Нитрофураны в урологической практике: все ли они одинаковые и почему мы возвращаемся к ним сегодня?
Автор: Перепанова Т.С.
Журнал: Экспериментальная и клиническая урология @ecuro
Рубрика: Инфекционно-воспалительные заболевания
Статья в выпуске: 3, 2018 года.
Бесплатный доступ
Увеличивающийся уровень антимикробной резистентности у микроорганизмов является одной из важных проблем здравоохранения. Основной движущей силой является повсеместное потребление антибиотиков. ВОЗ сигнализирует о росте антимикробной резистентности вместе с неуклонным снижением открытия новых антимикробных препаратов. Чтобы контролировать антимикробную резистентность необходимо более рационально использовать существующие препараты. Поэтому, необходима переоценка эффективности и побочных эффектов старых уже много лет известных нам антимикробных препаратов, таких как нитрофураны. Нитрофураны очень эффективны при острых инфекциях, в основном в мочевых путях с низкой концентрацией препарата в тканях, они не вызывают бактериальную резистентность так быстро, как другие антибиотики. Несмотря на имеющиеся сходства в строении молекул препаратов нитрофуранового ряда, каждая молекула имеет свои особенности, которые выражаются и в проявлении клинического эффекта и переносимости. Главное, что вызывает «новый» интерес в урологии к этой группе препаратов на сегодня - это редкое формирование резистентности и довольно широкий спектр антимикробной активности для применения в урологической практике.
Инфекции мочевых путей, нитрофурантоин, фуразидин, нифурател, острый цистит, посткоитальный цистит, рецидивирующий цистит
Короткий адрес: https://sciup.org/142216897
IDR: 142216897
Текст научной статьи Нитрофураны в урологической практике: все ли они одинаковые и почему мы возвращаемся к ним сегодня?

Следует обратить внимание также на то, что нитрофурантоин, рекомендованный для терапии инфекций нижних отделов мочевых путей, доступен в России только в микрокристаллическом виде, тогда как все клинические исследования проводились с его макрокристалли-ческой формой. Однако W. Brumfitt и соавт. провели анализ применения макро- и микрокристаллического нитрофурантоина при длительной профилактике рецидивов ИМП с 1972 по 1992 гг. [17]. Применяли 3 схемы профилактики – в группе А (N 43) пациентки принимали микрокристаллический нитрофурантоин в дозе 50 мг х 2 раза в день, в группе В (N 110)-100 мг макро-кристаллического нитрофурантоина (1 раз в день перед сном) и в группе С (N 66) макрокристаллический нитрофурантоин в дозе 50 мг перед сном. У большинства пациенток улучшение достигнуто через 6 месяцев лечения, у 84% пациенток рецидивов не было. Не отмечено различий в эффективности лечения между тремя группами. Число симптоматических эпизодов уменьшилось в 5,4 раза. В группе А 25,6% пациентов преждевременно прекратили лечение из-за побочных эффектов любого типа, тогда как в группе макрокристаллического нитрофурантоина – только 13% (p < 0,01). У пациентов пожилого возраста отмечено не больше НПР, чем у молодых. Ни одного жизнеугрожающего побочного эффекта не отмечено. Анализ фекальной флоры не показал повышенного роста нитрофу-рантоин-резистентных бактерий и элиминации чувствительных коли-форм. Также и S. Kalowski с соавт. при проведении сравнительного исследования микро- и макрокристал-лического нитрофурантоина не выявили различий в эффективности лечения хронических ИМП [18].
Последние работы 2018 г. показали, что в отличие от ципрофлоксацина лечение нитрофурантоином не оказывало влияния на распространенность ни ципрофлоксацин- резистентных штаммов, ни штаммов, продуцирующих БЛРС [29] . В систематическом обзоре 2017 г. проведен анализ 26 контролируемых исследований, включающих 3052 пациентов и 16 популяционных когортных исследований на выявление токсичности [30]. В целом, качество исследований признано плохим с высоким риском предвзятости. При сравнении с группами пациентов, не получавших профилактики, нитрофурантоин был эффективным в предотвращении ИМП (отношение риска 0,38 в пользу нитрофурантоина, 95% доверительный интервал, 0,30-0,48). Профилактическая эффективность нитрофурантоина выше, чем мете-намина гиппурата и сравнима с другими антибиотиками. При сравнении с пациентами, получавшими другие антимикробные препараты, у тех, кто получал нитрофурантоин был увеличенный риск 2,24 (95%CI 1,77-2,83) для несерьезных побочных реакций. Только у одного пациента из всех контролируемых исследований отмечен сильный побочный эффект (интерстициальная пневмония). В когортных исследованиях частота серьезных побочных реакций равна 0,02-1,5 на 1000 пациентов. Риск сильной токсичности увеличивается с длительностью применения нитрофурантоина.
