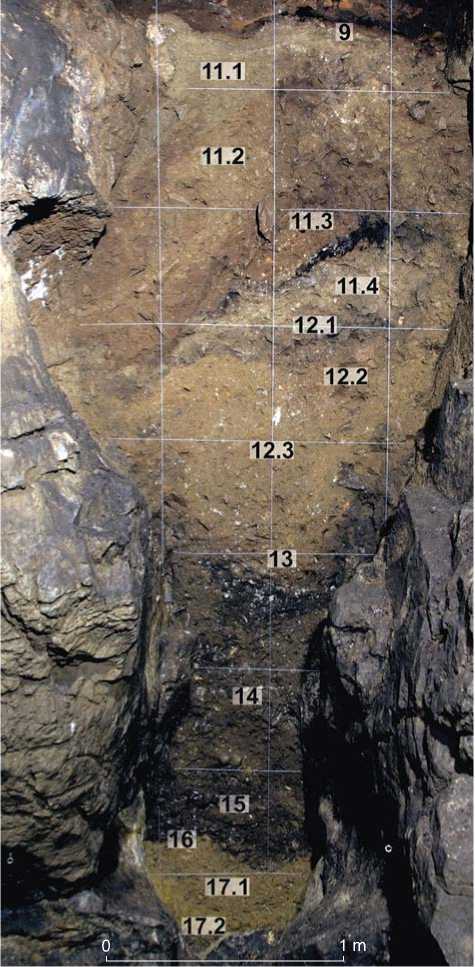
Fig. 2. Pleistocene deposits in the East Chamber of Denisova Cave.
(cave “terra rossa”) with inclusions of limestone rubble, boulders, and leached dripstone formations. The middle unit (layers 16–11.1) is lenticular-layered variegated soft loams saturated with boulder-rubble material. The upper unit (layer 9) includes soft loess-like loams with lenses and isolated inclusions of grus and fine rubble. It is characterized by a high saturation with sooty organic matter, although, the degree of saturation is lower than that in the overlying Holocene sediments. Pleistocene deposits of the East Chamber were accumulated mostly owing to the subaerial aeolian, deluvial, and eluvial processes, with a significant biogenic-anthropogenic effect.
Material and study methods
In total, 138 pollen samples were collected from the section in the East Chamber: 79 samples from layers 17.1–9 of the southeastern wall of the trench, and 59 samples from layers 17.1 and 17.2 of the northwestern wall.
Unlike subaqueous sediments (lake, marsh, floodplain), which are classic objects for pollen analysis, cave deposits show a relatively low concentration of pollen and spores. Hence, palynomorphs were isolated from 50-gram portions of a sample, using a modified version of the separation method developed for the isolation of pollen and spores from subaerial and plant-microremains-poor Pleistocene sediments in the Paleobotanical Laboratory of the Faculty of Geography of Moscow State University (Bolikhovskaya, 1995). Much attention was paid to achieving maximum dispersion of the processed sediments in order to free pollen and spores from the “shell” of amorphous silica, iron and manganese oxides, silty quartz particles, calcite, clay minerals, and organic compounds. When the amount of pollen and spores in the portion was insufficient for statistical calculations, these were isolated from another (50- or 100-gram) portion of the sample. If necessary, pollen concentrates were purified from a large amount of silt and pelitic particles using 40 % hydrofluoric acid (HF). A thorough pollen analysis of macerate solutions was carried out for 79 samples from lithological layers 17.1–9. Representative pollen data were derived for 51 samples. Most of these samples contain from 320 to 1381 grains of pollen and spores of the autochthonous complex. Analysis of ten samples from layer 17.2 showed a very low concentration of pollen and spores.
Palynotaphonomic studies and palynomorphological determinations were carried out with the aid of an Axio Imager D1 microscope. Fossil photographic images were made with an AxioCam digital camera. For each sample of Pleistocene deposits containing an amount of pollen and spores statistically sufficient to derive representative spectra, a collection of digital images of plant microfossils was compiled. These include well-preserved autochthonous pollen and spore grains; pollen and spore grains belonging to an allochthonous complex with mineralized, loose, thinned, torn shells damaged during diagenesis, long-distance transportation or multiple redeposition; and also pre-Cenozoic diatoms, dinoflagellate cysts, and other redeposited palynomorphs, as well as non-pollen microremains. The photo-collection of samples also includes pollen and spore grains belonging to the allochthonous complex, which had mineralized, loose, thinned, torn shells, damaged during diagenesis, long-distance transportation, or repeated redeposition.
