Mitochondrial therapy: direct visual assessment of the possibility of preventing myocardial infarction under chronic neurogenic pain and B16 melanoma growth in the experiment
Автор: Kit O.I., Shikhlyarova A.I., Frantsiyants E.M., Neskubina I.V., Kaplieva I.V., Zhukova G.V., Trepitaki L.K., Pogorelova Y.A., Bandovkina V.A., Surikova E.I., Popov I.A., Voronina T.N., Bykadorova O.V., Serdyukova E.V.
Журнал: Cardiometry @cardiometry
Рубрика: Review
Статья в выпуске: 22, 2022 года.
Бесплатный доступ
On models of chronic neurogenic pain (CNP) and the growth of a malignant tumor (metastasizing B16 melanoma) in male mice, we studied an effect produced by mitochondrial therapy (MCT) on the state of the myocardium. Some structural correlates of the compensatory-restorative effect by mitochondria transplanted from healthy recipient rats were revealed. It has been identified that MCT contributes to the preservation of the structural integrity of the myocardial tissue, the inclusion of an auxiliary link in the cellular mechanisms of tissue restoration: fibroblasts, histiocytes, lymphocytes, eosinophils and other connective tissue elements, which implement the intercellular mechanism of information transfer that provides the external regulatory function of MCT. The ability of mitochondria to prevent the DNA decay determines the possibility of initiation of the operation of the nuclear mechanisms of the cardiomyocyte division, which is characteristic of a population of young cells and which indicates the determining position of exogenous mitochondria.
Laboratory mice, chronic neurogenic pain, b16 melanoma, mitochondrial therapy, myocardium, pathomorphology, cardiac microscopy
Короткий адрес: https://sciup.org/148324619
IDR: 148324619 | DOI: 10.18137/cardiometry.2022.22.3849
Текст научной статьи Mitochondrial therapy: direct visual assessment of the possibility of preventing myocardial infarction under chronic neurogenic pain and B16 melanoma growth in the experiment
Oleg I. Kit, Alla I. Shikhlyarova, Elena M. Frantsiyants, Irina V. Neskubina, Irina V. Kaplieva, Gаlina V. Zhukova, Lidia K. Trepitaki, Yulia A. Pogorelova, Valeria A. Bandovkina, Ekaterina I. Surikova, Ivan A. Popov, Tamara N. Voronina, Oksana V. Bykadorova, Elizaveta V. Serdyukova. Mitochondrial therapy: direct visual assessment of the possibility of preventing myocardial infarction under chronic neurogenic pain and B16 melanoma growth in the experiment. Cardiometry; Issue 22; May 2022; p. 38-49; DOI: 10.18137/cardiometry.2022.22.3849; Available from:
Ischemic heart disease is a widespread disease and one of the leading causes of mortality [1]. Damage to mitochondria is the main link in the pathogenesis of myocardial ischemia, resulting in the death of cardiomyocytes and blocking the contractility of the heart [2]. Mitochondria are essential for the survival of cells, especially cardiomyocytes, due to their high energy requirements. It is known that mitochondria can change their metabolic phenotype to cope with the problems associated with high energy consumption and macromolecular synthesis [3]. It is common knowledge that mitochondria occupy 30% of the cell volume and produce a shocking 30 kg of ATP per day to maintain the normal mechanical functions of the heart [4]. Thus, mitochondria play a key role in myocardial damage and repair in response to ischemia caused by a restriction of blood flow to the heart that may lead to extensive myocardial damage, i.e. infarction [5].
In recent years, advances in molecular and biochemical methodologies have led to a better understanding of mitochondrial defects and their mechanisms as the cause of various diseases, but however treatments of mitochondrial disorders remain still insufficient. Several medical drugs have been evaluated to improve the mitochondrial function and remove symptoms of the mitochondrial dysfunction. Agents such as coenzyme Q10, idebenone, riboflavin, dichloroacetate and thiamine have been used to improve the ETC function, and creatine monohydrate has been used as an energy shuttle to move high energy phosphate from the mitochondria to the cytoplasm. In addition, antioxidants like vitamin C, vitamin E, and lipoic acid have been tested as supplements to detect mitochondrial disorders, and a number of other drugs are the subject of ongoing clinical trials [6, 7]. However, the agents used can provide a very limited protection, and most mitochondrial diseases are considered as irreversible because they are caused by damage, for instance, due to mtDNA mutation. In this regard, therapies using most agents are of certain limited usefulness.
For the myocardial regeneration, some studies have been focused on the 3D bio-printing technology, the regulation of long non-coding RNA, or release of VEGF and BMP9 from an injectable alginate-based composite hydrogel [8–10]. This sort of therapies may be effective in restoring the cardiac function after injury. In fact, ischemic injury causes significant disturbances in mitochondria, which have a detrimental effect on metabolic cellular processes and, finally, lead to the death of cardiomyocytes [11]. Therefore, the technologies to restore functional mitochondria may counteract the above damaging effects on cardiomyocytes and have a therapeutic potential in preventing ischemic heart injury.
The therapeutic efficacy of the mitochondrial transplantation has been demonstrated in a series of animal studies, and it has shown promising results in recent clinical applications [12–15]. Both in the experiment and under the clinical conditions, the post-ischemic transplantation of healthy mitochondria by a direct injection into the myocardium leads to a significant improvement in the contractile function and viability of the tissue of the damaged myocardium. However, although the mitochondrial transplantation by the direct injection into the donor mitochondrial tissue is therapeutically effective, it has some limitations that multiple injections and physical manipulations of the heart are required to adequately distribute mitochondria throughout the heart. An open access to the heart is also required that imposes limits on the potential patient population eligible for the mitochondrial transplantation [2].
An intracoronary delivery of mitochondria, as a minimally invasive procedure, avoids these shortcomings and is capable of distributing mitochondria throughout the infused area. Some previous studies using an isolated perfused heart model demonstrated that the intracoronary delivery of mitochondria resulted in a rapid absorption and an extensive distribution of transplanted mitochondria [14].