В Кохрейновском систематическом обзоре 19 исследований по профилактике рецидивов цистита было показано, что ежедневный прием или прием 3 раза в неделю антимикробных препаратов в низкой дозе значительно уменьшает риск рецидива инфекций до 85%, с 0,8-3,6 до 0-0,9 случаев в год. Эффективность разных антимикробных препаратов одинакова.
При профилактике рецидивов цистита у женщин период ремиссии был длиннее у пациентов, принимавших нитрофурантоин, по сравнению с цефалексином – 108 и 92 дня соответственно [31].
Широко и эффективно используется нитрофурантоин в дет- ской практике при лечении рецидивирующей инфекции мочевых путей [32-35]. Эффективность нитрофурантоина при профилактике рецидивов ИМП подтвердили в своих исследования T.A. Stamey и W.E. Stamm [36,37]. В метаанализе G. Williams показан эффект длительного (10-52 недели) применения антибактериальных препаратов, который превосходит плацебо, таким образом обоснованы рекомендации по использованию данного метода терапии [38].
НИФУРАТЕЛ
Нифурател – химиотерапевтический препарат (5 [(метилтио)-метил]-3[(5-нитрофурфурилиден) амино]-2-оксазолидинон. Обладает активностью в отношении E.coli, Klebsiella spp., Enterobacter spp, Enterococcus spp., Staphylococcus spp., Streptococcus faecalis, Proteus mirabilis, Citrobacter spp., Shigella spp., Bacillus subtilis, Salmonella spp. и др. с минимально ингибирующей концентрацией (МИК), составляющей 2,550 мкг/мл; выраженной трихомона-цидной активностью, также активен in vitro в отношении грибов с МИК в отношении Candida albicans 500 мкг/мл [39]. In vitro нифурател ингибировал T.vaginalis в концентрации 0,25 мкг/мл. Обладает активностью в отношении представителей семейства Mollicutes, и, в частности, против Ureaplasma urealyticum, значения МИК составили 31,2-125 мкг/мл для M. pneumoniae; 15,6-125 для M. hominis и 62,2-125 для U. Ure-alyticum (рис. 1) [40].
Активность в отношении три-хомонад практически не отличается от таковой у метронидазола, нифу-рател ингибирует почти 100% штаммов в концентрациях от 0,1 до 10 мкг/мл. Также ингибирует Bac-teroides fragilis и Clostridium perfrin-gens, хотя и в меньшей степени, чем метронидазол [41].
Нифурател не обладает активностью в отношении Lactobacillus spp. Это положительный факт
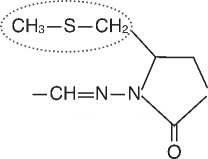
Нифурател (Макмирор)
производное нитрофурана с антибактериальными (E.coli, Klebsiella spp., Enterococcus spp., Staphylococcus spp., Proteus mirabilis и др.), противогрибковыми (Candida albicans) и противопротозойными свойствами
-
• Наличие в химической формуле нифуратела тиоэфирной группы обеспечивает:
-
- Широкий спектр системного действия (бактерии, простейшие, грибы)
-
- Выраженную бактерицидную и антипротозойную активность
-
- Низкую вероятность формирования резистентности
Рис. 1. Химическая формула и механизм действия Нифуратела.
для препарата, который применяется для лечения вагинальной и мочевой инфекции, не вызывая дисбиоза кишечника и влагалища, а также изменения рН влагалища. [42].
Нифурател в России выпускается в виде таблеток, 200 мг, и в виде вагинальных лекарственных форм, в комбинации с нистатитом (капсулы и крем). Плохо растворим в воде и ацетоне. Легко растворим в диметилформамиде. Антимикробная активность более широка, чем у нитрофурантоина. Фармакокинетические показатели нифуратела представлены в таблице 2.