Almost all samples of Pleistocene deposits in the East Chamber contain a considerable amount of microorganic remains in the form of carbonaceous and humified particles, as well as marine diatoms, dinocysts, and sponge spicules, which were imported to the deposits from the destroyed limestone bedrock of the cave. Along with pollen and spores of higher plants, the autochthonous complexes contain so-called non-pollen palynomorphs— spores of soil fungi, testate amoebae Arcella, fragments of insects and leaf blades of plants with stomata, and other microfossils. Among the autochthonous and allochthonous non-pollen palynomorphs, macerate solutions most often reveal Pleistocene plant microremains with stomata and redeposited pre-Cenozoic marine diatoms. Three samples from the lower part of the Pleistocene sequence contained the largest number of pre-Cenozoic diatoms: 86, 77.7, and 77.5 % of the total number of pollen, spores, and nonpollen palynomorphs. In other samples, the proportion of diatoms was much smaller: from 0.2–10.0 to 16–22 %. At the same time, no paleoclimatic correlation of the maximum content of pre-Cenozoic marine palynomorphs with either interglacial or cold stages in the deposition of Pleistocene layers in the East Chamber was noted.
When considering the taphonomic aspects of cave deposits palynology, it should be borne in mind that the preservation state of some sporopollenin membranes of pollen and spores in the samples was relatively average. This is due to the sedimentation character of subaerial deposits; another possibility is that before fossilization some pollen and spore grains experienced biogenic-chemical effects in the digestive system of herbivores and then predatory animals. Therefore, in order to obtain representative data in the process of pollen studies, palynotaphonomic analysis was carried out to differentiate autochthonous and allochthonous components in the Pleistocene pollen and spore grains. Pollen and spores with heavily destroyed shells were excluded from the composition of autochthonous complexes.
In addition to pollen and spores with destroyed shells, the samples contained pollen and spore grains with clots of amorphous silica. During the diagenesis of the Quaternary subaerial deposits, the organic matter of the cytoplasm of pollen and spores with apertures (pores, furrows, false furrows, slits, and other thinned or open areas) were replaced by amorphous silica. The morphological features of the exine of most of these grains were not distorted by these “new formations”, and were suitable for determining the genus and species of the pollen of woody and shrubby plants, and the family of the pollen of herbaceous plants; therefore, these were included in the autochthonous palynomorphs. In order to avoid errors in the pollen grain determination as woody or shrubby forms (since the grain sizes and morphology of the pore openings were distorted), some Betula pollen grains were excluded from the autochthonous palynoflora; these grains were completely filled with amorphous silica, distorting their morphological characteristics, or were almost completely covered with tiny mineral particles, mineral and organic-mineral shells.
Recent and subrecent pollen spectra as a basis for paleophytocenotic interpretation of pollen data from cave deposits
The study of pollen spectra from recent samples collected in caves (pollen samples from the air, collected with special traps and devices) and subfossil samples from cave sediments, along with pollen spectra from recent samples and samples of modern sediments from the vicinity of caves, has shown that pollen spectra from cave sediments adequately reflect the composition of zonal, regional, and local vegetation (De Porras, Mancini, Prieto, 2011; Fiacconi, Hunt, 2015; Gerasimenko, Ridush, Avdeyenko, 2019). Analysis of pollen in the air collected with the aid of the Tauber traps inside three caves and in the adjacent area in New York State, in the northeastern United States, has shown that the caves’ recent spectra not only correspond to the composition of regional and local vegetation, but also are close to those of subrecent lake samples (Burney D.A., Burney L.P., 1993). A study of recent pollen samples from four cave sites at Creswell Crags (Sheffield, England) and beyond them, has shown that the cave pollen complexes reliably represented vegetation in the immediate vicinity of the caves and in the broader surroundings (Coles, Gilbertson, 1994). The proportions of tree-shrub and herbaceous-shrub pollen in recent spectra from the caves corresponded to the proportions of forest and non-forest areas both in the immediate vicinity and within a 5 km radius. It was noted that in two caves the number of grains in the samples decreased in the more rear parts of the cave, while in another cave, vice versa, the number of pollen grains was greater in samples from the rear parts of the cave.