Most studies emphasize that the isolation of separated mitochondria must be completed within a short time at a low temperature, since they are very sensitive and their activity and survival loss tend to occur rapidly. In addition, there is currently no method for a long-term storage and preservation of mitochondria, so they should be used immediately after their isolation. Therefore, a protocol for an optimal method of the mitochondrial isolation and storage to properly maintain the mitochondrial integrity and ensure their longer survival should be developed in order to enable their clinical use [16].
We have found that the growth of a malignant tumor against the background of chronic neurogenic pain (CNP) is accompanied by the mitochondrial dysfunction, including that of cardiomyocytes, leading to myocardial infarction, which is not observed under the CNP-free tumor growth [17-19]. In an attempt to prevent the development of infarctions in animals with the growth of a malignant tumor against the background of CNP, it was decided to use the transplantation of live mitochondria.
The aim hereof is to study the morphological changes in the heart muscle in mice during mitochondrial therapy under the growth of a malignant tumor against the background of chronic neurogenic pain.
Materials and methods
Male mice (n=37) of the C57BL/6 line, aged 8 weeks, with an initial individual body weight of 28–3 g, were used in our research work. The animals were supplied by the Federal State Budgetary Institution International Research Center for Biomedical Technologies “An-dreevka” at FMBA (Moscow Region). The experimental groups were composed as follows: the mice with chronic neurogenic pain (CNP) + B16/F10 melanoma (n=27); the mice with CNB + B16/F10 melanoma + mitochondrial therapy (MС therapy) (n=10).
The laboratory animals were kept under natural light conditions with free access to water and food. The work was carried out in accordance with the rules of the European Convention for the Protection of Vertebrate Animals used for Experimental and other Scientific Purposes (Directive 86/609/EEC) as well as in accordance with the International Guiding Principles for Biomedical Research Involving Animals and
Order No. 267 “Approval of the Rules of Laboratory Practice” dated June, 19, 2003 issued by the Ministry of Health of the Russian Federation.
Model of chronic neuropathic pain
All manipulations with the animals were carried out in a box. Tools, utensils, hands were disinfected in the conventional way, observing all asepsis conditions.
The model of chronic neurogenic pain was reproduced by applying a ligature to the sciatic nerve on both sides under xylazolethyl anesthesia [20]. Anesthesia: xyl-zoletyl, 10 minutes before the main anesthesia; premedication: xylazine (Xila preparation) intramuscularly, at a dose of 0.05 ml/kg of body weight (according to the instructions), then after 10 minutes, Zoletil-50 was administered at a dose of 10 mg per 100 g of body weight. On post-operative day 14, mechanical allodynia and hyperalgesia were measured.
-
2 weeks after the reproduction of chronic neurogenic pain, B16/F10 melanoma was transplanted into the C57BL/6 mice by a standard subcutaneous injection of tumor suspension cells under the right shoulder blade in a volume of 0.5 ml of the cell suspension in a 1:10 dilution with saline solution. The tumor strain of mouse melanoma B16/F10 was ordered from the Russian National Medical Research Center of Oncology named after N.N. Blokhin, at the Ministry of Health, the Russian Federation.
Isolation of mitochondria
An intact rat was sacrificed with a guillotine, and the heart was harvested on ice, which was perfused with ice-cold sterile 0.9% KCl solution. Mitochondria were isolated using the differential centrifugation with high-speed refrigerated centrifuge Avanti J-E, BEC-MAN COULTER, USA according to the method by Egorova M.V. and Afanasiev S.A. 2011 [21]. To destroy the intercellular bonds, the cell wall and plasma membranes, mechanical processing of tissues was used with grinding with scissors and homogenization in a glass homogenizer with a Teflon pestle (Potter-Elve-heim homogenizer). Per gram of tissue, 10 ml of sterile isolation medium (0.22 M mannitol, 0.3 M sucrose, 1 mM EDTA, 2 mM TRIS-HCL, 10 mM HEPES, pH 7.4) was added. The tissues were homogenized and centrifuged for the first time for 10 min at a speed of 1000 g, at a temperature 0-2°C; the second and third centrifugations were carried out at 20000 g, for 20 min, at a temperature 0-2°C. Between the centrif- 40 | Cardiometry | Issue 22. May 2022
ugations, the mitochondrial pellet was resuspended in the isolation medium. Mitochondria were further extra purified from lysosomes, peroxisomes, melano-somes, etc. by centrifugation in a 23% Percoll gradient. The suspension of the subcellular structures was layered on a Percoll gradient, centrifuged for 15 min at 21000 g, after which the separation into 3 phases was observed; the lower layer of mitochondria was left and resuspended in the isolation medium. The next washing of mitochondria was carried out by centrifugation for 10 min at 15000 g, at a temperature 0–2°C. Mitochondrial samples were diluted with 0.9% NaCl solution to a protein concentration of 3.3 mg of protein in 0.3 ml of the saline solution.
Bio-therapy with mitochondria
24 hours after the B16/F10 melanoma inoculation, the mice were intraperitoneally injected with freshly isolated heart mitochondria (3.3 mg of protein per animal in 0.3 ml of the saline solution). Further, mitochondrial therapy was carried out daily until the end of the experiment – 3 weeks of the B16/F10 melanoma growth.
The male C57BL/6 mice with B16/F10 melanoma, growing against the background of chronic neurogenic pain, which were injected intraperitoneally with 0.3 ml of the saline solution daily, were taken as the reference.
The animals were decapitated 3 weeks after the B16/F10 melanoma cell inoculation. After the decapitation, the isolated heart preparations were processed according to the stages of morphological preparation for staining sections with hematoxylin-eosin, followed by the morphological examination of the structure with the Leica DM LS2 microscope furnished with an Olympus optical.C-5050 Zoom video camera and Morfotest software. Photographing was carried out with magnification x10, x40, x100.