Таблица 2. Фармакокинетика нифуратела
|
Пероральная абсорбция |
Неизвестно |
|
C max 200 мг перорально |
0,005-0,02 мг/л через 2 часа |
|
Период полувыведения из плазмы |
2,75 час |
|
Обьем распределения |
15 л |
|
Связь с белками плазмы |
30% |
Антибактериальная активность в моче обусловлена активными метаболитами. При использовании вагинальных суппозиториев нет системной абсорбции или она небольшая.
При инфекциях нижних мочевых путей дозы взрослым перорально 200-400 мг 3 раза в день. В литературе имеются данные о применении нифуратела при остром неосложненном цистите 400 мг 3 раза в день в первые 1-2 суток с дальнейшим переходом на поддерживающую терапевтическую дозу 200 мг 3 раза в день в последующие 3-4 дня [43,44].
R.N. Gruneberg в своем исследовании сравнивал бактериологические параметры нифуратела с нитрофурантоином в отношении 409 уропатогенов ( E.coli, P.mirabilis, Klebsiella spp, E. faecalis, P. aeruginosa, Staphylococci ) [45]. Минимально ингибирующие концентрации у нифуратела были ниже, чем у нитрофурантоина. Бактерицидные концентрации составили 3,23 для нитрофурантоина против 3,16 для нифуратела. Выраженный бактерицидный эффект для обоих препаратов наблюдали при рН =7, различий в степени выраженности их бактерицидного действия выявлено не было.
Y.M.T. Hamilton-Miller с соавт. исследовали активность пяти препаратов нитрофуранового ряда (нитрофурантоин, нифурател, нит-рофуразон, фуразолидон, SQ 18,506) в отношении 201 микробного штамма [46]. E.coli, Enterococcus faecalis, micrococci, Staphylococcus epidermidis были чувствительны ко всем 5 соединениям. Наиболее активными были фуразолидон и SQ 18,506. Штаммы Proteus spp, Providentia stu-artii, P.aeruginosa обладали рези- стентностью. Нифурател ингибировал несколько штаммов Candida albicans. В целом, антимикробные свойства нифуратела превосходили таковые у нитрофурантоина.
F. Dubini, P. Furneri исследовали активность нифуратела против возбудителей инфекций, передаваемых половым путем: Сhlamydia trachomatis, Micoplasma hominis, Micoplasma pneumoniae, Candida, Haemophilus vaginalis, Trichomonas vaginalis [47]. Нифурател обладал выраженным ингибирующим действием в отношении Chlamydia trachomatis. Отмечено ингибирование типичных внутриклеточных включений в концентрациях от 15,6 до 62,5 мкг/мл, что в 30 и 8 раз, соответственно, ниже цитотоксической дозы для данных клеток (цитопатогенный эффект при дозе 500 мкг/мл).
W. Mendling, F. Mailland в 2002 году отметили сильную антитрихо-монадную активность нифуратела, равную метронидазолу, но в отличие от него, обладающему широким антибактериальным спектром активности, включающим грам-отри-цательные и грам-положительные микроорганизмы, а также активность против Chlamydia trachomatis и Mycoplasma spp., Candida spp. и ми-цеты [48]. Нифурател имеет очень низкий токсикологический профиль, практически не токсичен в остром тесте у мышей и крыс и хорошо переносится при повторном пероральном и вагинальном приемах. Ни-фурател не обладает тератогенным эффектом. Сравнение ранних и недавних клинических исследований подтвердило, что в противоположность метронидазолу, нет сообщений о развитии резистентности к нифурателу. Препарат может применяться у беременных, так как у него не отмечено тератогенных эффектов. Более того, у пациенток с вульвагинальной инфекцией нифу-рател показывает благоприятное соотношение риск/польза [49].
При взаимодействии нифура-тела с алкоголем возможны ди-сульфирам-подобные реакции. Из
3 201 8
нежелательных побочных реакций, в основном, отмечают нарушения со стороны желудочно-кишечного тракта. Основными противопоказаниями пероральному назначению и приему нифуратела являются: дефицит сахарозы/изомальто-зы, непереносимость фруктозы, глюкозо-галактозная мальабсорбция, гиперчувствительность.