The palynotaphonomy study of archaeological cave sites in Kurdish Iraq (Fiacconi, Hunt, 2015) and in Patagonia in southern Argentina (De Porras, Mancini, Prieto, 2011) has led to similar conclusions. Analysis of subrecent pollen spectra from Shanidar Cave and the adjacent territory has shown the complete correspondence of pollen complexes from the interior of the cave and its environs with those from the adjacent territory (Fiacconi, Hunt, 2015). At the same time, a large proportion of anemophilous plant pollen was observed in the samples collected at the cave’s entrance, and an increased proportion of entomophilous plant pollen in samples from the cave’s interior. Similar results were achieved in the studies of subfossil spectra from Bukovynka Cave and the surrounding area in the south of the Eastern Carpathian foothills (Gerasimenko, Ridush, Avdeyenko, 2019).
The results of pollen analysis of cave hyena coprolites are also used for the reconstruction of environmental settings: these reflect not only the local vegetation in the vicinity of caves, but also the vegetation cover over a larger area of the predator’s hunting grounds (Scott, 1987; Carrión et al., 1999, 2018; Yll et al., 2006; Gerasimenko, Ridush, Avdeyenko, 2019).
Recent and subrecent pollen spectra as a representation of modern phytocenoses and a basis for the pollen identification of Pleistocene vegetation in the vicinity of Denisova Cave
Denisova Cave is located in the mountain-taiga belt of the geobotanical zoning scheme (Atlas…, 1991). In the valley of the upper Anui, mid-mountain forest-steppe and forest landscapes predominate. The right-side slope of the valley, containing the cave, shows solitary tracts or areas of sparse birch-pine forest. The left side of the valley opposite the cave is covered with a dense birch-larch forest. The vegetation cover of the upper Anui valley, from the bottom to the watershed, includes floodplain-meadow, meadow-steppe, forest (with birch, pine, and larch), mountain-steppe, and mountain-tundra communities. Floodplain areas show meadow and grass associations. Large areas of the riverbed parts of the floodplain and the low above-floodplain terrace are covered with willowbirch forests with shrub-willow undergrowth of currant, caragana (pea shrub), bird cherry, and other vegetation. Meadow-steppe associations are common at absolute altitudes from 680 to 1100 m. Meadow grass-forb and sedge-grass-forb steppes occupy areas of floodplains and adjacent slopes. The shrub steppe communities include spirea, caragana, honeysuckle, rose hips, barberry, gooseberry, and cotoneaster (Ogureeva, 1980). Meadow steppe with shrub thickets is developed on low terraces and gentle slopes; the thickets’ co-edificators are Dasiphora Dasiphora fruticosa and Siberian grass Sibiraea altaiensis (laevigata) (Kuminova, 1960). Larch-birch forests with a shrub layer of caragana, spirea, currant, honeysuckle, and dasiphora are spread on the shaded and most humid slopes of the northern exposure at an absolute altitude of 700–1300 m. Birch-pine forests, sometimes with an admixture of larch, and with Siberian spruce and Siberian pine in the areas close to the tops cover the slopes of southeastern and southwestern exposure at an altitude of 650–1200 m. Siberian pine forests, with an admixture of spruce, larch, and fir, are widespread in small valleys and on slopes at an altitude of 1500–2000 m (Smagin et al., 1980). Above the mountain-taiga belt, there are subalpine Siberian pine and larch forests, with a typical representative of the subalpine and mountaintundra belts—the round-leaved birch shrub Betula rotundifolia—in the undergrowth. Dwarf communities, with a predominance of round-leaved birch and not so numerous spirea, juniper, and shrub willow, form shrub tundra on high-mountain plateaus, smooth passes, and in saddles at an altitude of 1800–2300 m. Subalpine and alpine meadow associations, moss-lichen, dryad, lichenrubble, and other tundra communities are also present in high-mountain landscapes.