Results
Anti-metastatic effect produced by MCT
The idea of the need to use a living mitochondrial substrate to prevent high risk of myocardial infarction, which is macroscopically regularly recorded in experiments in animals with B16 melanoma against the background of chronic neurogenic pain, has been realized with achieving a double effect, covering both the cardiotropic and anti-metastatic effects of mito- chondrial therapy (MCT). The results obtained are submitted in detail in the present issue of this journal in paper “Biological effects of mitochondrial therapy: preventing development of myocardial infarction and blocking metastatic aggression of B16/F10 melanoma”.
Nevertheless, we use the opportunity to emphasize the important role of MCT not only in the targeted coronary influence, but also as an outstanding agent of a systemic influence on the organism and the tumor , changing at the level of subcellular structures the genetically programmed properties of metastasis of the stably aggressive B16 melanoma cell strain in the mice. The cancellation of this genetically determined sign of melanoma in the vast majority of animals upon completion of MCT was proven not only macroscopically during a thorough examination of the dissected lungs and other organs, but also by a 100% rate of metastases found in the lungs and other organs in the reference mice (without MCT) with B16 melanoma growing against the background of CNP.
The attempts made to get closer to an understanding the possibility of preventing ischemic heart damage using MCT were implemented during the initial microscopic study of the myocardial tissue in a mouse, as a subject of self-assessment of the effect obtained.
Morphological changes in the myocardium
In classical morphology, it is considered a generally accepted fact that in each age period there are different mechanisms for increasing the mass of the myocardium with an increased functional load: in the early age period it takes place due to an increase in the number of cardiomyocytes, which, as they grow older, are “replaced” by the mechanisms of the hypertrophic transformation of the muscle cells. In the adult state, there are no cambial, stem cell elements in the myocardium, therefore, the main morphological manifestation of the myocardial hyperfunction and regenerative reaction in response to the damaging effect of strong stimuli and overloads is the development of various rearrangements of intracellular structures (the physiological intracellular regeneration). These properties of the reversibility of the dystrophic changes in the myocardium are recorded in various pathological processes: rheumatism, renal failure, after clinical death, in cardiopathy, thyrotoxicosis that has been stated 35 years ago in the fundamental works by D.S. Sarkisov (1987) [22].
The conducted morphological study of some changes in the myocardium under the influence of organotropic mitochondrial therapy in the B16 melanoma tumor-bearing mice with the tumor cells transplanted against the background of induced chronic neurogenic pain primarily indirectly indicated a significant compensatory extension in the myocardium, both from the base of the heart and from its apex. This could be seen when comparing a pair of upper illustrations taken from the Atlas “Pathological Morphology of the Mouse” (2010) [23], which shows the structures of the base and the apex of the heart in an intact mice, with the lower pair of the images taken after the application of mitochondrial therapy in a tumor-bearing mice with reproduced neurogenic pain (see Figure 1 and 2 herein).
A number of studies have identified the fact of the lack of correlation between the extent of the myocardial hypertrophy and the degree of functional load accompanied by lagging. This non-correlation can be observed in cases of a more successful execution of an increased load by the heart, as well as in cases of more favorable general conditions of the performance [22] (cited by D.S. Sarkisov, 1987), during which the male rats withstand the functional loading worse than it is the case with the female rats [18,20].
At the forefront of our research was the therapeutic effect produced by MC therapy, which, apparently, determined the recovery processes, and acted as an alternative to the damaging effect made by chronic neurogenic pain in combination with the development of a malignant tumor. Chronic pain and tumor development is just not that functional load, which can be successfully compensated by any medical drugs to stimulate some biosynthetic processes. We are dealing with much deeper pathological changes in metabolism at various hierarchical levels, including the dysregula-tion of the life maintenance systems, primarily the energy status with the destruction of the mitochondrial matrix in cells of various organs, including the heart [24-29]. The introduction of “live” mitochondria from healthy recipients was aimed at replenishing the energy resources, however, considering the high degree of the self-organization and the regulatory capabilities of mitochondria, one could expect an expansion of the field of an influence of this sort on the myocardium .
The completed studies confirmed the possibility of complete blocking of the deep dystrophic and necrotic processes in the myocardium by introducing the living mitochondrial substrate, which were abundantly found in the heart in animals not receiving MCT (see
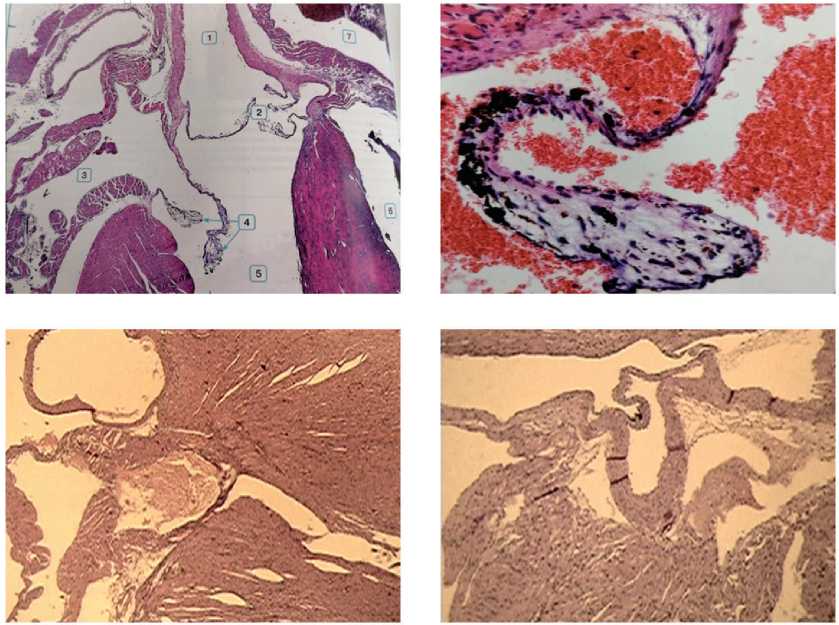
Figure 1. Fragments of the base of the heart in an intact mouse (upper two images) and during MCT under the conditions of B16 melanoma growth in combination with CNP (bottom images): elements of compensatory hypertrophy of the structures of the aortic and mitral valves in males are visible (left image) and normalization of the valve system in females (right image). Stained with hematoxylin-eosin, magnification x10, x100.