В ходе проведенных экспериментальных исследований для лечения вагинальных инфекций, вызванных Trichomonas vaginalis, отработаны дозы для комбинированной пероральной и интравагинальной терапии (600 мг/день перорально в течение 7 дней и 250-500 мг/день интравагинально на протяжении 10 дней). Эффективность комбинированной пероральной и интрава-гинальной терапии всегда выше, чем при применении только перорально (600-800 мг/день) или только интравагинально (250-500 мг/день). Для достижения лучшего результата рекомендован параллельный пероральный прием препарата нифура-тел сексуальным партнером. При вульвовагините, вызванном Trichomonas vaginalis, Candida albicans или смешанной бактериальной флорой, введение нифуратела перорально и интравагинально, дополняющиеся местным применением препарата в виде мази, обеспечивало очень хорошие результаты.
Для лечения негонококкового уретрита у мужчин нифурател эффективен при пероральном приеме по 800-1200 мг/день в течение 7-10 дней.
Эффективность нифуратела, перорально по 1200 мг/день на протяжении 1-3 недель для лечения инфекций мочевыводящих путей, вызванных преимущественно Escherichia coli, была оценена у более чем 600 пациентов; более высокие показатели эффективности лечения наблюдались при острых ИМП (62-93%), тогда как у пациентов с хроническими ИМП эффективность отмечена лишь в 50% случаев.
При оценке безопасности ни-фуратела были проанализированы результаты 62 исследований, в которых приняли участие 6319 пациентов. Из них 8 исследований были контролируемыми и оценивали эффективность нифуратела с контрольными препаратами (931 пациент); 49 – открытые, неконтролируемые исследования у пациентов с ИМП (перорально, интравагиналь-но или комбинировано) всего обследовано 5185 пациентов; 5 исследований проведены у больных с протозойными кишечными инфекциями при пероральном приеме ни-фуратела (203 пациента). Всего у 2,2% пациентов зафиксированы нежелательные побочные реакции. Из них в 11,6% случаев отмечали изжогу, 35,8% – тошноту, расстройства пищеварения отмече-ны у 10,8%, боли в животе –у 1,7%, метеоризм – у 5,8%, зуд, сыпь и крапивница, экзантема, покраснение лица – у 1,7%-5,8%, жжение и раздражение во влагалище – у 14,2%. Число пациентов, прервавших лечение из-за НПР равно 0,11% [50,51].
Нифурател можно безопасно назначать беременным женщинам, как при инфекции мочевых путей, так и при трихомонадной и канди-дозной инфекциях. При лечении более чем 150 беременных женщин серьезных нежелательных побочных действий не отмечено [52].
Клиническая эффективность применения нифуратела 600 мг/сут в течение 7 дней перорально и 250500 мг/сут. в течение 10 дней интра-вагинально) при вагинальных инфекциях, вызванных Trichomonas vaginalis, Gardnerella vaginalis, Candida spp, E.coli и смешанной бактериальной флорой более чем у 800 пролеченных пациенток достигала 82-85%, микробиологическая эффективность – 88-96%. Вагинальное применение нифуратела рекомендуют в дозах 250-500 мг/сут, чаще всего, в течение 8-10 дней; его, в основном, применяют при трихомонадном или кандидозном вагинитах у беременных [53].
Эффективность перорального приема нифуратела в дозе 1200 (400 мг каждые 8 часов) мг/сут в течение 7 дней при лечении инфекций мочевых путей, вызванных E.coli, у более чем 600 пациентов была показана в 62-93% случаев. По эффективности он схож с действием нитрофурантоина (100 мг каждые 8 часов в течение 7 дней).
Таким образом, антибактериальная активность препарата примерно равна или превышает активность нитрофурантоина, что делает его пригодным для эффективного лечения инфекций мочевых путей. Профиль безопасности препарата благоприятный, нежелательные побочные реакции возникали редко, в основном со стороны желудочно-кишечного трака, он хорошо переносился, аллергические реакции были крайне редки.
ФУРАЗИДИН
Фуразидин представляет собой аналог нитрофурантоина, который имеет более сильный эффект, чем его исходное соединение, на грамположительные и грамотри-цательные бактерии, а также по сравнению с сульфонамидом и некоторыми антибиотикорезистентными патогенными штаммами. Активность фуразидина увеличивается в кислой моче. Чем выше значение рН, тем больше уменьшается его эффективность. Фурази-дин используется как при острых, так и при хронических инфекциях мочевыводящих путей [54-56].