The pollen determinations of the Pleistocene landscape and climatic conditions were made from the results of pollen analysis of 115 subrecent samples of subaerial deposits of modern soils and subaqueous sediments collected in the areas of mountain-taiga, mountainforest-steppe, and mountain-steppe belts in the valley of the Anui River and its tributaries, as well as in the areas of mountain-tundra and mountain-forest-tundra plant communities of the nearest ridges. The results of the pollen analysis have shown that the composition and percentage of components in the pollen spectra of samples of modern subaerial deposits reflect quite properly the composition and percentage of pollen and spores of the yielding plants in the sample sites that characterize the zonal, regional, and local features of the plant communities of the Anui valley (Table 1). The analytical data from a large number of subrecent soil samples from the Anui valley also demonstrate that their spectra correspond to the composition and percentage ratio of plants in the phytocenoses of the sample sites. In the mountain-tundra spectra with high content of pollen of dwarf birch Betula rotundifolia and Siberian pine Pinus sibirica , the pollen of shrubs and herbaceous-dwarf shrub taxa predominates, illustrating the development of open landscapes. The pollen spectra of mountain-forest communities are dominated by pollen of Scotch pine Pinus sylvestris , silver birch Betula pendula , and Siberian pine Pinus sibirica . The pollen composition spectra of the mountain-forest-steppe belt, similarly to those of the flat forest-steppe regions of Northern Eurasia, show close values of the contents of two predominant groups: pollen of trees and grass-shrub plants (Bolikhovskaya, Ogureeva, Rudaya, 2005). In the steppe spectra, pollen of grasses and shrubs (cereals, wormwood, and forbs) prevails, and in the arboreal group, pollen of birch and pine, which vegetate forest areas on mountain slopes of steppe landscapes of the northwestern Altai.
At the same time, there is an increased content of tree pollen in the overall composition of spectra in subrecent soil samples collected outside the Anui basin in open and forested areas of the tundra and steppe belts, and in non-forested areas of high-mountain steppe basins. First of all, a high content of Siberian pine pollen is recorded in subrecent samples of tundra and steppe soils collected in the areas near upper and lower boundaries of the mountain-taiga belt (Pelankova, Chytrý, 2009) and in the bottoms of high-mountain steppe depressions, the surrounding mountains of which are vegetated with Siberian pine forests. The spectra with high content of
Table 1. Examples of pollen spectra of subrecent soil samples collected in the Anui valley, %
|
Indicator |
Vegetation type |
|||||
|
mountain-taiga |
mountain-forest-steppe |
mountain-steppe |
||||
|