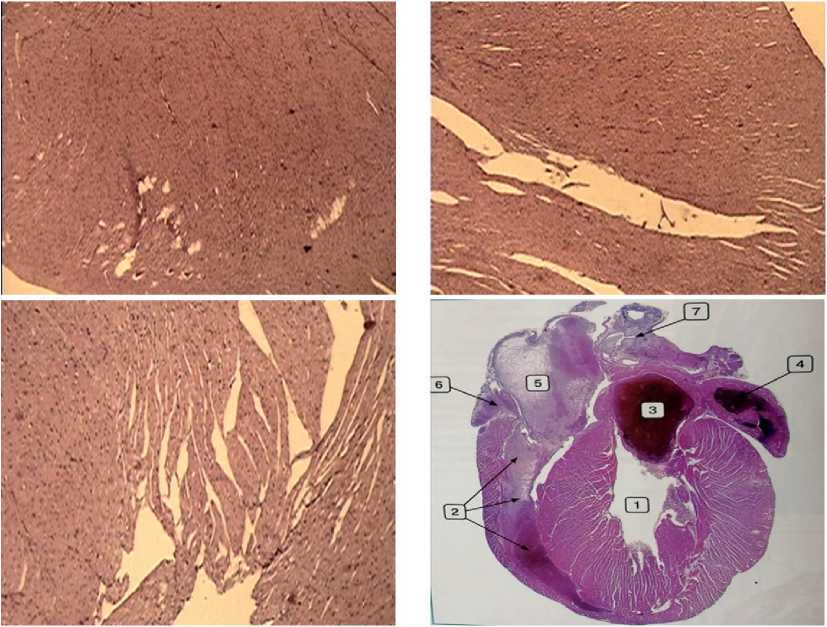
Figure 2. The structure of the heart in an intact mouse according to the Atlas “Pathological morphology of the mouse” [23] (the lower right image: 1 – left ventricular cavity, 2 – right ventricular cavity, 3 – left atrium, 4 – left atrial appendage, 5 – right atrium, 6 – right atrial appendage, 7 – veins). The structure of the myocardium after MCT under the conditions of B16 melanoma growth in combination with CNP (the upper and lower left images): compensatory hypertrophy of the left ventricular wall without pronounced areas of damage, narrowing of the lumen of the ventricular cavity in a female mouse. Stained with hematoxylin-eosin. Magnification x 10.
the respective data in our paper “Structural myocardial catastrophe under the influence of chronic neurogenic pain due to development of B16 melanoma in female mice” published in the present issue of this journal). Additional testing of myocardial histological preparations with high-magnification microscopy has provided very convincing evidence thereof and is essential for an understanding of the compensatory-adaptive nature of the heart muscle rehabilitation processes under the influence made by MCT (see Figure 3 herein).
In the images taken with the same magnification (x40) in different parts of the myocardium in the experimental male mice, it was possible to note the absence of fundamental differences from the cellular structure in the reference sample, represented by an identical density of the cardiomyocyte bundles and comparable sizes and staining intensity of the nuclei. With this magnification, neither small nor, especially, large areas of the necrotic processes were revealed (see Figure 3 herein).
However, when viewing all experimental samples of the myocardium in the male mice, large intraventricular hemorrhages were found in one case only (see Figure 4 herein), while none of the experimental animals had hemorrhages in the heart. A possible cause of hemorrhage in that individual case could be an individualized feature of the state of the endothelium of the coronary vessels or a manifestation of another factor of unknown etiology. We believe that the absence of hemorrhages in almost all mice demonstrates the safety of mitochondrial biotherapy.
In our study of the heart preparations with a magnification of the objective x100, some events were analyzed, which could indicate the mechanisms and points of the application of mitochondrial therapy. It is less difficult to trace the signs of the reparative regeneration with electron microscopy of the cell than it is the case with histological sections, when destructive changes under the influence of pathogenic factors (pain, tumor growth) can appear partially. It is known that the most stable cell structure against pathogenic influences is the cell nucleus, which induces and regulates all biosynthetic processes in the cell. At high magnification, we succeeded to fix not only some changes in the nucleus of cardiomyocytes, but mainly to make sure of a completely new mode of the intercellular interactions arising under the influence of MCT. In the presented photo session (see Figures 5-7), we have tried to demonstrate this new “information” mechanism of the MCT influence on intercellular interactions.
The presented photos clearly show the fibers of cardiomyocytes, which are in close contact with actively growing fibroblasts and histiocytes, in a literal
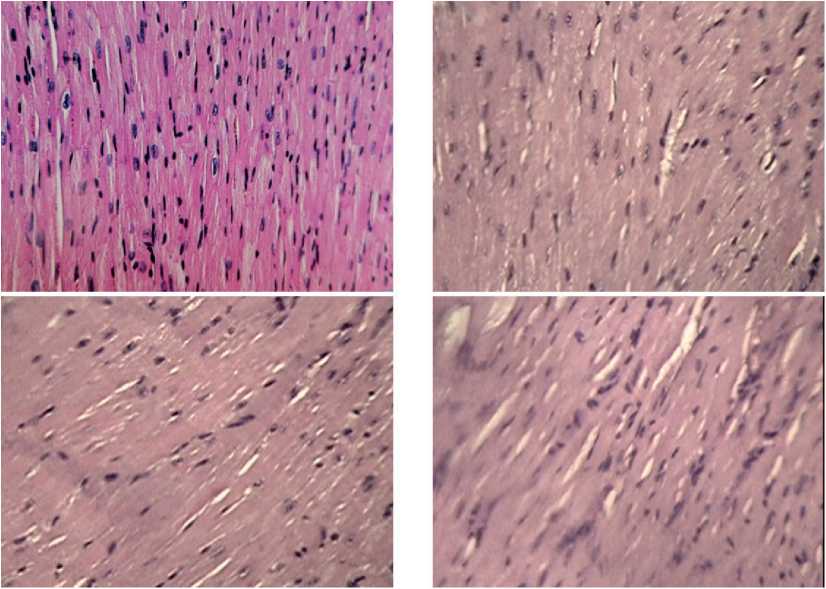
Figure 3. The structure of cardiomyocytes in an intact mouse according to the Atlas “Pathological morphology of mice” [23] (upper left image) and samples of mouse myocardium similar in cellular structure upon completion of MCT under the conditions of chronic neurogenic pain and the development of B16 melanoma. Stained with hematoxylin-eosin, magnification x 40.