После приема внутрь фурази-дин быстро всасывается из пищеварительного тракта, достигая максимальной концентрации в плазме в течение получаса (максимальной терапевтической концентрации после 1,5-2 часов) и сохраняется в течение 4-5 часов.
Примерно 13-40% от дозы фу-разидина выводится в неизмененном виде в течение первых суток после введения. Средние концентрации фуразидина в моче (от 0,45 до 6,25 мг/мл) находятся в пределах значений минимальных подавляющих концентраций (МПК) для 80% патогенных штаммов кишечной палочки.
Взаимодействие с другими лекарственными препаратами
Фуразидин является антагонистом налидиксовой кислоты, ингибируя ее бактериостатическую активность. Урикозурические препараты, такие как пробеницид (в больших дозах) и сульфинпиразон уменьшают канальцевую секрецию производных нитрофурана и могут вызвать накопление фуразидина в организме, увеличивая его токсичность и снижая концентрацию в моче ниже минимальной бактериостатической, что приводит к снижению терапевтической эффективности данного препарата. Одновременное введение ощелачивающих средств, содержащих три-силат магния, ухудшает всасывание фуразидина.
Нежелательные побочные действия фуразидина такие же как и для всей группы нитрофуранов. Со стороны нервной системы на фоне лечения нитрофуранами отмечены головокружение, головные боли, сонливость, нарушения зрения, периферическая нейропатия [57].
Особые предостережения
У пациентов с почечной недостаточностью, анемией, дефицитом витамина В и фолиевой кислоты, а также с заболеваниями легких необходимо соблюдать особую осторожность. У пациентов с сахарным диабетом может развиться полинейропатия. Клинические исследования показали отрицательное влияние на функцию яичек. Они могут снижать подвижность сперматозоидов, уменьшать секрецию спермы и вызывать патологические изменения в морфологии сперматозоидов.
Особые категории пациентов
Резюме: Summary:Nitrofurans in the urological practice: are they all the
Увеличивающийся уровень антимикробной резистентно- same and why are we getting back to them today?
сти у микроорганизмов является одной из важных проблем здравоохранения. Основной движущей силой является повсе- Perepanova T.S.
местное потребление антибиотиков. ВОЗ сигнализирует о
The article comprises a literature review on nitrofurans,
росте антимикробной резистентности вместе с неуклонным снижением открытия новых антимикробных препаратов. Чтобы контролировать антимикробную резистентность необходимо более рационально использовать существующие препараты. Поэтому, необходима переоценка эффективности и побочных эффектов старых уже много лет известных нам антимикробных препаратов, таких как нитрофураны.
Нитрофураны очень эффективны при острых инфекциях, в основном в мочевых путях с низкой концентрацией препарата в тканях, они не вызывают бактериальную резистентность так быстро, как другие антибиотики.
Несмотря на имеющиеся сходства в строении молекул препаратов нитрофуранового ряда, каждая молекула имеет свои особенности, которые выражаются и в проявлении клинического эффекта и переносимости. Главное, что вызывает «новый» интерес в урологии к этой группе препаратов на сегодня – это редкое формирование резистентности и довольно широкий спектр антимикробной активности для применения в урологической практике.
Автор заявляет об отсутствии конфликта интересов.
which are chemotherapeutic drugs with antimicrobial action.
Due to the growth of resistance to broad-spectrum antibiotics – cephalosporins and fluoroquinolones, the use of which is sharply limited during the lower urinary tract infections, nitrofurans, along with fosfomycin trometamol, remain the first-choice drugs for treating acute and recurrent lower urinary tract infections (LUTI).
This paper presents the microbiological, pharmacokinetic and clinical data of various representatives of the nitrofuran group: nitrofurantoin, nifuratel and furazidin. A Cochrane modern systematic review on the use of nitrofurans in the prevention of recurrent LUTI is shown. The data on undesirable side effects of administration of nitrofurans, on the safety of their use and interaction with other drugs, as well as on the use of this group of drugs in special categories of patients are provided.
Author declare lack of the possible conflicts of interests.
Список литературы Нитрофураны в урологической практике: все ли они одинаковые и почему мы возвращаемся к ним сегодня?
- Goossens H, Ferech M, Vander Stichele R, Elseviers M, ESAC Project Group. Outpatient antibiotic use in Europe and association with resistance: a crossnational database study. Lancet 2005;365(9459):579e87. Tacconelli E. Antimicrobial use: risk driver of multidrug resistant microorganisms in healthcare settings. Curr Opin Infect Dis 2009;22:352e8).