t го .ГГ^ § о g “ о £ 8 с о с = о л г ± ° ^ Ф со го w о. ф с г ГО Н ГО CL > СО |
Е о ч— Н1§ го Е о > О Ф OI Го — ГО 1- ГО СО СО |
л ® 1 о сл д > if 2 > « ” о * щ Е 8. о. ГО £ О Е 1- S s и W |
О « О СЛ
ГО О S^Va ® n го 1— ГО Q- <л СО |
О ф со £ g > £ у 2 ГО о го 5s oz СО ±± ГО СО -ГО CL го и Е Е h со t И |
Е ч— 1« Осо >с Ф о ГО О 5 ^ -е z со ±± £ го ГО CL ВВ ” Го Н СО CD W |
|
|
Arboreal pollen Shrub pollen Grass and small shrub pollen Spores Pollen of trees and shrubs: Abies sibirica Picea obovata Pinus sibirica Larix sibirica Pinus sylvestris Betula pendula Betula rotundifolia Alnaster/Duschekia Salix spp. Juniperus spp. Padus avium cf. Spiraea Ribes alpinum Pollen of grasses and small shrubs: Ericales Poaceae Cyperaceae Cannabis Artemisia (subgenera) Chenopodiaceae Herbetum mixtum Pollen of aquatic plants Spores: Bryales Sphagnum Polypodiaceae Dryopteris sp., D. fragrans Botrychium Lycopodium sp. Equisetum Total pollen and spore grains |
28.5 16.5 47.5 7.5 6 3.4 42 3.4 3.4 6 36 0.5 0.6 – – – – 1 25 25 – 8 3 37 – 37 13 22 – 9 – 19 716 |
75 9 14 2 1 2 74 1.3 1 10 10.3 0.2 – – – – 0.5 5 16 – – 54 5 20 – 4* – 3* 2* – – – 453 |
64 – 28 8 11 1.5 16 24 28 19 – – – 0.3 – – – – 20 17 – 20 8 35 – 23 0 70 – – – 7 532 |
34 1 58 6 4 3 6 6 57.5 19 – – 3 0 0.5 – – – 34 14 0.5 1.2 1.2 43 6 2* 1* 2* – – – 18* 360 |
49 – 47 4 5 1.3 13 10.5 32 38 – – – – – – – – 36 2 – 21 2 39 – 23 19 56 – – 2 – 1088 |
24.5 0.5 71 4 9 1 10 0 50 28 – – – – – 1 1 – 11 3 – 25 2 59 – 4* – 3* – – – 9* 398 |
*Number of grains.
Table 2. Composition of pollen and spores in subfossil samples from the Altai Mountains, %
|
Indicator |
Forest-steppe |
Steppe |
|||
|
The Anui valley close to Karama |
The Ursul valley close to Ongudai |
Kurai basin |
Kan basin |
||
|
Soil |
Soil |
Soil |
Soil from flood plain |
Soil from terrace |
|
|
1 |
2 |
3 |
4 |
5 |
6 |
|
Pollen of trees and shrubs |
32.9 |
88.9 |
91.0 |
43.7 |
51.4 |
|
Pollen of grass and small shrubs |
57.2 |
9.2 |
8.5 |
50.5 |
43.5 |
|
Spores |
9.9 |
1.8 |
0.5 |
5.8 |
5.2 |
|
Pollen of trees and shrubs: |
|||||
|
Abies sibirica |
8.1 |
1.5 |
0.8 |
2.2 |
4.3 |
|
Picea obovata |
1.3 |
4.1 |
9.9 |
8.1 |
2.9 |
|
Pinus sibirica |
6.0 |
91.2 |
78.9 |
47.7 |
68.1 |
|
Pinus sylvestris |
43.0 |
0.1 |
6.4 |
1.1 |
|
|
Larix sp. |
1.3 |
1.2 |
8.8 |
3.2 |
4.3 |
|
Betula sp. |
– |
1.4 |
0.3 |
– |
– |
|
B. pendula |
30.2 |
– |
– |
27.1 |
16.5 |
|
B. cf. rotundifolia |
– |
– |
0.3 |
0.5 |
1.8 |
|
Salix spp. |
– |
– |
0.1 |
4.6 |
– |
|
Grossulariaceae |
0.7 |
0.1 |
0.1 |
– |
– |
|
Viburnum sp. |
– |
– |
– |
0.2 |
– |
|
Lonicera sp. |
– |
0.1 |
– |
– |
– |
|
Rosaceae |
– |
0.1 |
– |
– |
– |
|
Spiraea sp. |
9.4 |
– |
– |
– |
– |
|
Zygophyllaceae |
0.1 |
– |
1.1 |
||
|
Pollen of grass and small shrubs: |
|||||
|
Poaceae |
45.6 |
8.0 |
4.8 |
22.8 |
15.7 |
|
Cyperaceae |
2.3 |
13.3 |
56.6 |
40.2 |
11.4 |
|
Ephedra sp. |
– |
– |
2.4 |
– |
1.3 |
|
Artemisia s.g. Euartemisia |