Figure 4. A single case of a large intraventricular hemorrhage in a male mouse during MCT under the conditions of chronic neurogenic pain and the development of B16 melanoma. Stained with hematoxylin-eosin, magnification x 10.
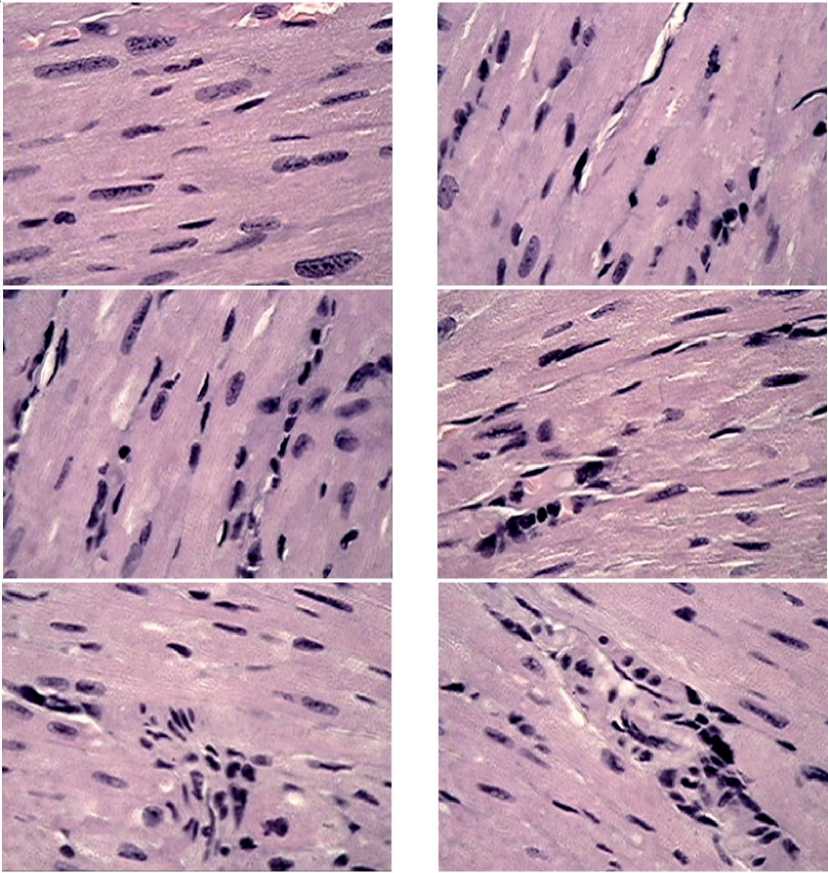
Figure 5. Fragments of myocardial tissue in male mice after MCT under the growing melanoma B16 against the background of chronic neurogenic pain. Close packing of cardiomyocytes with clear nuclei and division activation elements. In most fields of vision, a significant activation of connective tissue cells entering the intercellular relationships, the formation of strands of young connective tissue are visible. Stained with hematoxylin-eosin, magnification x 100.
sense “sewing together” the fibers and restoring the integrity of the heart tissue. The shape of the nuclei of young fibroblasts could be oval with a large amount of basophilic cytoplasm surrounding the nucleus.
44 | Cardiometry | Issue 22. May 2022
It should be noted that an important distinguishing property of fibroblasts is their secretory activity. It is known that these cells secrete not through a specific area, but through many pores over the entire surface
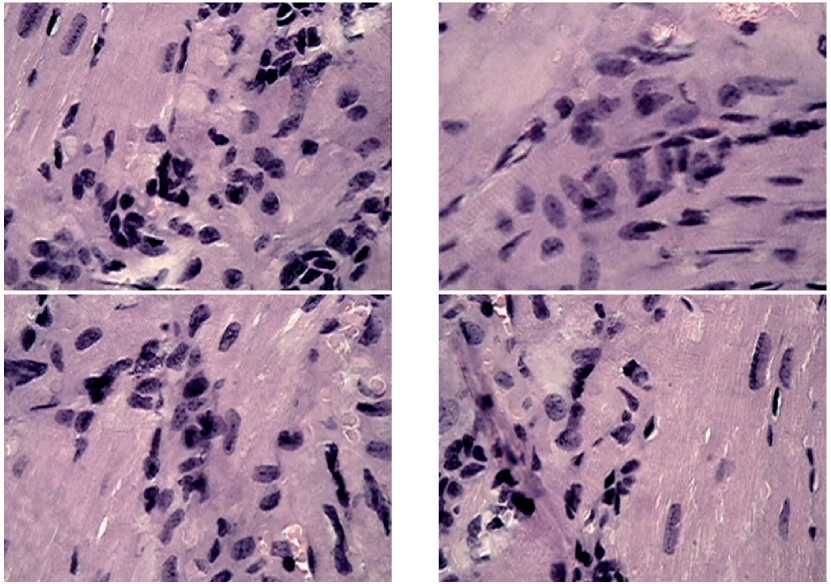
Figure 6. Fragments of myocardial tissue in male mice after MCT under the growing B16 melanoma against the background of chronic neurogenic pain. Significant accumulations of structure-forming elements: fibroblasts, histiocytes, lymphocytes, forming associate complexes, areas of loose connective tissue are found. Stained with hematoxylin-eosin, magnification x 100.
of the plasma membrane, delivering procollagen, glycosaminoglycans and proelastin, as well as secreted microfibrillar protein for elastic fibers. These gentle repair events in the development of young connective tissue are well reflected in the sections stained with he-matoxylin-eosin. On the preparations in the areas of development of young connective tissue, a significant accumulation of lymphocytes, monocytes, eosinophils was found, which formed associate complexes necessary for the transmission of signal information regarding the self-organization of the myocardial structure (see Figure 6 herein).