- World Health Organisation. Global action plan on antimicrobial resistance. Geneva: WHO; 2015. www.who.int/drugresistance/en.)
- Федеральные клинические рекомендации «Антимикробная терапия и профилактика инфекций почек, мочевыводящих путей и мужских половых органов», Москва, 2017г, 70с.
- Многоцентровое исследование резистентности возбудителей амбулаторных инфекций мочевыводящих путей (UTIAP-3). Научный отчет НИИ антимикробной химиотерапии ГОУ ВПО «Смоленская государственная медицинская академия» Федерального агентства по здравоохранению и социальному развитию. М. 2006
- Albert X, Huertas I, Pereiro II, Sanfelix J, Gosalbes V, Perrota C. Antibiotics for preventing recurrent urinary tract infection in non-pregnant women. Cochrane Database Syst Rev 2004;(3):CD001209
- Sanchez GV, Baird AM, Karlowsky JA, Master RN, Bordon JM. Nitrofurantoin retains antimicrobial activity against multidrug-resistant urinary Escherichia coli from US outpatients. JAntimicrob Chemother 2014;69:3259e62).
- Nicolle LE, Bradley S, Colgan R, et al. Infectious Diseases Society of America guidelines for the diagnosis and treatment of asymptomatic bacteriuria in adults. Clin Infect Dis 2005;40:643-54.
- Nicolle LE, Harding GK, Thomson M, et al. Efficacy of five years of continuous, low-dose trimethoprim-sulfamethoxazole prophylaxis for urinary tract infection. J Infect Dis 1988;157:1239
- Stamm WE, Counts GW, McKevitt M, Turck M, Holmes KK.Urinary prophylaxis with trimethoprim and trimethoprim-sulfamethoxazole: efficacy, influence on the natural history of recurrent bacteriuria, and cost control. Rev Infect Dis, 1982;4(2):450-5.
- A.E.Muller, E.M.Verhaegh, S. Harbarth, J.W.Mouton, A. Huttner. Nitrofurantoin’s efficacy and safety as prophylaxis for urinary tract infections: a systematic review of the literature and meta-analysis of controlled trials. Clinical Microbiology and infection 23 (2017) 355-362
- «Old» is not always bad.T.J. Verheij, S.E. Geerlings. http://dx.doi.o DOI: rg/10.1016/j.cmi.2016.08.003
- Dorota Olender, Justyna Zwawiak, and Lucjusz Zaprutko, Pharmaceuticals (Basel). 2018 Jun; 11(2): 54 DOI: 10.3390/ph11020054
- Практическое руководство по антиинфекционной химиотерапии/под ред. Л.С. Страчунского, Ю.Б.Белоусова, С.Н. Козлова, Смоленск: МАКМАХ,2007.-с.128)
- Roger G. Finch, David Greenwood, S. Ragnar Norrby, Richard J Whitley. Livigstone, 2003,328-334s
- Holmberg L, Boman G. Pulmonary reactions to nitrofurantoin. 447 cases reported to the Swedish Adverse Drug Reaction Committee 1966-1976. Eur J Respir Dis. 1981 Jun;62(3):180-9
- Holmberg L, Boman G, Böttiger LE, Eriksson B, Spross R, Wessling A Adverse reactions to nitrofurantoin. Analysis of 921 reports. Am J Med. 1980 Nov;69(5):733-8.
- Brumfitt W., Hamilton-Miller J.M. Efficacy and safety profile of long-term nitrofurantoin in urinary infections: 18 years' experience. J Antimicrob Chemother. 1998. 42 (3): 363-71
- Kalowski S, Radford N, Kincaid-Smith P. Crystalline and macrocrystalline nitrofurantoin in the treatment of urinary-tract infection. N Engl J Med. 1974 Feb 14;290(7):385-7
- Guay D.R. An update on the role of nitrofurans in the management of urinary tract infections. Drugs. 2001; 61(3):353-64., Cunha B.A. Antibiotic side effects. Med Clin North Am. 2001. 85 (1): 149-85.