13.9 |
26.7 |
12.0 |
19.5 |
35.2 |
|
A . s.g. Dracunculus |
– |
– |
– |
0.2 |
– |
|
A . s.g. Seriphidium |
– |
– |
– |
3.8 |
3.4 |
|
Chenopodiaceae |
4.6 |
25.3 |
12.0 |
3.2 |
6.8 |
|
Rosaceae |
3.1 |
4.0 |
– |
1.5 |
– |
|
Sanguisorba sp. |
0.8 |
– |
– |
– |
– |
|
Apiaceae |
0.8 |
– |
– |
0.2 |
0.4 |
|
Rubiaceae |
0.8 |
– |
– |
– |
– |
|
Brassicaceae |
– |
– |
– |
0.4 |
– |
|
Plantaginaceae |
– |
– |
– |
1.3 |
|
|
Polygonaceae |
– |
2.7 |
3.6 |
1.3 |
|
|
Gentianaceae |
– |
– |
– |
0.2 |
– |
|
Onagraceae |
0.4 |
– |
– |
0.4 |
0.4 |
|
Primulaceae |
– |
– |
– |
0.2 |
0.4 |
|
Lysimachia maritima |
– |
– |
– |
– |
0.4 |
|
Ranunculaceae |
1.2 |
– |
– |
– |
– |
|
Ranunculus sp. |
– |
– |
– |
0.2 |
– |
Table 2 (end)
|
1 |
2 |
3 |
4 |
5 |
6 |
|
Thalictrum sp. |
– |
– |
– |
0.2 |
1.3 |
|
Polemoniaceae |
0.4 |
– |
– |
– |
– |
|
Lamiaceae |
1.2 |
5.3 |
0.2 |
0.4 |
|
|
Caryophyllaceae |
– |
5.3 |
1.2 |
– |
1.3 |
|
Fabaceae |
3.1 |
4.0 |
2.4 |
– |
1.3 |
|
Liliaceae |
– |
2.7 |
– |
0.6 |
0.8 |
|
Urtica sp. |
– |
– |
– |
0.6 |
– |
|
Zygophillaceae |
0.8 |
– |
– |
– |
– |
|
Plumbaginaceae |
– |
1.3 |
2.4 |
– |
– |
|
Saxifragaceae |
– |
– |
– |
0.4 |
– |
|
Juncaceae |
– |
– |
– |
0.6 |
0.8 |
|
Euphorbiaceae |
– |
– |
– |
0.2 |
– |
|
Asteraceae |
4.2 |
– |
1.2 |
3.8 |
13.1 |
|
Cirsium sp. |
– |
– |
– |
0.4 |
– |
|
Echinops sp. |
1.2 |
– |
– |
– |
– |
|
Chenopodiaceae |
13.9 |
1.3 |
– |
– |
3.0 |
|
Indifferent herbs |
1.9 |
1.2 |
– |
– |
|
|
Spores: |
|||||
|
Bryales |
13.3 |
10 * |
1 * |
40.7 |
22 * |
|
Sphagnum |
1 * |
– |
– |
1 * |
|
|
Polypophyta |
13.3 |
3 * |
3 * |
– |
2 * |
|
Cryptogramma crispa |
– |
1 * |
– |
– |
– |
|
Botrychium sp. |
– |
– |
– |
– |
1 * |
|
B. lunaria |
– |
– |
– |
– |
1 * |
|
Diphaziastrum alpinum |
– |
– |
1 * |
– |
– |
|
Equisetum sp. |
73.3 |
– |
– |
59.3 |
1 * |
|
Total pollen and spore grains |
453 |
814 |
975 |
936 |
543 |
*Number of grains.
Pinus sibirica showing a noticeable admixture of pollen of wormwood Artemisia and amaranth Amaranthaceae and a low content of spores, usually represented by single grains, indicate their affiliation to steppe and forest-steppe phytocenoses (Table 2).
The pollen composition of soil samples from the Denisova Cave deposits and the composition of subrecent samples from its environs point to a high representativeness of the pollen spectra. To study the features of cave deposit sedimentation and the influx of pollen and spores in the soil, a sample was taken from the surface layer of eluvial-subaerial fine earth accumulated on a small ledge in the wall of bedrock at the cave’s entrance. The derived pollen spectrum corresponds to those of surface samples of subaerial deposits (soils) collected on the sample sites of plant communities in the areas of the valley closest to the cave, in particular, to their botanical zonal affiliation and the composition of the plants yielding pollen and spores (Table 3).