Our attention was drawn to the clearly manifested structures of some cylindrical fibers of the connective tissue, where there was no transverse striation characteristic of collagen fibers. These fibers had a homogeneous amorphous structure and, obviously, were built from elastin in the immediate vicinity of fibroblasts, forming their own framework. Thus, it was possible to see some morphological correlates referred to invisible, but extremely important functional rearrangements in the myocardium, initiated by mitochondrial bio-therapy. The cellular and non-cellular components of the basic substance of the connective tissue, which received powerful energy and regulatory support provided by the functionally complete mito- chondria from an exogenous source, were able to implement their genetically determined program of the myocardial structural restoration. In our experiments, it was shown that the depth of ischemic and necrotic myocardial damage under CNP and tumor growth left no chance of survival that manifested itself from the first week of the experiment in the mice without MCT.
In full accordance with the recovery dynamics of the highly organized structure of cardiomyocytes, changes in the vascular system of the coronary circulation were observed, as well as “sewing together” some isolated fibrous areas. In many fields of view (Figure 7), one could see a complex cooperative “work” on the formation of the walls of arterioles and venules, apparently performed by smooth muscle cells of a fusiform shape with a centrally located elongated nucleus, lined up in a sequence of “braids” round the periphery of a vessel or elastic fibers, which clearly delimited the line of the territorial integrity of the myocardium and the lumen of the blood vessel.
We could not leave without comments some features of the rearrangement of the cell population of cardiomyocytes in the male mice, which, in our opinion, might be attributed to various nuclear factors of hormonal supply.
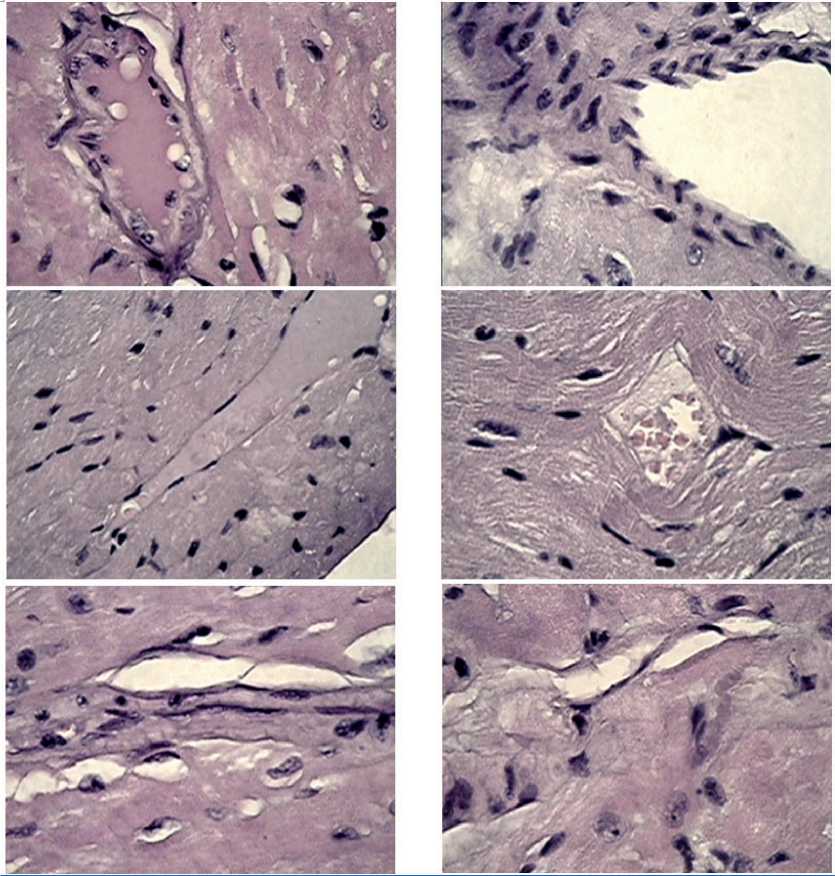
Figure 7. Fragments of myocardial tissue in male mice after MCT under growing B16 melanoma against the background of chronic neurogenic pain. Formation of the walls of arterioles and venules by spindle-shaped smooth muscle cells and elastic fibers, “sewing together” isolated fibrosed small areas of the myocardium against the background of the development of loose connective tissue. Stained with hematoxylin-zosin, magnification x 100.
In our opinion, the male mice, received MCT, have exhibited the compensatory and regenerative processes related to the nucleus of cardiomyocytes that can be judged by the preservation of a sufficient density of the nuclear consistency and a clear spatial organization of the chromatin grains, which are arranged in a linear sequence along the central axis of the ovoid core structure.
One of the structural features of the myocardium in the male mice after the intraperitoneal transplantation of the mitochondrial suspension was the detection of some areas of the intercellular and intracellular space filled with a finely dotted network of an aggregated substance. Considering the ability of living mitochondria to be released into the extracellular space and transported to cells of various 46 | Cardiometry | Issue 22. May 2022
systems [30], we believe that the identified conglomerations demonstrate the implementation of the effect of the mitochondrial penetration into the myocardial structure. In other words, according to the revealed morphological features, this network may correspond to the megamitochondrial structure, i.e. the therapeutic substrate that provides metabolic (energetic) and regulatory assistance to cardiomyocytes damaged under CNP and the melanoma growth. The lower images (Figure 8) clearly show the filling of cardiomyocytes and intercellular “islands” with dotted structures of the mitochondrial nature. This confirms the possibility of replacing damaged mitochondria, and, consequently, the inclusion of the life mainenance program and the restoration of the impaired functions.