- Brumfitt W., Hamilton-Miller J.M. Efficacy and safety profile of long-term nitrofurantoin in urinary infections: 18 years' experience. J Antimicrob Chemother. 1998. 42 (3): 363-71;
- Karpman E., Kurzrock E.A. Adverse reactions of nitrofurantoin, trimethoprim and sulfamethoxazole in children. J Urol. 2004. 172 (2): 448-53
- Roger G. Finch, David Greenwood, S. Ragnar Norrby, Richard J Whitley. Antibiotic and Chemotherapy. Anti-infective agents and their use in therapy. Churchill Livigstone, 2003,328-334s
- Goettsch W.G., Janknegt R., Herings R.M. Increased treatment failure after 3-days' courses of nitrofurantoin and trimethoprim for urinary tract infections in women: a population-based retrospective cohort study using the PHARMO database. Br J Clin Pharmacol. 2004. 58 (2): 184-9
- Hoang P, Salbu RL, Updated Nitrofurantoin Recommendations in the Elderly: A closer Look at the Evidence. Consult Pharm.2016; 31:381-4
- Ho PL, Ng KY, Lo WU, Law PY, Lai EL, Wang Y et al. Plasmid-mediated oqxAB is an important mechanism for Nitrofurantoin resistance in Escherichia coli. Antimicrob Agents Chemother. 2015; 60:537-43
- Huttner A, Verhaegh EM, Harbarth S, Muller AE, Theuretzbacher U, Mouton JW. Nitrofurantoin revisited: a systematic review and meta-analysis of controlled trials. J Antimicrob Chemother;2015;70:2456-64
- Scherwin J, Holm P. Long-term treatment with sulphamethoxazole/trimethoprim (Bactrim) and nitrofurantoinin chronic urinary tract infections. A controlled clinical trial. Chemotherapy. 1977;23(4):282-8
- Uhari M, Nuutinen M, Turtinen J. Pediatr Infect Dis J. 1996 May;15(5):404-8.)
- Stewardson A.J., Vervoot J, Adriaenssens N, Coenen S, Godycki-Cwirko M,Kowalczyk A., Huttner B., Lammens C., Malhotra-Kumar S., Goossens H., Harbarth S.; SATURN WP1 AND wp3 Study Groups. Effect of outpatients antibiotics for utinary tract infections on antimicrobial resistance aming commensal Enterobacteriaceae: a multinational prospective cohort study. Clin Microbiol Infect.2018 Jan 10
- A.E.Muller, E.M.Verhaegh, S. Harbarth, J.W.Mouton, A. Huttner. Nitrofurantoin’s efficacy and safety as prophylaxis for urinary tract infections: a systematic review of the literature and meta-analysis of controlled trials. Clinical Microbiology and infection 23 (2017) 355-362
- Sourander L, Saarimaa H. Effect of long-term treatment of urinary tract infection with a single dose in the evening. Chemotherapy. 1975;21(1):52-5
- Iravani A, Klimberg I, Briefer C, Munera C, Kowalsky SF, Echols RM. J Antimicrob Chemother. 1999 Mar;43 Suppl A:67-75.A trial comparing low-dose, short-course ciprofloxacin and standard 7 day therapy with co-trimoxazole or nitrofurantoin in the treatment of uncomplicated urinary tract infection.
- Yüksel S, Oztürk B, Kavaz A, Ozçakar ZB, Acar B, Güriz H, Aysev D, Ekim M, Yalçinkaya F. Antibiotic resistance of urinary tract pathogens and evaluation of empirical treatment in Turkish children with urinary tract infections. Int J Antimicrob Agents. 2006 Nov;28(5):413-6. Epub 2006 Sep 26;
- Helin I, Three-day therapy with cephalexin for lower urinary tract infections in children. Scand J Infect Dis. 1984;16(3):305-7;
- Baka-Ostrowska M. Pol Merkur Lekarski. 2008;24 Suppl 4:95-7. Review. Polish. Vesicoureteral reflux and urinary tract infections).
- Stamey TA, Condy M, Mihara Prophylactic efficacy of nitrofurantoin macrocrystals and trimethoprim-sulfamethoxazole in urinary infections. Biologic effects on the vaginal and rectal flora. G.N Engl J Med. 1977 Apr 7;296(14):780-3.
- Stamm WE, Counts GW, McKevitt M, Turck M, Holmes KK.Urinary prophylaxis with trimethoprim and trimethoprim-sulfamethoxazole: efficacy, influence on the natural history of recurrent bacteriuria, and cost control. Rev Infect Dis. 1982 Mar-Apr;4(2):450-5.