All the pollen spectra of the subrecent samples collected beyond the cave show tree pollen domination (62–93 %); this group includes the following co-edificators: Scotch pine Pinus sylvestris , Siberian larch Larix sibirica , and silver birch Betula pendula , which indicates a zonal mountain-taiga type of vegetation in this section of the Anui valley and the composition of edificators of forest associations in the sample sites (see Table 1). Notably, in cases where sample areas were established in small forest associations, the percentage of tree pollen composition in these spectra corresponded to the composition of the forest association dominating on this slope of the valley.
The pollen spectrum of the recent sample from Denisova Cave reflects adequately the zonal type of mountain-taiga vegetation in the surroundings of the cave and the composition of local forest association near the site. The cave entrance zone of the slope is vegetated with larch-birch-pine sparse forest. The spectrum
Table 3. Results of pollen analysis carried out on surface samples of subaerial deposits in Denisova Cave and the adjacent areas in the mountain-taiga zone of the Anui valley, %
|
Indicator |
Sampling places |
||||
|
At the cave’s entrance |
Right side of the valley. Soil from the high floodplain covered by birchlarch forest |
Left side of the valley. Soil from the slope covered by birch-larch forest |
Right side of the valley. Soil from the high floodplain covered by birchpine forest |
Right side of the valley. Soil from the slope covered by pine-birch-larch forest |
|
|
Sample No. 1/2015 |
Sample No. 3/06 |
Sample No. 10/06 |
Sample No. 42/06 |
Sample No. 1/09 |
|
|
Tree pollen |
54 |
62 |
70 |
72 |
93 |
|
Shrub pollen |
16 |
1 |
6 |
0.2 |
1 |
|
Grass and small shrub |
|||||
|
pollen |
21 |
28 |
21 |
21 |
4 |
|
Spores |
9 |
9 |
3 |
7 |
2 |
|
Pollen of trees and shrubs: |
|||||
|
Abies sibirica |
– |
5 |
2.5 |
9 |
9.4 |
|
Picea obovata |
0.75 |
4 |
1 |
2 |
5.6 |
|
Pinus sibirica |
12 |
5 |
3 |
12 |
4 |
|
Larix sibirica |
4 |
13 |
13 |
6 |
5 |
|
Pinus sylvestris |
34 |
43 |
26 |
42 |
61.5 |
|
Betula pendula |
26.5 |
26 |
47 |
29 |
13.2 |
|
Betula cf. sect. |
|||||
|
Nanae |
– |
– |
0.2 |
0.2 |
– |
|
Alnaster / Duschekia |
– |
0.1 |
– |
– |
– |
|
Salix spp. |
– |
3.5 |
– |
– |
– |
|
Juniperus spp. |
0.75 |
1.5 |
– |
– |
– |
|
Lonicera tatarica |
– |
– |
0.5 |
0.2 |
1 |
|
Rosaceae, Dasiphora |
|||||
|
fruticosa |
– |
– |
7 |
– |
0.3 |
|
Humulus lupulus |
22 |
– |
– |
– |
– |
|
Pollen of grass and small shrubs: |
|||||
|
Poaceae |
20 |
22 |
12 |
52 |
46 |
|
Cyperaceae |
– |
1.5 |
3 |
21 |
– |
|
Artemisia (subgenera) |
12.5 |
16 |
3 |
5 |
34 |
|
Amaranthaceae |
10 |
2 |
– |
1 |
10 |
|
Herbetum mixtum |
57.5 |
55 |
84 |
21 |
10 |
|
Pollen of aquatic |
|||||
|
plants |
– |
3.5 |
– |
– |
– |
|
Spores: |
|||||
|
Bryales |
3 * |
34 |
5 * |
37 |
16 |
|
Polypodiophyta |
15 * |
9 |
9 * |
37 |
77.5 |
|
Pteridium aquilinum |
– |
31 |
– |
– |
– |
|
Ophioglossaceae |
– |
– |
3 * |
– |
– |
|
Lycopodium sp. |
– |
– |
– |
2 |
6.5 |
|
Equisetum |
– |
26 |
– |
24 |
– |
|
Total pollen and |
|||||
|
spore grains |
190 |
971 |
619 |
678 |
1253 |
*Number of grains.