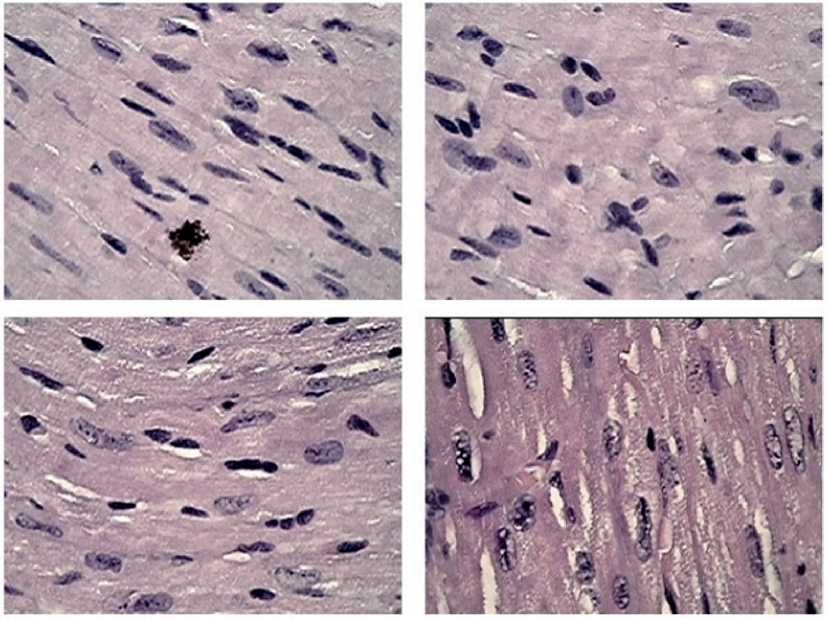
Figure 8. Fragments of myocardial tissue in male mice after MCT under growing melanoma B16 against the background of chronic neurogenic pain. Close intercellular interactions between the fibrohistiocytic link of cells activated by MCT and the population of cardtomyocytes. Preservation of a uniform color density of the nuclear contents and clear large chromatin grains with a central linearly organized spatial arrangement in cardiomyocytes. Stained with hematoxylin-eosin, magnification x 100.
Conclusion
Thus, using a routine morphological analysis of the state of the myocardium in the male mice with induced pathological processes of the growth of the malignant tumor against the background of pain stimulation, it was possible to identify the structural correlates of the compensatory-restorative effect produced by mitochondria, transplanted from healthy recipient rats. The mechanisms implemented by mitochondrial therapy are associated with the prevention of acute bio-energetic hypoxia with complete inhibition of the respiratory chain and the formation of an energy-deficient state with necrosis at its output that has been observed in our experiments on models of CNP and the B16 melanoma growth without MCT.
What is the main result of the influence made by MCT? Firstly, it is the preservation of the structural integrity of the myocardial tissue in the experimental mice, the absence of fiber disruptions and the compaction of the intercellular space.
Secondly, it is an inclusion of an auxiliary link in the cellular mechanisms of the tissue restoration: fibroblasts, histiocytes, lymphocytes, eosinophils and other elements of the connective tissue, which se- crete the required “construction” materials (procollagen, glycosaminoglycans and proelastin as well as secreted microfibrillar protein) and implement the intercellular mechanism transmission of information, which provides the external regulatory function of MCT.
Thirdly, MCT prevents the DNA decay and determines the possibility of initiating the operation of the nuclear mechanisms of cardiomyocyte division, which is characteristic of a population of young cells and which indicates the internal determining function of MCT.
Of course, our visual impressions and considerations of the morphological events in the myocardium in the animals after MCT are not complete, and they need a further deep study with obtaining evidence data at the biochemical and molecular-genetic levels. However, firmly relying on the positions of the unity of the functional and structural principles of the living tissues in assessing the pathological and regenerative processes, we can state that the possibilities of mitochondria as the basis for providing the energy and life regulation programs, represent an inexhaustible reserve for therapy of the future.
Statement on ethical issues
Research involving people and/or animals is in full compliance with current national and international ethical standards.
Conflict of interest
None declared.
Author contributions
The authors read the ICMJE criteria for authorship and approved the final manuscript.
Список литературы Mitochondrial therapy: direct visual assessment of the possibility of preventing myocardial infarction under chronic neurogenic pain and B16 melanoma growth in the experiment
- Benjamin EJ, Blaha MJ, Chiuve SE. American Heart Association Statistics Committee and Stroke Statistics Subcommittee Heart disease and stroke statistics–2017 update: a report from the American Heart Association. Circulation. 2017;135:e146–e603.
- Shin B, et al. Novel Biological Strategy for Myocardial Protection by Intracoronary Delivery of Mitochondria: Safety and Efficacy. JACC. Basic to translational science. 2019;4(8);871–888. doi:10.1016/j.jacbts.2019.08.007.
- Zong WX, Rabinowitz JD, White E. Mitochondria and Cancer. Mol Cell. 2016; 61(5): 667–676. doi:10.1016/j. molcel.2016.02.011.
- Hall AR, Burke N, Dongworth RK, Hausenloy DJ. Mitochondrial fusion and fission proteins: novel therapeutic targets for combating cardiovascular disease. Br. J. Pharmacol. 2014;171:1890–1906. doi: 10.1111/bph.12516.
- Sun X, et al. Alda-1 treatment promotes the therapeutic effect of mitochondrial transplantation for myocardial ischemia-reperfusion injury. Bioactive materials. 2021;6(7):2058–2069. doi:10.1016/j.bioactmat. 2020.12.024.