- Williams G, Craig JC. Long-term antibiotics for preventing recurrent urinary tract infection in children. Cochrane Database Syst Rev. 2011 Mar 16;(3):CD001534. Review DOI: 10.1002/14651858.CD001534.pub3
- Coppi G. Nifuratel: pharmacokinetics in rat and rabbit. Archives of Polichem-Report of November 30th 1992
- Delivenneri A, Conrado M, Dzik S et al. Terapeutica das infeccioes vaginais com novo quimiotherapico Nifuratel. Rev Bras Med 1969; 26;261
- Di Paola M, Di Tocco M, Suppa F. Sulla terapia della tricomoniasi vaginale con nifuratel. Min Ginecol 1970; 22 (N 17);853
- Fowler W, Hussain M. Nifuratel in trichomonal vaginitis. Brit J Ven Dis,1968; 44; 331
- J.Kladensky Nifuratel in treatment of acute uncomplicated urinary tract in-fections//Czech urology 1998. -5:8-10
- J.Kladensky Benefit of Nifuratel in treating acute uncomplicated urinary tract infections//Urologie pro praxi. -2006. -3:108-110
- Gruneberg R.N. The use of nitrofurans in the treatment of urinary tract infections with some observations on the in vitro properties of nifuratel. In: F. Gasparri, G.Gargani, P.Periti, Firenze, Italy, 1972, pp.109-112
- Hamilton-Miller JMT, Brumfitt W, Williams RJ. Comparative in vitro activity of five nitrofurans. Chemotherapy 1978;24; 161
- Dubini F, Furneri P Attivita antimicrobica del NIfuratel. G Ital Chemoter 1985; 32:545
- Mendling W, Mailland F. Microbiological and Pharmaco-toxicological Profile of Nifuratel and vits Favourable Risk/Benefit Ratio for the Treatment of Vulvo Vginal Infections/Arzneim Forsch/drug Res 2002;52(1):8-13
- Gagliardi, S.; Consonni, S.; Ronzoni, A.; Bulgheroni, A.; Ceriani, D. Nifuratel Sulfoxide for Use in the Treatment of Bacterial Infections. EP Patent EP 2797914 B1, 16 September 2015
- Arnold M. Vergleich von Nifuratel un Tinidazol bei Trichomonaden vaginitis. Ther Umsch 1974; 31:202;
- Brumfitt W. The use of nifuratel in asymptomatic bacteriuria in pregnancy and the frequency-disuria syndrome in general practice.//In: F. Gasparri, G. Gargani, P.Periti.Diagnosis and Chemotherapy of urogenital infections. Edizioni Medicine P.Periti, Firenze, Italy 1972, pp.411-416.
- Aure J Chr, Gjonnaess. Treatment of candida vaginitis with nifuratel. Acte Obst et Gynec Scandinav 1969; 49-95; Carrera Macia JM, Dexeus S. Tratamento de la leucorrea infecciosa. Rev Esp de Obstet y Gynecol 1966; 25:308;
- Liang Q, Li N, Song S, Zhang A, Duan Y. High-dose nifuratel for simple and mixed aerobic vaginitis: a single -center prospective open-label cohort study. J Obstet Gynecol Res 2016 Oct; 42 (10):1354-1360
- Bains, A.; Buna, D.; Hoag, N.A. A retrospective review assessing the efficacy and safety of nitrofurantoin in renal impairment. Can. Pharm. J. 2009, 142, 248-252.
- Cunha, B.A.; Schoch, P.E.; Hage, J.R. Nitrofurantoin: preferred empiric therapy for community-acquired lower urinary tract infections. Mayo Clin. Proc. 2011, 86, 1243-1244.
- El-Zaher, A.A.; Mahrouse, M.A. A validated spectrofluoremetric method for the determination of nifuroxazide through coumarin formation using experimental design. Chem. Cent. J. 2013, 7, 90
- Männistö P, Karttunen P. Pharmacokinetics of furagin, a new nitrofurantoin congener, on human volunteers. Int J Clin Pharmacol Biopharm. 1979 Jun;17(6):264-70
- Czeizel AE, Rockenbauer M, S0rensen HT, Olsen J.A population-based case-control teratologic study of furazidine, a nitrofuran-derivative treatment during pregnancy. Clin Nephrol. 2000 Apr;53(4):257-63