is dominated by pollen of trees and shrubs (70 % in total): Scotch pine Pinus sylvestris , silver birch Betula pendula , and Siberian larch Larix sibirica (see Table 3). The proportion of pollen of Siberian pine Pinus sibirica (12 %) corresponds to the presence of this species in the upper belt of mountain forests in this area. A noticeable proportion (22 %) in this group (arboreal pollen) of the spectrum of hop pollen Humulus lupulus is explained by its flowering during sampling. The composition and percentage of pollen from grass-shrub plants—cereals Poaceae, forbs Herbetum mixtum, wormwood Artemisia , and amaranth Amaranthaceae—indicate grass-forb associations, clumps of wormwood, and goosefoot Chenopodioideae growing near the cave. The presence of spores of fern and green mosses in the sample points to the forest type of the composition spectrum of this sample. In addition to pollen and spores of modern plants, a large number of carbonaceous organic microparticles and pre-Cenozoic marine palynomorphs were noted in the preparations of the recent sample. Each preparation contained approximately 30–35 palynomorphs (diatom valves, dinoflagellate cysts, sponge spicules, etc.) and 7–8 pollen grains. In general, the content of preCenozoic palynomorphs amounted to 55 % of the total number of pollen objects in the recent sample. In terms of preservation and taphonomic features, pollen and spores from modern cave deposits do not differ from those of subrecent samples of modern soils on the slopes in the vicinity of the cave. These findings indicate the representativeness of the pollen data obtained from the Pleistocene deposits of Denisova Cave. A distinctive feature of subrecent samples of modern soils from the Anui valley slopes is the absence of palynomorphs from the bedrock of its surrounding mountains.
Conclusions
Palynotaphonomic studies of pollen objects and palynomorphological determinations of pollen and spores from Pleistocene sediments of the East Chamber of Denisova Cave have shown a high degree of correlation between the derived pollen data. Analysis of pollen spectra from recent, subrecent, and fossil samples from cave sediments, as well as spectra from samples of modern subaerial deposits collected at sample sites of various phytocenoses of all environmental zones of the Anui valley, indicates that the pollen spectra from cave sediments adequately reflect the composition of the zonal, regional, and local vegetation of the surrounding area. The palynotaphonomic studies of cave deposits have shown that the main agents transporting pollen and spores into the cave cavities were subaqueous and eolian processes, along with humans, large and small mammals, birds, and insects—bees and bumblebees, transferring pollen of entomophilous plants. Plant microparticles (objects of pollen study) were transferred with the air mass through the cave entrance, located in the steep rock wall of the southwestern exposure, as well as through a hole in the western part of the vault of the Main Chamber.
Numerous marine diatoms, dinocysts, and spicules of Porifera in the recent samples and their almost constant (from 0.2–10.0 to 78–86 % of the total number of pollen objects) occurrence in samples from the Pleistocene deposit sequence suggest a significant role of loose decomposed limestones, which are the bedrock containing the karst cavity of the cave, in the composition of fine fractions of sediments in the East Chamber. Marine palynomorphs were also added to Pleistocene deposits in the form of mineral coprolites, when large mammals used weathered salt-bearing marine sediments as kudyurites.
Acknowledgements
This study was supported by the Russian Science Foundation, Grant No. 24-18-00069,
Pollen studies carried out by N.S. Bolikhovskaya are part of the research federal themes of the Faculty of Geography of the Lomonosov Moscow State University “Paleogeographic Reconstructions of Natural Geosystems and Forecasting of Their Future Changes” (121051100135-0) and “Evolution of the Natural Environment in the Cenozoic, Relief Dynamics, Geomorphologic Hazards, and Risks of Nature Management” (121040100323-5).
The authors express their gratitude to N.A. Rudaya and E.G. Lapteva for the participation in determination of the botanical composition of herbaceous vegetation at the sites for collecting subrecent samples.