- Potgieter M, Pretorius E, Pepper MS. Primary and secondary coenzyme Q10 deficiency: The role of therapeutic supplementation. Nutr. Rev. 2013;71:180–188. doi: 10.1111/nure.12011.
- El-Hattab AW, Zarante AM, Almannai M, Scaglia F. Therapies for mitochondrial diseases and current clinical trials. Mol. Genet. Metab. 2017;122:1–9. doi: 10.1016/j.ymgme.2017.09.009.
- Ponnusamy M, et al. Long noncoding RNA CPR (cardiomyocyte proliferation regulator) regulates cardiomyocyte proliferation and cardiac repair. Circulation. 2019;139:2668– 684.doi:10.1161/CIRCULATIONAHA.118.035832.
- Liu N, et al. Advances in 3D bioprinting technology for cardiac tissue engineering and regeneration. Bioact. Mater. 2021;6:1388–1401. doi: 10.1016/j.bioactmat. 2020.10.021.
- Wu Y, et al. Release of VEGF and BMP9 from injectable alginate based composite hydrogel for treatment of myocardial infarction. Bioact. Mater. 2021; 6:520–528. doi: 10.1016/j.bioactmat.2020.08.031.
- Kunecki M, Plazak W, Podolec P, Golba KS. Effects of endogenous cardioprotective mechanisms on ischemia-reperfusion injury. Postepy Hig. Med. Dosw. 2017;71:20–31. doi: 10.5604/17322693.1228267.
- Emani SM, et al. Autologous mitochondrial transplantation for dysfunction after ischemia-reperfusion injury. J Thorac Cardiovasc Surg. 2017;154:286–289. doi: 10.1016/j.jtcvs.2017.02.018.
- McCully JD, et al. Mitochondrial transplantation: from animal models to clinical use in humans. Mitochondrion. 2017;34:127–134. doi: 10.1016/j.mito.2017.03.004.
- Cowan DB, et al. Transit and integration of extracellular mitochondria in human heart cells. Sci Rep. 2017;7:17450. doi: 10.1038/s41598-017-17813-0.
- Kaza AK, Wamala I, Friehs I. Myocardial rescue with autologous mitochondrial transplantation in a porcine model of ischemia/reperfusion. J Thorac Cardiovasc Surg. 2017; 153:934–943. doi: 10.1016/j.jtcvs.2016.10.077.
- Park A, et al. Mitochondrial Transplantation as a Novel Therapeutic Strategy for Mitochondrial Diseases. International journal of molecular sciences. 2021; 22(9): 4793. https://doi.org/10.3390/ijms22094793.
- Frantsiyants EM, et al. The functional state of mitochondria of ca rdiomyocytes in a malignant process against the background of comorbid pathology in the experiment. South Russian journal of oncology. 2021;2( 3):13-22. doi: 10.37748/2686-9039-2021-2-32. [in Russian]
- Kit OI, et al. The effect of chronic neuropathic pain on the course of the malignant process of B16/F10 melanoma in male mice. News of higher educational institutions. North Caucasian region. Series: Natural Sciences. 2019; (201):106-111. doi: 10.23683/0321-3005-2019-1-106-111. [in Russian]
- Frantsiyants EM, et al. Content of apoptosis factors and self-organization processes in the mitochondria of heart cells in female mice C57BL/6 under growth of melanoma b16 / f10 linked with comorbid pathology. Cardiometry. 2021; 18:121-30.
- Kotieva IM, et al. An increase in the degree of malignancy of melanoma against the background of chronic pain in female mice. Malignant tumors. 2017; 7( 3S1): 123-4. [in Russian]
- Egorova MV, Afanasiev SA. Isolation of mitochondria from cells and tissues of animals and humans: Modern methodological techniques. Siberian medical journal. 2011; 26(1-1):22-28. [in Russian]
- Sarkisova DS. Structural bases of adaptation and compensation of disturbed functions. Мoscow, 1987. 445p. [in Russian]
- Atlas “Pathological morphology of the mouse”. 2010. V.3, 432p. [in Russian]
- Kit OI, et al. The processes of self-organization of mitochondria during the growth of experimental tumors under conditions of chronic neurogenic pain. News of higher educational institutions. North Caucasian region. Series: Natural Sciences. 2019; 2 (202):97-105. [in Russian]
- Frantsiyants ЕМ, et al. Effect of urokinase gene knockout on melanoma growth in experiment. Siberian scientific medical journal. 2019;39; 4:62-70. doi: 10.15372/SSMJ20190408. [in Russian]
- Frantsiyants ЕМ, et al. Vascular endothelial growth factors and receptors in the development of transplanted melanoma B16/F10. Russian journal of oncology. 2015. 20; 2:32-7. [in Russian]
- Kotieva IM, et al. Influence of chronic pain on the level of sex hormones, prolactin and gonadotropic hormones in blood serum and pathologically changed skin in female mice in the dynamics of malignant melanoma growth. News of higher educational institutions. North Caucasian region. Series: Natural Sciences. 2018; 2 (198):106-16. doi: 10.23683/0321-3005-2018-2-106-116. [in Russian]
- Kit ОI, et al. Neurotransmitter systems of the brain of female mice in the dynamics of the growth of malignant melanoma reproduced against the background of chronic pain. Pathogenesis. 2017; 15; 4:49-55. doi: 10.25557/GM.2018.4.9749. [in Russian]
- Kit ОI, et al. Dynamics of the tissue system of plasminogen regulators in skin melanoma against the background of chronic pain in female mice. Translational medicine. 2018. 5; 2:38-46. [in Russian]
- Lopez-Crisosto C, et al. Sarcoplasmic reticulum- mitochondria communication in cardiovascular pathophysiology. Nat. Rev. Cardiol. 2017; 14:342–360. doi: 10.1038/nrcardio.2017.23.


